Abstract
Using transgenic mice that replicate hepatitis B virus (HBV) in their livers, we previously showed that passively transferred HBV-specific cytotoxic T cells (CTLs) recruit antigen-nonspecific lymphomononuclear and polymorphonuclear inflammatory cells that contribute to the pathogenesis of liver disease. This process is chemokine-dependent, because we recently showed that blocking the chemokines CXCL9 and CXCL10 reduces the recruitment of antigen-nonspecific lymphomononuclear cells and the severity of liver disease after CTL injection. In the current study we show that the severity of the CTL-initiated liver disease is also ameliorated by the depletion of neutrophils. Interestingly, depletion of neutrophils does not affect the intrahepatic migration or antiviral activity of CTLs, but it profoundly inhibits the recruitment of all antigen-nonspecific cells into the liver. This effect occurs in face of high intrahepatic levels of chemokine gene expression, suggesting that neutrophil-dependent functions other than chemokine induction are necessary for the recruitment process to occur. The notion that depletion of neutrophils is associated with maintenance of antiviral effects but diminished tissue damage may be significant for the development of immunotherapeutic approaches for the treatment of chronic HBV infection.
The hepatitis B virus (HBV) is an enveloped DNA virus that causes acute and chronic liver disease characterized by a necroinflammatory cell infiltrate and Kupffer cell hyperplasia (1). HBV is not directly cytopathic for the hepatocyte, and therefore the immune response to viral antigens is thought to be responsible for both liver disease and viral clearance after HBV infection. Using transgenic mice that sustain high-level HBV replication in the liver (2), we have shown that most of the antiviral potential of virus-specific cytotoxic T cells (CTLs) is mediated by noncytolytic mechanisms that involve the intrahepatic production of IFN-γ by the CTLs after antigen recognition (3–5). We also showed that the direct cytopathic potential of the CTLs is limited, involving the apoptotic death of a small number of hepatocytes and resulting in widely scattered, acidophilic, Councilman bodies (apoptotic hepatocytes) that are typical of acute viral hepatitis in humans (6). This process is followed by the intrahepatic recruitment of many host-derived inflammatory cells that contribute to the formation of necroinflammatory foci in which apoptotic hepatocytes and CTLs are outnumbered by host-derived lymphomononuclear and polymorphonuclear cells (6, 7).
The recruitment process is thought to be a chemokine-dependent event, because we recently showed that blocking the chemokines CXCL9 (a monokine induced by IFN-γ, Mig) and CXCL10 (a chemokine responsive to γ-2/IFN-γ-inducible protein, IP10) reduces the migration of antigen-nonspecific lymphomononuclear cells and the severity of liver disease after CTL injection (8). The association of reduced liver disease with reduced recruitment of antigen-nonspecific lymphomononuclear cells implies that these cells can amplify the liver damage initiated by the antigen-specific CTLs. It is noteworthy also that those studies showed that the severity of liver disease was reduced but not abolished by the passive neutralization of CXCL9 and CXCL10, and the recruitment of polymorphonuclear neutrophils (PMNs) was basically unaffected (8). These results suggest that the residual liver damage observed in those animals could be due to the CTLs, the PMNs, or both.
PMNs are the most abundant leukocytes in the body and play a fundamental role in host defense by phagocytosing invading microorganisms (9). It is becoming clear that PMNs may be involved in many more biological functions than just phagocytosis. Indeed, PMNs can be induced to express a large number of genes involved in immune regulatory processes, and they include cytokines, cytokine receptors, growth factors [such as IFN-α, tumor necrosis factor (TNF)-α, IL-1-β, IL-6, IL-1Rα, IL-12, granulocyte/macrophage colony-stimulating factor, and vascular endothelial growth factor], chemokines (such as CXCL1, CXCL8, CXCL9, CXCL10, CCL2, CCL3, and CCL4), complement components, and even molecules with known antiviral activity such as Mx1 and PKR (10–12). Although the role of PMNs in the pathogenesis of HBV infection is poorly understood, it has been shown previously that the migration of PMNs into the liver of mice infected with adenovirus is associated with exacerbation of hepatitis (13, 14). An essential involvement of PMNs in perpetuating a T cell-mediated inflammatory reaction in the eye has also been proposed in the case of herpetic stromal keratitis (15).
To test the role of PMNs in our system, we depleted them from HBV transgenic mice before CTL transfer, and we monitored the ability of passively transferred CTLs to induce antiviral activity, liver disease, chemokine expression, and recruitment of inflammatory cells into the liver.
Materials and Methods
Mice.
HBV transgenic mouse lineage 1.3.32 used in this study has been described (2). Lineage 1.3.32 (inbred C57BL/6, H-2b) was bred one generation against B10D2 mice (H-2d) to produce H-2bxd F1 hybrids before injection of H-2d-restricted hepatitis B surface antigen (HBsAg)-specific CTLs. In all experiments, the mice were matched for age (8 weeks), sex (female), and hepatitis B e antigen (HBeAg) levels in their serum before experimental manipulations. All animals were housed in pathogen-free rooms under strict barrier conditions. These studies were approved by the review board of The Scripps Research Institute.
