Abstract
We summarize all original research in the field of critical care nephrology published in 2004 or accepted for publication in Critical Care and, when considered relevant or directly linked to this research, in other journals. Articles were grouped into four categories to facilitate a rapid overview. First, regarding the definition of acute renal failure (ARF), the RIFLE criteria (risk, injury, failure, loss, ESKD [end-stage kidney disease]) for diagnosis of ARF were defined by the Acute Dialysis Quality Initiative workgroup and applied in clinical practice by some authors. The second category is acid–base disorders in ARF; the Stewart–Figge quantitative approach to acidosis in critically ill patients has been utilized by two groups of researchers, with similar results but different conclusions. In the third category – blood markers during ARF – cystatin C as an early marker of ARF and procalcitonin as a sepsis marker during continuous venovenous haemofiltration were examined. Finally, in the extracorporeal treatment of ARF, the ability of two types of high cutoff haemofilters to influence blood levels of middle- and high-molecular-weight toxins showed promise.
Introduction
During 2004 Critical Care accepted and published original research articles focused on nephrology and renal replacement therapy (RRT). These studies included reports on various aspects of acute renal failure (ARF), acid–base approach and treatment, and RRT insights into specific blood purification issues. We present a review of these papers and other key articles on critical care nephrology published in 2004.
Definition of acute renal failure
Despite several advances in treatment and in our understanding of the pathogenesis of ARF, many important issues in this field remain subject to controversy, confusion and lack of consensus. One such issue is the definition of ARF. In fact, because ARF is mostly an artificial concept, it can neither be proved nor disproved that an individual has ARF unless one agrees what the term means in advance. A clear consensual definition is needed if we are to describe and understand the epidemiology of ARF, randomize patients in controlled trials, test therapies in similar groups of patients, develop animal models and validate diagnostic tests. In this regard ARF is no different from acute respiratory distress syndrome, severe sepsis, or septic shock.
In order to make consensus based recommendations and delineate key questions for future studies, the Acute Dialysis Quality Initiative workgroup identified topics relevant to the field of ARF [1], among which a definition/classification system for ARF was ranked highest in terms of importance and clinical impact [2]. The workgroup considered the definition of ARF to require the following features: ease of use and clinical applicability in different centres; high sensitivity and specificity for different populations and research questions; consideration of creatinine change from baseline; and implementation of classifications for acute on chronic renal disease. A classification system should therefore include and differentiate mild and severe, and early and late cases. This would allow such a classification to identify patients in whom renal function is mildly affected (high sensitivity for the detection of kidney dysfunction but limited specificity for its presence) and patients in whom renal function is markedly affected (high specificity for true renal dysfunction but limited sensitivity in detecting early and more subtle loss of function). Accordingly, a multilevel classification system was proposed, in which a wide range of disease spectra can be included, embodied in the acronym RIFLE (Risk of renal dysfunction, Injury to the kidney, Failure or Loss of kidney function, and End-stage kidney disease; Fig. 1).
Figure 1.
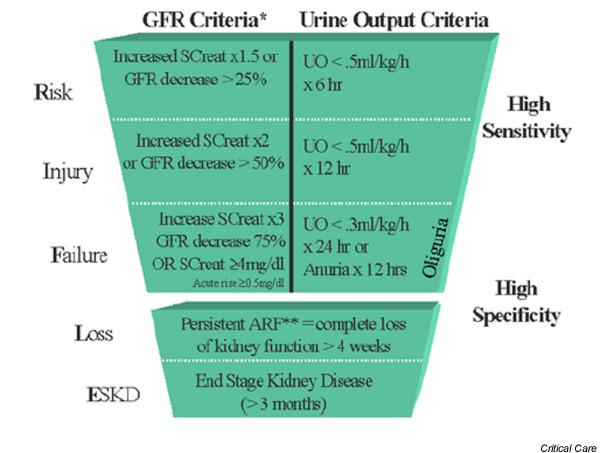
Proposed classification scheme for ARF. The classification system includes separate criteria for creatinine and urine output (UO). A patient can fulfil the criteria through changes in serum creatinine (SCreat) or changes in UO, or both. The criteria that lead to the worst possible classification should be used. Note that the F component of RIFLE (Risk of renal dysfunction, Injury to the kidney, Failure or Loss of kidney function, and End-stage kidney disease) is present even if the increase in SCreat is under threefold, as long as the new SCreat is greater than 4.0 mg/dl (350 μmol/l) in the setting of an acute increase of at least 0.5 mg/dl (44 μmol/l). The designation RIFLE-FC should be used in this case to denote 'acute on chronic disease'. Similarly, when the RIFLE-F classification is achieved by UO criteria, a designation of RIFLE-FO should be used to denote oliguria. The shape of the figure highlights the fact that more patients (high sensitivity) will be included in the mild category, including some who do not actually have ARF (less specificity). In contrast, at the bottom of the figure the criteria are strict and therefore specific, but some patients will be missed. ARF, acute renal failure; GFR, glomerular filtration rate.