Injection of HBsAg-Specific CTL Clones.
HBV transgenic mice were injected with an HBsAg-specific, H-2d-restricted, CD8+ CTL clone (designated 6C2) that recognize an epitope (IPQSLDSWWTSL) located between residues 28 and 39 of HBsAg (7). Clone 6C2 was maintained as described (7). Five days after the last stimulation, the cells were washed, counted, and injected intravenously (1 × 107 per mouse) into HBV transgenic mice. Groups of mice (three per group) were killed on days 1, 2, and 5 after injection, and their livers were perfused and harvested for histological and flow-cytometry analyses, or they were snap-frozen in liquid nitrogen and stored at −80°C for subsequent molecular analyses (see below).
Antineutrophil Abs.
Rat IgG2b monoclonal Abs specific for mouse Ly-6G (Gr-1) (clone RB6-8C5) and rat IgG2b control Abs (clone A95-1) (BD PharMingen, San Diego) were used. Mice that were killed on day 1 after CTL injection were injected i.p. twice with anti-Gr-1 or control Abs (100 μg per mouse), first 16 h before and then simultaneously with the i.v. injection of the CTLs. A third injection (100 μg per mouse) was administered 24 h later to mice that were killed on day 2 after CTL injection. Two more injections (50 μg per mouse) were administered on days 3 and 4 to mice that were killed on day 5 after CTL injection.
Tissue DNA and RNA Analyses.
Total DNA and RNA were isolated from frozen livers (left lobe) and analyzed for HBV DNA by Southern blot and cytokine and chemokine mRNAs by RNase protection exactly as described (2, 3, 8). The relative abundance of specific DNA and RNA molecules was determined by phosphorimaging analysis using the OPTIQUANT image-analysis software (Packard).
Biochemical and Histological Analyses.
The extent of hepatocellular injury was monitored by measuring serum alanine aminotransferase (sALT) activity at multiple time points after treatment. sALT activity was measured in a Paramax chemical analyzer (Baxter Diagnostics, McGaw Park, IL) exactly as described (3). For histological analysis, livers were fixed in 10% zinc-buffered formalin (Anatech, Battle Creek, MI), embedded in paraffin, sectioned (3 μm), and stained with hematoxylin/eosin (3). Quantitative morphometric analysis of the number and size of intrahepatic inflammatory foci (scored as apoptotic hepatocytes and inflammatory cells) was performed by counting ≈100 high-power (×400) fields representing 4 mm2 of liver tissue.
Isolation and Analysis of Intrahepatic Leukocytes (IHLs).
Mouse livers were weighed at the time of autopsy. Single-cell suspensions were prepared from two liver lobes of known weight, and analysis of the IHL population was performed by flow cytometry exactly as described (8). The cells were surface-stained with FITC-, phycoerythrin-, or allophycocyanin-labeled anti-CD4, anti-CD8, anti-CD3, anti-T cell antigen receptor (TCR), anti-natural killer (NK)1.1, anti-CD19, anti-Gr-1, anti-CD11b, and anti-CD11c Abs (PharMingen) for the detection of NK1.1+/CD3− cells (NK cells), CD8+/TCR+ cells (mostly CTLs), CD4+/TCR+ (mostly T helper cells), CD11b−/CD11c+ (mostly lymphoid dendritic cells), CD11b+/CD11c+ cells (mostly myeloid dendritic cells), Gr-1−/CD11b+/CD11c− (mostly macrophages), CD19+ (B cells), and Gr-1+/CD11b+ cells (mostly PMNs). Samples were acquired on a FACSCalibur flow cytometer, and the data were analyzed by using CELLQUEST software (Becton Dickinson Immunocytometry Systems).
Quantitative Analyses of the Intrahepatic Content of the Transferred CTLs.