If patients are admitted with ARF without any baseline measure of renal function, then a theoretical baseline serum creatinine value for a given patient, assuming normal glomerular filtration rate (GFR), should be estimated. By normalizing the GFR to body surface area, and assuming a GFR of approximately 75–100 ml/min per 1.73 m2, the simplified MDRD (modification of diet in renal disease) formula was selected by the workgroup to provide an estimate of GFR relative to serum creatinine, based on age, race and sex [3]:
Estimated GFR = 75 (ml/min per 1.73 m2) = (186 × serum creatinine) - (1.154 × age) - (0.0203 [× 0.742 if female] [× 1.210 if black])
Of note, the RIFLE criteria were intended only to be used as a classification/definition for ARF, but some authors have already applied it to the clinical evaluation of ARF [4,5]. Hoste and colleagues [4] prospectively analyzed data from 5313 patients admitted to an intensive care unit (ICU) and found the clinical severity of the RIFLE criteria to correlate with increasing mortality. A similar conclusion was drawn by Bell and coworkers [5]. Those investigators examined data from 8152 consecutive patients who had been admitted to the ICU of a university hospital; 207 patients were treated by continuous RRT, and those who were in the RIFLE-F category had a significantly higher mortality than those in the RIFLE-R and RIFLE-I categories.
Acid–base disorders during acute renal failure
During 2004 Critical Care published a series of reviews covering many aspects of acid–base disorders in critically ill patients [6-12]. Interest in this important field of critical care medicine has recently brought many researchers to evaluate a quantitative approach to interpreting acid–base derangements, namely the Stewart–Figge methodology. Acid–base disorders, especially metabolic acidosis, are considered to be common in patients with ARF. The nature of this acidosis is only indirectly understood, and this lack of information has typically led to the assumption that the acidosis of ARF is mostly an anion gap (AG) acidosis, which is essentially secondary to accumulation of unexcreted acids. This is unlikely in the critically ill, in which other disorders of acid–base physiology might also be present. A more specific view might lead clinicians to more accurate physiological diagnoses and could perhaps influence their treatment choices.
The Stewart–Figge method first involves the calculation of the apparent strong ion difference (SIDa; mEq/l):
SIDa = [Na+] + [K+] + [Mg2+] + [Ca2+] - [Cl-] - [lactate]
However, this equation does not take into account the role played by weak acids (CO2, albumin and phosphate) in the balance of electrical charges in plasma water. This is expressed through the calculation of the effective strong ion difference (SIDe). The formula is as follows (with PCO2 [partial carbon dioxide tension] expressed in mmHg, albumin in g/l and phosphate in mmol/l):
SIDe = 1000 × 2.46 × 10-11 × PCO2/(10-pH) + [albumin] × (0.12 × pH - 0.631) + [phosphate] × (0.39 × pH - 0.469)
The SIDe formula quantitatively includes the contribution of weak acids to the electrical charge equilibrium in plasma. The SIDa to SIDe difference should equal zero (electrical charge neutrality) unless there are unmeasured changes to explain this 'ion gap'. Such charges are described by the strong ion gap (SIG) = SIDa - SIDe. A positive value for the SIG must represent unmeasured anions (sulphate, keto acids, citrate, pyruvate, acetate, gluconate, etc.), which must be considered to account for the measured pH. The traditional AG is calculated using the following formula:
AG = [Na+] + [K+] - [Cl-] - [HCO3-]
To examine the nature of acid–base disorders using Stewart's quantitative biophysical methods [13] and modified by Figge and colleagues [14], a retrospective study was carried out by Rocktaeschel and coworkers [15] in critically ill patients suffering from ARF and requiring continuous RRT, match controlled by two groups of patients without ARF. Those investigators found that ICU patients with ARF had a mild acidaemia (mean pH 7.30 ± 0.13) secondary to metabolic acidosis, with a mean base excess of -7.5 ± 7.2 mEq/l. However, half of these patients had a normal AG. Quantitative acid–base assessment revealed multiple metabolic acid–base processes compared with control individuals, which contributed to the overall acidosis. These included high levels of unmeasured anions (13.4 ± 5.5 mEq/l), hyperphosphataemia (2.08 ± 0.92 mEq/l) and the alkalinizing effect of hypoalbuminaemia (22.6 ± 6.3 g/l). In other words, this acidosis was the result of the net balance of acidifying forces due to the accumulation of unmeasured anions, phosphate, and the attenuating effect of metabolic alkalosis secondary to hypoalbuminaemia. In ARF patients the compensatory responses are inadequate, both at the respiratory and metabolic levels.
However, starting from the concept that the importance of a raised SIG in clinical practice is unknown and that normal levels for the SIG in critically ill patients are unknown, Moviat and coworkers [16] prospectively studied 50 consecutive patients admitted to an ICU with a metabolic acidosis, with the purpose of comparing two different methods of quantifying metabolic acidosis in patients admitted to an ICU: the Stewart–Figge quantitative analysis and the AG corrected for albumin and lactate (AGcorr). Metabolic acidosis was defined as standard base excess of -5 or less. Twenty-nine patients exhibited evidence of decreased renal function. AGcorr was calculated with using the following formula: AGcorr = AG + 0.25 × (40 - [albumin]) - lactate. The main finding of the study was a very strong correlation between the AGcorr and the SIG (r2 = 0.934; P < 0.001) in these critically ill patients with metabolic acidosis. The authors concluded that, although the SIG is a gold standard, the time consuming calculation of this parameter, in accordance with the Stewart methodology, is unnecessary for clinical purposes because multiple mechanisms underlying metabolic acidosis in most ICU patients were reliably determined using the lactate-corrected and albumin-corrected AG.
Blood markers during acute renal failure
The Acute Dialysis Quality Initiative workgroup highlighted that creatinine excretion is much greater than the filtered load, resulting in a potentially large overestimation of the GFR. However, for clinical purposes it is important to determine whether renal function is stable or becoming worse or better. This can usually be done by monitoring serum creatinine alone. Like creatinine clearance (CCr), serum creatinine is not an accurate reflection of GFR in the non-steady-state condition of ARF. The degree to which serum creatinine changes from baseline, however, does reasonably reflect change in GFR. Serum creatinine is readily and easily measured, and it is specific for renal function. Nevertheless, in unstable, critically ill patients, acute changes in renal function can render accurate real-time evaluations crucial to timely diagnosis and early treatment.
Villa and coworkers [17] conducted an evaluation of serum cystatin C concentration as a real-time marker of ARF in critically ill patients. Cystatin C is a nonglycosylated protein that belongs to the cysteine protease inhibitors, and it is produced at a constant rate by nucleated cells. It is found in relatively high concentrations in many body fluids, and its low molecular weight (13.3 kDa) and positive charge at physiological pH levels facilitate its glomerular filtration. Subsequently, it is reabsorbed and almost completely catabolized in the proximal renal tubule. Therefore, because of its constant rate of production, its serum concentration is determined by glomerular filtration. Moreover, its concentration is unaffected by infections, liver disease and inflammatory disease. Villa and coworkers measured serum creatinine, serum cystatin C and 24-hour CCr in 50 critically ill patients at risk for developing renal dysfunction. Twenty-four-hour body surface adjusted CCr was used as a control. Serum cystatin C correlated better with GFR than did creatinine, and cystatin C was diagnostically superior to creatinine (area under the curve for cystatin C = 0.927, 95% confidence interval = 86.1–99.4; area under the curve for for creatinine = 0.694, 95% confidence interval = 54.1–84.6). Twenty-five of the 50 patients had acute renal dysfunction, defined as CCr below 80 ml/min. Only five (20%) of these 25 patients had elevated serum creatinine, whereas 76% had elevated serum cystatin C levels (P = 0.032). According to these data, cystatin C appeared to be an accurate marker of subtle changes in GFR. Unfortunately, the authors did not evaluate whether cystatin C can be used to detect renal dysfunction before creatinine values become abnormal.