Male CTLs (clone 6C2) were injected into female transgenic mice, and the animals were killed at different times after injection. Total liver DNA was extracted as described (2). Fifty micrograms of DNA were digested with HindIII, electrophoresed overnight on a 1% agarose gel, and stained with ethidium bromide. Regions of gels that contained DNA molecules between 3.6 and 4 kb in length were excised, and DNA was purified by using QIAquick columns (Qiagen, Valencia, CA) according to manufacturer instructions. Primers specific for the Sry gene contained in the mouse Y chromosome (16) (GenBank accession no. X67204) (sense, 5′-GCAGTTGCCTCAACAAAACTGT-3′, and antisense, 5′-AGGTGTGCAGCTCTACTCCAG-3′) and primers specific for the internal control cholecystokinin type-A receptor (CCKAR) (17) (GenBank accession no. D85605) contained in the mouse chromosome 5 (sense, 5′TCATTCTGCCCTCTAAACCC-3′, and antisense, 5′-GTGATAACCAGCGTGTTCCC-3′) were selected within Sry- and CCKAR-restricted DNA regions of 3,811 and 3,718 bp in length, respectively. Fifty nanograms of excised DNA were subjected to quantitative PCR by using Sry- and CCKAR-specific primers in an iCycler apparatus (Bio-Rad) and SYBR green was used as the fluorescent dye. Each sample was run in triplicate with both primers. Sry- and CCKAR-specific amplicons were also cloned into pGEM-T Easy vector (Promega), and standard curves were produced by amplifying known copy numbers of Sry-specific (104, 103, 102, 50, 25, 12.5, and 6.25 copies per well) and CCKAR-specific (108, 107, 106, 105, 104, 103, and 102 copies per well) DNA. After normalization with the CCKAR internal control, the average copy number of Sry-specific amplicons in each group of mice (three mice per group) was calculated on a 10,000-liver-cell genome basis.
Results and Discussion
Anti-Gr-1 Treatment Depletes PMNs in Vivo.
To monitor whether the anti-Gr-1 treatment depleted PMNs in the mice that received virus-specific CTLs, the absolute number of intrahepatic Gr-1+/CD11b+ cells was quantitated by fluorescence-activated cell sorter analysis in groups of age- and serum HBeAg-matched female transgenic mice from lineage 1.3.32 (three mice per group) that were injected with 1 × 107 HBsAg-specific CTLs (clone 6C2) and anti-Gr-1 Abs. Mice were bled, killed, and perfused, and livers were harvested on days 1, 2, and 5 after CTL transfer. The results were compared with additional groups of transgenic mice (three mice per group) that were injected with either saline (NaCl) alone or CTL and irrelevant (Irr) control Abs and killed at the same time points.
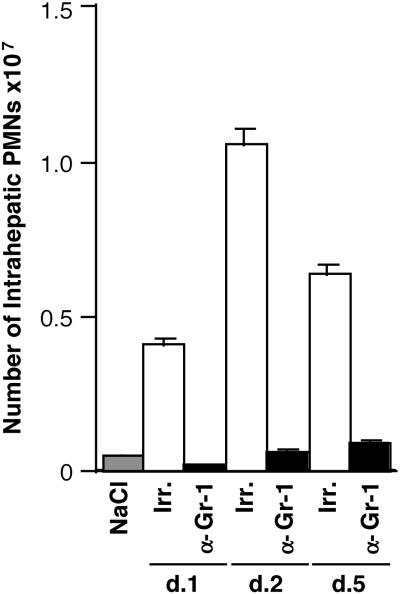
When compared with NaCl-injected controls (Fig. 1, gray bars), the total number of Gr-1+/CD11b+ cells significantly increased in the liver of CTL-treated mice that received Irr Abs (Fig. 1, white bars). The number of Gr-1+/CD11b+ cells increased 8.7-, 21.3-, and 13.6-fold on days 1, 2, and 5, respectively, whereas little or no increase in the number of Gr-1+/CD11b+ cells (0.47-, 1.36-, and 1.98-fold on days 1, 2, and 5, respectively) was observed in mice that received anti-Gr-1 Abs (Fig. 1, black bars). A strong increase in the number of Gr-1+/CD11b+ cells was observed also in the blood of CTL-treated mice that received Irr Abs, and this effect was blocked completely by the anti-Gr-1 treatment (data not shown). To confirm that the PMNs were depleted and not simply masked by the infused anti-Gr-1 Ab (clone RB6-8C5), peripheral blood mononuclear cells (PBMCs) were stained with an FITC-labeled anti-rat IgG2b that was specific for clone RB6-8C5 (clone G15-337, BD PharMingen) to detect cell-bound anti-anti-Gr-1. No PBMCs from anti-Gr-1-treated mice could be detected with this Ab, in contrast to PBMCs that were incubated with anti-Gr-1 in vitro and then stained (data not shown). These results indicate that the anti-Gr-1 treatment efficiently depleted PMNs in vivo.
Figure 1.
Anti-Gr-1 treatment quantitatively depletes PMNs in vivo. Nine age- and serum HBeAg-matched female transgenic mice from lineage 1.3.32 (three mice per group) were injected with anti-Gr-1 Abs and 1 × 107 HBsAg-specific CTLs (clone 6C2). Mice were bled and killed, and livers were harvested at days 1, 2, and 5 after CTL transfer. Livers were weighed at the time of autopsy. IHLs were isolated from two liver lobes of a known weight and analyzed by flow cytometry. The indicated numbers of Gr-1+/CD11b+ cells (PMNs) represent the numbers detected in the whole liver. The results were compared with additional groups of transgenic mice (three mice per group) that were injected with either saline (NaCl) alone or CTL and Irr control Abs.
Depletion of PMNs Does Not Affect the Intrahepatic Recruitment of HBV-Specific CTLs.
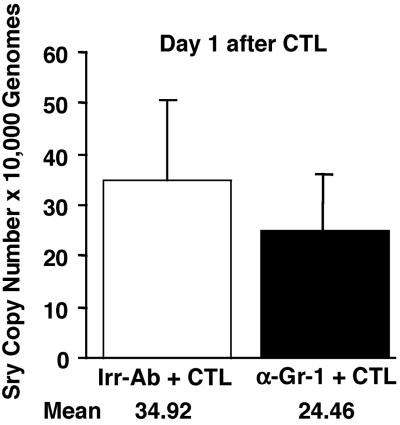
To determine whether the depletion of PMNs affected the recruitment of HBV-specific CTLs into the liver, we monitored the ability of anti-Gr-1 Abs to modulate this process. The recruitment of the passively transferred clone 6C2 [originally produced in male mice (7)] was measured by quantifying the amount of Sry-specific sequences [Sry is a gene contained in the Y chromosome (16)] and CCKAR-specific sequences [CCKAR is a single-copy gene present in chromosome 5 that was used as internal control (17)] in the liver of the same HBV transgenic female mice described above.
As shown in Fig. 2, the number of Sry-specific amplicons was very similar in the liver of mice that were killed on day 1 and received Irr or anti-Gr-1 Abs. Indeed, after normalization with the CCKAR internal control, we calculated that the average copy number (per 10,000 liver-cell genomes) detected in the liver of Irr- (white bars) versus anti-Gr-1-treated (black bars) mice was ≈35 and 24, respectively (Fig. 2). These results indicate that similar numbers of CTLs reached the liver at this time point, and therefore PMN depletion did not affect their intrahepatic recruitment. This conclusion is supported also by the finding that the levels of IFN-γ mRNA [a marker of antigen recognition by CTLs (3, 4, 8)] were virtually identical in Irr- and anti-Gr-1-treated mice (Fig. 3), reiterating the fact that the recruitment of the passively transferred CTLs did not depend on PMNs.
Figure 2.
Depletion of PMNs does not affect the intrahepatic recruitment of HBV-specific CTLs. The recruitment of the passively transferred clone 6C2 (originally produced in male mice) was measured in the same livers described in the legend to Fig. 1 by quantifying the amount of Sry- and CCKAR-specific sequences by real-time PCR. The indicated numbers represent the average copy numbers of Sry-specific amplicon per 10,000 liver-cell genomes.
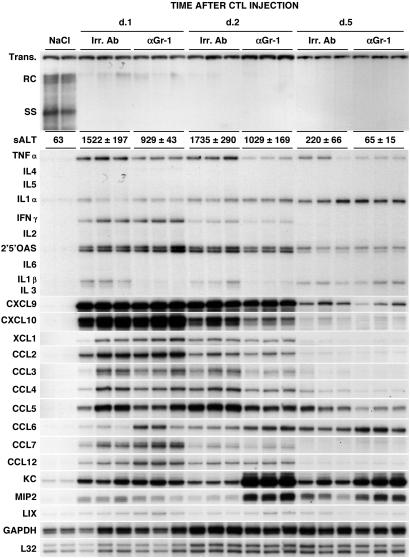
Figure 3.
Effect of PMN depletion on HBV replication, liver disease, and expression of cytokines and chemokines in the liver of CTL-injected HBV transgenic mice. Age- and serum HBeAg-matched female transgenic mice (three mice per group) described in the legend to Fig. 1 were killed at the indicated time points, and total hepatic DNA was analyzed for HBV replication by Southern blot analysis. Bands corresponding to the integrated transgene, relaxed-circular (RC), and single-stranded (SS) linear HBV DNA replicative forms are indicated. The integrated transgene can be used to normalize the amount of DNA bound to the membrane. The mean sALT activity (± standard deviation), measured at the time of autopsy, is indicated for each group and is expressed in units/liter. Total hepatic RNA from the same mice was analyzed also by RNase protection assay for the expression of various cytokines and chemokines as indicated. The housekeeping mRNAs encoding GAPDH and the ribosomal protein L32 were used to normalize the amount of RNA loaded in each lane. Results were compared with those observed in livers pooled from two age-, sex-, and serum HBeAg-matched transgenic littermates injected with saline (NaCl).
No detection of Sry-specific amplicons was observed by days 2 and 5 after transfer (data not shown), indicating that the number of CTLs that were present in the liver at these time points was below the detection limit of our assay (four Sry-specific amplicons per 10,000 liver-cell genomes). This result presumably reflects activation-induced cell death of the CTLs, because it has been shown in other systems as well (18, 19).
Depletion of PMNs Does Not Affect the Antiviral Potential of HBV-Specific CTLs but Ameliorates the Severity of Liver Disease.