Interestingly, Herget-Rosenthal and coworkers [18] evaluated early detection of ARF by cystatin C and showed that the increase in blood levels of this marker blood significantly preceded that of creatinine. According to the R, I and F criteria of RIFLE, cystatin C detected renal dysfunction 2 days earlier than did creatinine, with a high diagnostic value, and predicted RRT in the longer term of ARF moderately well.
Procalcitonin (PCT) is another blood marker that has recently attracted considerable interest. PCT is induced in the plasma of patients with sepsis and septic shock, and is specifically increased in generalized bacterial or fungal infections. This polypeptide is a very useful marker with which to monitor treatment in critically ill patients. Some authors recently demonstrated that PCT amplifies nitric oxide synthase gene expression and nitric oxide production, which might account for the observed correlation between PCT concentration and the fatal outcome in multiple organ dysfunction syndrome and septic shock [19]. Elimination of PCT is not well understood. Like other plasma proteins, PCT is probably degraded by proteolysis. Renal excretion of PCT plays a minor role, and there is no accumulation of PCT in patients with severe renal failure.
Level and coworkers [20] evaluated the mass transfer and clearance of PCT during continuous venovenous haemofiltration (CVVH) with a postfilter substitution reinfusion rate of 1.5–2 l/hour and with a high flux membrane in patients with septic shock. These researchers also aimed to identify the mechanism of elimination of PCT and its impact on plasma concentrations during the course of convective therapy. Level and coworkers concluded that PCT is removed from the plasma of patients with septic shock during CVVH. They found a PCT sieving coefficient of 0.07, and stated that most of the mass was eliminated by a coinvective clearance (K) of 1.85–5.01 ml/min. However, according to their data, adsorption appeared to contribute impressively to PCT elimination, especially during the first hours of CVVH. In fact, the reported range of plasma clearance of 37.4–31.5 ml/min is almost double that in previous studies; bearing in mind the presence of such a convective K, the adsorbtive K should have accounted for about 35–25 ml/min. Nonetheless, confirming the findings of previous studies, the effect of PCT removal by an extracorporeal conventional treatment did not appear to affect plasma concentrations of PCT, establishing PCT as a useful diagnostic marker in septic patients treated with CVVH. The impact of high volume haemofiltration on the PCT clearance, mass transfer and plasma concentration remains to be evaluated.
Extracorporeal treatment of acute renal failure
In a case report, Naka and coworkers [21] tested the ability of a novel super high flux (SHF) membrane with a relatively large pore size to clear myoglobin from serum. A patient with serotonin syndrome complicated with rhabdomyolysis and oliguric ARF was treated by CVVH at 2 l/hour ultrafiltration (UF) with a standard polysulphone 1.4 m2 membrane (cutoff point 20 kDa), followed by CVVH with a SHF membrane (cutoff point 100 kDa) at 2 l/hour UF, and then at 3 l/hour UF and at 4 l/hour UF, in order to clear myoglobin from the patient's blood. The authors found that the myoglobin concentration in the ultrafiltrate at 2 l/hour exchange was at least five times greater with the SHF membrane than with the conventional one (>100,000 μg/l versus 23,000 μg/l). The sieving coefficient with the SHF membrane at 3 l/hour UF and 4 l/hour UF were 72.2% and 68.8%, respectively. The amount of myoglobin removed with the SHF membrane was about five times greater than with the conventional membrane. The SHF membrane achieved a K in excess of 56 l/day, and achieved a reduction in serum myoglobin concentration from over 100,000 to 16,000 μg/l in 48 hours. SHF haemofiltration resulted in a much greater clearance of myoglobin than conventional haemofiltration, and its feasibility as a potential modality for the treatment of myoglobinuric ARF was demonstrated. Taking the rationale from this interesting case report, a controlled trial aiming to demonstrate the clinical impact of such a treatment versus traditional CVVH is now necessary.