To define the role of PMNs in the antiviral potential of HBV-specific CTLs, we monitored the content of HBV-replicative intermediates in the same livers described above. As shown in Fig. 3 Upper, administration of anti-Gr-1 Abs did not block the CTL-induced inhibition of hepatic HBV replication observed at days 1, 2, and 5 that also was detected in control mice that received CTLs plus Irr Abs. This outcome is not surprising, because similar transcript levels for IFN-γ and other cytokines [i.e., tumor necrosis factor-α and IFN-α/β (the induction of which was monitored by quantitating the content of 2′,5′-oligoadenylate synthetase mRNA)] with known antiviral activity against HBV (3, 4) were detected in the two groups of animals at each time point (Fig. 3). In keeping with the notion that activated PMNs can produce tumor necrosis factor-α and IL-1-β (10, 11), it is also noteworthy that the expression of these cytokines was reduced somewhat (particularly at days 1 and 2 after CTL transfer) in anti-Gr-1-treated mice as compared with the controls (Fig. 3). These results demonstrate that depletion of PMNs does not affect the antiviral potential of HBV-specific CTLs, and they also indicate that PMNs do not directly inhibit HBV replication themselves, nor do they regulate the antiviral potential of HBV-specific CTLs in our system.
The liver disease in these animals was monitored biochemically (and histologically, see below) at multiple time points after CTL or NaCl injection by quantitating the activity of sALT, a hepatocellular enzyme that is released into the circulation by necrotic hepatocytes. Similar to previous studies with this and other CTL clones (3, 7, 8, 20, 21), the liver disease in mice that received Irr Abs was transient, reached maximum severity at day 2, and subsided thereafter (Fig. 3). Treatment with anti-Gr-1 Abs diminished the severity of liver disease at all time points. Indeed, at the time of autopsy, the sALT activity of mice that received anti-Gr-1 Abs was reduced by ≈30% when compared with control animals at days 1, 2, and 5 after CTL (Fig. 3). The association of reduced liver disease with PMN depletion and unaffected recruitment of virus-specific CTLs implies that either PMNs contribute directly to the liver disease or that these cells are necessary for the intrahepatic recruitment of antigen-nonspecific lymphomononuclear cells that are known to amplify the CTL-induced liver-cell damage (8).
Depletion of PMNs Does Not Affect Intrahepatic Chemokine Expression.
We also monitored the effect of anti-Gr-1 treatment on the intrahepatic induction of CXCL9, CXCL10, and other chemokines, because we recently showed that blocking the chemokines CXCL9 and CXCL10 reduces the recruitment of host-derived lymphomononuclear cells into the liver and the severity of liver disease in this model (8).
As shown in Fig. 3, the messages for CXCL9 and CXCL10 [chemoattractants for lymphomononuclear cells (22, 23)] were induced at similar high levels in CTL-injected mice that received either Irr Abs or anti-Gr-1 Abs and were killed at different time points after CTL transfer. CXCL9 and CXCL10 were induced in both groups over 100-, 15-, and 3-fold on days 1, 2, and 5, respectively, when compared by phosphorimaging analysis with NaCl-injected controls. Likewise, other chemoattractants for lymphomononuclear cells such as XCL1 (lymphotactin, LTN), CCL2 (monocyte chemotactic protein, MCP-1), CCL3 (macrophage inflammatory protein, MIP1-α), CCL4 (MIP1-β), CCL5 (regulated on activation, normal T-cell expressed and secreted, RANTES), CCL6 (C10), CCL7 (MCP-3), and CCL12 (MCP-5) were found to be induced similarly in CTL-injected animals that received either Irr Abs or anti-Gr-1 Abs (Fig. 3). These results indicate that PMN depletion did not affect the intrahepatic activation of CXCL9, CXCL10, and other chemoattractants for lymphomononuclear cells, and thus these cells should be recruited in the liver of anti-Gr-1-treated animals.
The CXC chemokines KC, MIP2, and lipopolysaccharide-induced chemokine (LIX) [chemoattractants for polymorphonuclear cells (22, 23)] were also up-regulated similarly in Irr- and anti-Gr-1-treated mice that were killed on day 1 after CTL transfer (Fig. 3). Interestingly, higher levels of these chemokines were observed in anti-Gr-1-treated animals that were killed on days 2 and 5 (Fig. 3). The association between the absence of PMNs and higher levels of PMN chemoattractants suggests that PMNs may participate in the down-regulation of their own chemoattractants in the liver. Furthermore, the fact that depletion of PMNs did not diminish the intrahepatic induction of those chemokines (CXCL9, CXCL10, CCL2, CCL3, CCL4, KC, and MIP2) that the PMNs themselves can produce (10–12) suggests that the contribution of PMNs to the production of these chemokines in our system is quite limited.
Depletion of PMNs Blocks the Recruitment of Antigen-Nonspecific Cells into the Liver.
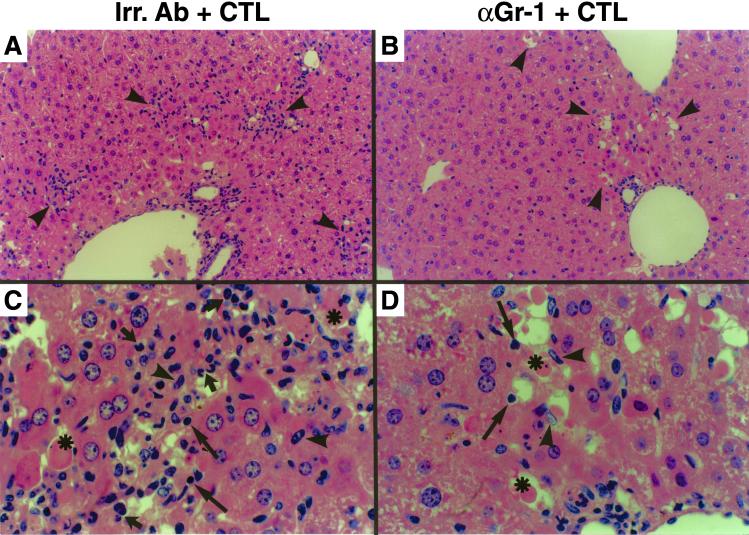
Surprisingly, when compared with mice injected with Irr Abs, treatment with anti-Gr-1 Abs completely blocked the recruitment of all antigen-nonspecific cells into the liver. As shown in Fig. 4, histological analysis of the livers from animals treated with Irr Abs and killed 24 h after the CTL transfer demonstrated necroinflammatory foci scattered throughout the liver parenchyma (Fig. 4A, arrowheads) containing apoptotic hepatocytes (Fig. 4C, asterisks), lymphomononuclear cells resembling T cells (long arrows) or macrophages (arrowheads) and polymorphonuclear cells (short arrows). Anti-Gr-1 treatment did not significantly reduce the extent of hepatocellular apoptosis and drop-out or the number of lymphomononuclear cells resembling CTLs in the liver (Fig. 4B) on days 1 and 2 after CTL injection, as measured by quantitative morphometric analysis [67 ± 12.4 (day 1) and 85 ± 21.8 (day 2) in anti-Gr-1-treated mice versus 89 ± 31.8 (day 1) and 123 ± 29.9 (day 2) in control animals: these numbers represent the mean ± SEM per 100 high-power fields, corresponding to ≈4 mm2 of liver tissue]. This again indicates that similar numbers of CTLs reached the liver in both groups of animals. However, anti-Gr-1 treatment dramatically reduced the recruitment of antigen-nonspecific cells into the liver (Fig. 4 B and D) such that cells morphologically resembling the transferred CTLs (Fig. 4D, long arrows) and macrophages (Fig. 4D, arrowheads) were the only inflammatory cell subsets detectable in these livers. Note that these macrophages could represent the resident population of the liver (Kupffer cells), and they were hyperplastic and physically adjacent to apoptotic hepatocytes (Fig. 4D, asterisks), indicating that they may assume a prominent role as phagocytic cells in the absence of PMNs.
Figure 4.
Depletion of PMNs blocks the recruitment of antigen-nonspecific cells into the liver: histological features. (A and B) Histological analysis of the necroinflammatory foci (arrowheads) detected in livers from animals treated either with control Irr Abs (A) or anti-Gr-1 Abs (α-Gr-1, B) that were killed 1 day after CTL transfer. (C and D) Histological analysis of the same livers at higher magnification. Cells displaying the histological features of apoptotic hepatocytes (asterisks), lymphocytes (long arrows), macrophages (arrowheads), and PMNs (short arrows) are indicated. Note that much fewer inflammatory cells (lymphocytes and macrophages) were detected along with apoptotic hepatocytes in the foci of anti-Gr-1-treated mice. Original magnification, ×100 (A and B) and ×600 (C and D).
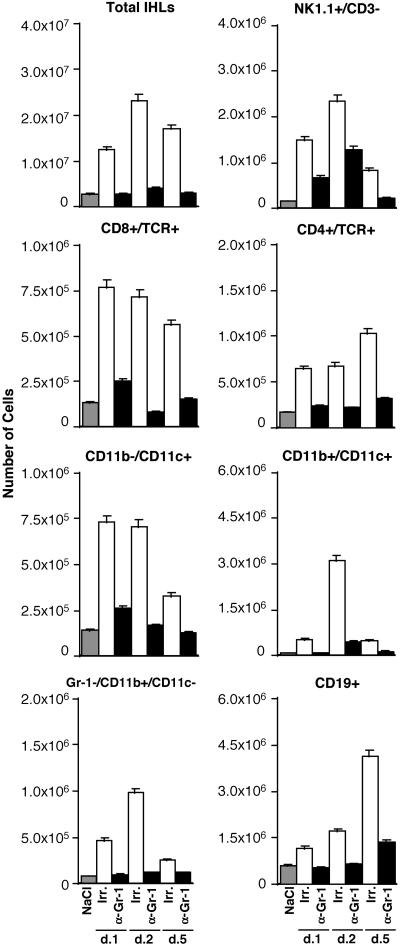
To determine the characteristics of the intrahepatic inflammatory infiltrate in the same livers, the absolute number of IHLs recovered was quantitated, and the phenotype of the recruited inflammatory cell subsets was determined by fluorescence-activated cell sorter analysis. Three additional groups of transgenic control mice (three mice per group) were injected with NaCl alone to produce a baseline for the analysis of the intrahepatic infiltrate by flow cytometry.