High cutoff haemofilters with a cutoff point of approximately 60 kDa were also used for RRT by Morgera and coworkers [22,23]. Patients were randomly allocated to CVVH with either an UF rate of 1 l/hour (group 1) or one of 2.5 l/hour (group 2) or to continuous venovenous haemodialysis (CVVHD) with a dialysate flow rate of 1 l/hour (group 3) or 2.5 l/hour (group 4). IL-1 receptor antagonist, IL-1β, IL-6, tumour necrosis factor-α, and plasma proteins were measured daily. CVVH achieved significantly greater IL-1 receptor antagonist clearance compared with CVVHD (P = 0.0003). No difference was found for IL-6. Increasing UF volume or dialysate flow led to a highly significant increase in IL-1 receptor antagonist and IL-6 clearance rates (P < 0.00001). This filter allowed remarkable peak clearances for IL-1 receptor antagonist and IL-6 of 46 ml/min and 51 ml/min, respectively. Tumour necrosis factor-α clearance was poor for both CVVH and CVVHD. A significant decline in plasma IL-1 receptor antagonist and IL-6 clearance was observed only in patients with high baseline levels. Protein and albumin losses were greatest during the 2.5 l/hour CVVH mode. Of note, convection and diffusion did not exhibit the expected difference in terms of clearance of middle- to high-molecular-weight solutes, whereas using diffusion instead of convection significantly reduced the loss of proteins while maintaining good cytokine clearance rates. High cutoff haemofiltration can be viewed as a reasonable alternative to more complex techniques (coupled plasma filtration adsorption, plasmapheresis, and haemoperfusion) recently evaluated as methods with which to remove inflammatory mediators [24]. The optimal clinical applications of such promising membranes remain to be determined.
Conclusion
Reports on definition of ARF, acid–base approach and treatment, blood markers during ARF and RRT newest technology published by Critical Care in 2004 were reviewed. The presented papers provide interesting insights to various aspects of critical care nephrology.
Abbreviations
AG = anion gap; ARF = acute renal failure; CCr = creatinine clearance; CVVH = continuous veno-venous haemofiltration; CVVHD = continuous veno-venous haemodialysis; GFR = glomerular filtration rate; ICU = intensive care unit; IL = interleukin; K = ultrafiltration clearance; PCT = procalcitonin; RIFLE = risk, injury, failure, loss, ESKD (end-stage kidney disease); RRT = renal replacement therapy; SHF = super high flux; SIDa = apparent strong ion difference; SIDe = effective strong ion difference; SIG = strong ion gap; UF = ultrafiltration.
Competing interests
The author(s) declare that they have no competing interests.
Contributor Information
Zaccaria Ricci, Email: z.ricci@libero.it.
Claudio Ronco, Email: cronco@goldnet.it.
References
- Ronco C. Acute Dialysis Quality Initiative (ADQI): the PASSPORT project. Int J Artif Organs. 2005;28:438–440. doi: 10.1177/039139880502800502. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, the ADQI workgroup Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Kidney Foundation K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification. Am J Kidney Dis. 2002. pp. S76–S92. [PubMed]
- Hoste E, Clermont G, Kersten A, Venkataraman R, Kaldas H, Angus D, Kellum JA. Clinical evaluation of the new RIFLE criteria for acute renal failure. 24th International Symposium on Intensive Care and Emergency Medicine. Crit Care. 2004. p. P161.