When compared with NaCl-injected controls (Fig. 5, gray bars), the total number of IHLs as well as most cell subsets significantly increased in the liver of CTL-treated mice that received Irr Abs (Fig. 5, white bars). The total number of IHLs increased over 4.0-, 7.9-, and 5.8-fold on days 1, 2, and 5 after CTL injection, respectively, which corresponded with a commensurate increase in: (i) NK1.1+/CD3− (NK cells) (9.3-, 15.0-, and 5.1-fold); (ii) CD8+/TCR+ cells (mostly CTLs) (5.9-, 5.5-, and 4.3-fold); (iii) CD4+/TCR+ (mostly T helper cells) (3.8-, 4.0-, and 5.8-fold); (iv) CD11b−/CD11c+ (mostly lymphoid dendritic cells) (5.2-, 5.0-, and 2.3-fold); (v) CD11b+/CD11c+ cells (mostly myeloid dendritic cells) (7.1-, 40.7-, and 6.5-fold); (vi) Gr-1−/CD11b+/CD11c− (mostly macrophages) (5.8-, 12.0-, and 3.2-fold); and (vii) CD19+ (B cells) (1.9-, 2.7-, and 6.2-fold) at the same time points. As also shown in Fig. 5, anti-Gr-1 treatment reduced the number of total IHLs recruited (black bars) by ≈3.1-, 6.5-, and 4.8-fold on days 1, 2, and 5, respectively. Along with this, the number of all cell subsets recruited was reduced profoundly in these mice (Fig. 5, black bars) such that their numbers (with the exception of NK cells that were reduced to a lesser extent), in most cases, were similar or slightly higher than those detected in NaCl-injected controls (Fig. 5, gray bars).
Figure 5.
Depletion of PMNs blocks the recruitment of antigen-nonspecific cells into the liver: IHL analysis. IHL analysis in the same animals described in the legend to Fig. 4. Livers were weighed at the time of autopsy. IHLs were isolated from two liver lobes of a known weight and analyzed by flow cytometry. The indicated numbers of total IHLs and different cell subsets represent the numbers detected in the whole liver.
Collectively, these results indicate that together with the reduction of sALT elevation (Fig. 3), PMN depletion blocked the recruitment of most cell subsets into the liver (Figs. 4 and 5). This effect, coupled with the fact that the number of HBV-specific CTLs was unaffected by the anti-Gr-1 treatment (Fig. 2), suggests that PMN may participate in the pathogenesis of liver disease either directly or indirectly by promoting the migration of antigen-nonspecific lymphomononuclear cells into the liver. It is interesting also that antigen-nonspecific lymphomononuclear cells did not migrate into the liver of anti-Gr-1-treated mice despite the strong intrahepatic induction of their own chemoattractants (Fig. 3), which suggests that PMN-dependent functions other than chemokine production are necessary for the recruitment process to occur. The pathogenetic mechanisms whereby antigen-nonspecific inflammatory cells may induce liver damage are not understood. Future studies may address this important issue. It also is noteworthy that recent reports have shown that Gr-1 can be expressed on certain subsets of macrophages (24) and plasmacytoid DCs (25), and it is possible, therefore, that anti-Gr-1 treatment may have depleted these cells as well. Future studies may be aimed at determining whether these cell subsets also play a role in the intrahepatic recruitment of antigen-nonspecific cells.
In conclusion, we found that PMNs are dispensable for the migratory and antiviral activity of virus-specific CTLs, but they are necessary for the intrahepatic recruitment of antigen-nonspecific cells that amplify the CTL-dependent liver damage. A similar role of PMNs may be played in the pathogenesis of viral hepatitis in humans, where the intrahepatic number of HBV-specific T cells is also outnumbered by recruited non-virus-specific T cells (26, 27) and other inflammatory cells (28). Furthermore, the fact that PMN depletion is associated with maintenance of CTL-dependent antiviral effects but diminished tissue damage may help the design of potential immunotherapeutic approaches for the treatment of HBV infection in chronically infected patients. This should also be viewed in light of the fact that PMN depletion is a relatively simple procedure that has been performed in humans for the treatment of conditions such as inflammatory bowel disease and ulcerative colitis (29).