- Bell M, Liljestam E, Granath F, Fryckstedt J, Ekbom A, Martling C. Optimal follow-up time after Continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant. 2005;20:354–360. doi: 10.1093/ndt/gfh581. [DOI] [PubMed] [Google Scholar]
- Naka T, Bellomo R. Bench-to-bedside review: Treating acid–base abnormalities in the intensive care unit – the role of renal replacement therapy. Crit Care. 2004;8:108–114. doi: 10.1186/cc2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story DA. Bench-to-bedside review: A brief history of clinical acid–base. Crit Care. 2004;8:253–258. doi: 10.1186/cc2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlbach BK, Schmidt GA. Bench-to-bedside review: Treating acid–base abnormalities in the intensive care unit – the role of buffers. Crit Care. 2004;8:259–265. doi: 10.1186/cc2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum JA, Song M, Li J. Science review: Extracellular acidosis and the immune response: clinical and physiologic implications. Crit Care. 2004;8:331–336. doi: 10.1186/cc2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten EW. Science review: Quantitative acid–base physiology using the Stewart model. Crit Care. 2004;8:448–452. doi: 10.1186/cc2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TJ. Clinical review: The meaning of acid–base abnormalities in the intensive care unit – effects of fluid administration. Crit Care. 2005;9:204–211. doi: 10.1186/cc2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan LJ, Frangos S. Clinical review: Acid–base abnormalities in the intensive care unit. Crit Care. 2005;9:198–203. doi: 10.1186/cc2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PA. Modern quantitative acid–base chemistry. Can J Physiol Pharmacol. 1983;61:1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- Figge J, Mydosh T, Fencl V. Serum proteins and acid–base equilibria: a follow-up. J Lab Clin Med. 1992;120:713–719. [PubMed] [Google Scholar]
- Rocktaeschel J, Morimatsu H, Uchino S, Goldsmith D, Poustie S, Story D, Gutteridge G, Bellomo R. Acid–base status of critically ill patients with acute renal failure: analysis based on Stewart–Figge methodology. Crit Care. 2003;7:R41–R45. doi: 10.1186/cc2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moviat M, van Haren F, van der Hoeven H. Conventional or physicochemical approach in intensive care unit patients with metabolic acidosis. Crit Care. 2003;7:R41–R45. doi: 10.1186/cc2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P, Jiménez M, Soriano M, Manzanares J, Casasnovas P. Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit Care. 2005;9:R139–R143. doi: 10.1186/cc3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, Philipp T, Kribben A. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann G, Czechowski M, Schoesser M, Schobersberger W. Procalcitonin amplifies inductible nitric oxyde synthase gene expression and nitric oxide production in vascular smooth muscle cells. Crit Care Med. 2002;30:2091–2095. doi: 10.1097/00003246-200209000-00023. [DOI] [PubMed] [Google Scholar]
- Level C, Chauveau P, Guisset O, Cazin MC, Lasseur C, Gabinsky C, Winnock S, Montaudon D, Bedry R, Nouts C, et al. Mass transfer, clearance and plasma concentration of procalcitonin during continuous venovenous hemofiltration in patients with septic shock and acute oliguric renal failure. Crit Care. 2003;7:R160–R166. doi: 10.1186/cc2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka T, Jones D, Baldwin I, Fealy N, Bates S, Goehl H, Morgera S, Neumayer HH, Bellomo R. Myoglobin clearance by super high-flux hemofiltration in a case of severe rhabdomyolysis: a case report. Crit Care. 2005;9:R90–R95. doi: 10.1186/cc3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgera S, Melzer C, Sobottke V, Vargas-Hein O, Zuckermann-Becker H, Bellomo R, Neumayer H. Renal replacement therapy with high cutoff hemofilters: impact of convection and diffusive on cytokine clearances and protein status. 24th International Symposium on Intensive Care and Emergency Medicine. Crit Care. 2004;(Suppl 1):P151. doi: 10.1186/cc2618. [DOI] [Google Scholar]
- Morgera S, Slowinski T, Melzer C, Sobottke V, Vargas-Hein O, Volk T, Zuckermann-Becker H, Wegner B, Muller JM, Baumann G, et al. Renal replacement therapy with high cutoff hemofilters: impact of convection and diffusive on cytokine clearances and protein status. Am J Kidney Dis. 2004;43:444–453. doi: 10.1053/j.ajkd.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Venkataraman R, Subramanian S, Kellum JA. Clinical review: Extracorporeal blood purification in severe sepsis. Crit Care. 2003;7:139–145. doi: 10.1186/cc1889. [DOI] [PMC free article] [PubMed] [Google Scholar]