Acknowledgments
We thank Monte Hobbs and Iain Campbell for providing the cytokine and chemokine probe sets used in the RNase protection assays; and Heike Mendez, Rick Koch, and Margie Chadwell for excellent technical assistance. This work was supported by National Institutes of Health Grants CA40489 (to F.V.C.) and AI40696 (to L.G.G.). This is manuscript number 15114-MEM from The Scripps Research Institute.
Abbreviations
- HBV
hepatitis B virus
- CTL
cytotoxic T cell
- PMN
polymorphonuclear neutrophil
- HBsAg
hepatitis B surface antigen
- HBeAg
hepatitis B e antigen
- sALT
serum alanine aminotransferase
- IHL
intrahepatic leukocyte
- TCR
T cell antigen receptor
- NK
natural killer
- CCKAR
control cholecystokinin type-A receptor
- Irr
irrelevant
References
- 1.Chisari F V, Ferrari C. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 2.Guidotti L G, Matzke B, Schaller H, Chisari F V. J Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 4.McClary H, Koch R, Chisari F V, Guidotti L G. J Virol. 2000;74:2255–2264. doi: 10.1128/jvi.74.5.2255-2264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidotti L G, Chisari F V. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 6.Ando K, Guidotti L G, Wirth S, Ishikawa T, Missale G, Moriyama T, Schreiber R D, Schlicht H J, Huang S, Chisari F V. J Immunol. 1994;152:3245–3253. [PubMed] [Google Scholar]
- 7.Ando K, Moriyama T, Guidotti L G, Wirth S, Schreiber R D, Schlicht H J, Huang S, Chisari F V. J Exp Med. 1993;178:1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakimi K, Lane T E, Wieland S, Asensio V C, Campbell I L, Chisari F V, Guidotti L G. J Exp Med. 2001;194:1755–1766. doi: 10.1084/jem.194.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mollinedo F, Borregaard N, Boxer L A. Immunol Today. 1999;20:535–537. doi: 10.1016/s0167-5699(99)01500-5. [DOI] [PubMed] [Google Scholar]
- 10.Cassatella M A. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 11.Scapini P, Lapinet-Vera J A, Gasperini S, Calzetti F, Bazzoni F, Cassatella M A. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 12.Fessler M B, Malcolm K C, Duncan M W, Worthen G S. J Biol Chem. 2002;277:31291–31302. doi: 10.1074/jbc.M200755200. [DOI] [PubMed] [Google Scholar]
- 13.Maher J J, Scott M K, Saito J M, Burton M C. Hepatology. 1997;25:624–630. doi: 10.1002/hep.510250322. [DOI] [PubMed] [Google Scholar]
- 14.Muruve D A, Barnes M J, Stillman I E, Libermann T A. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 15.Thomas J, Gangappa S, Kanangat S, Rouse B T. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 16.Gubbay J, Vivian N, Economou A, Jackson D, Goodfellow P, Lovell-Badge R. Proc Natl Acad Sci USA. 1992;89:7953–7957. doi: 10.1073/pnas.89.17.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takata Y, Takiguchi S, Kataoka K, Funakoshi A, Miyasaka K, Kono A. Gene. 1997;187:267–271. doi: 10.1016/s0378-1119(96)00765-2. [DOI] [PubMed] [Google Scholar]
- 18.Borrow P. J Viral Hepat. 1997;4:16–24. doi: 10.1111/j.1365-2893.1997.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 19.Hunziker L, Klenerman P, Zinkernagel R M, Ehl S. Eur J Immunol. 2002;32:374–382. doi: 10.1002/1521-4141(200202)32:2<374::AID-IMMU374>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Nakamoto Y, Guidotti L G, Pasquetto V, Schreiber R D, Chisari F V. J Immunol. 1997;158:5692–5697. [PubMed] [Google Scholar]
- 21.Ishikawa T, Kono D, Chung J, Fowler P, Theofilopoulos A, Kakumu S, Chisari F V. J Immunol. 1998;161:5842–5850. [PubMed] [Google Scholar]
- 22.Rossi D, Zlotnik A. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 23.Zlotnik A, Yoshie O. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 24.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo N P, Zanovello P. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano H, Yanagita M, Gunn M D. J Exp Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maini M K, Boni C, Lee C K, Larrubia J R, Reignat S, Ogg G S, King A S, Herberg J, Gilson R, Alisa A, et al. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertoletti A, Maini M K. Curr Opin Immunol. 2000;12:403–408. doi: 10.1016/s0952-7915(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 28.Ishak K G. Am J Clin Pathol. 1976;65:787–827. [PubMed] [Google Scholar]
- 29.Shimoyama T, Sawada K, Hiwatashi N, Sawada T, Matsueda K, Munakata A, Asakura H, Tanaka T, Kasukawa R, Kimura K, et al. J Clin Apheresis. 2001;16:1–9. doi: 10.1002/jca.1000. [DOI] [PubMed] [Google Scholar]