Abstract
Peptide immunotherapy using multiple predominant allergen-specific T cell epitopes is a safe and promising strategy for the control of type I allergy. In this study, we developed transgenic rice plants expressing mouse dominant T cell epitope peptides of Cry j I and Cry j II allergens of Japanese cedar pollen as a fusion protein with the soybean seed storage protein glycinin. Under the control of the rice seed storage protein glutelin GluB-1 promoter, the fusion protein was specifically expressed and accumulated in seeds at a level of 0.5% of the total seed protein. Oral feeding to mice of transgenic rice seeds expressing the T cell epitope peptides of Cry j I and Cry j II before systemic challenge with total protein of cedar pollen inhibited the development of allergen-specific serum IgE and IgG antibody and CD4+ T cell proliferative responses. The levels of allergen-specific CD4+ T cell-derived allergy-associated T helper 2 cytokine production of IL-4, IL-5, and IL-13 and histamine release in serum were significantly decreased. Moreover, the development of pollen-induced clinical symptoms was inhibited in our experimental sneezing mouse model. These results indicate the potential of transgenic rice seeds in production and mucosal delivery of allergen-specific T cell epitope peptides for the induction of oral tolerance to pollen allergens.
Keywords: Japanese cedar pollinosis, peptide immunotherapy, seed-specific expression
Immunotherapy using allergen-specific T cell epitope peptides has been shown to be a safe and effective treatment for the control of IgE-mediated allergic diseases (1-3). Immunodominant epitopes derived from several allergens have been shown to possess therapeutic effects in both animal models and human clinical trials (4-8). Japanese cedar (Cryptomeria japonica) pollen is a major cause of pollinosis that elicits allergic disorders such as rhinitis and conjunctivitis in Japan (9). Two major allergens, designated Cry j I and Cry j II, were isolated from the pollen (9-13), and multiple domains of T cell epitope for humans and mice were identified from them (14-16). It has been reported that oral feeding to mice of a chemically synthesized major T cell epitope peptide of Cry j II reduces levels of Cry j II-specific IgE and IgG antibody responses via a decrease in the production of allergy-associated IL-4 in mice (15). These results open new possibilities for the development of allergen peptide-based immunotherapy for the control of Japanese cedar-induced pollinosis. Thus, oral vaccination with the major T cell epitope peptides derived from Cry j I and/or Cry j II pollen allergens is considered to be a practical and effective method of immunotherapy for the inhibition of pollinosis-associated type I hypersensitivity.
Plants have recently been recognized as a form of bioreactor for the cost-effective production of large-scale recombinant proteins (17-19). Compared to other expression systems such as bacteria and mammalian cell cultures, plants have a much lower risk of contamination by human pathogens, such as animal virus and prions (17-19). Furthermore, the edible tissues of plants further provide the significant benefit of achieving a simple method for mucosal delivery of vaccines and immunogens without the need for complicated purification steps (20-22).
Cereal crop seeds are essentially edible tissues and have the capacity to produce relatively large amounts of recombinant products (23, 24). Recombinant products accumulated in seeds have been shown to be stable for 6 months, even when stored at room temperature (19). Rice, a staple food in Asia, can be considered as an attractive system, compared to other cereals, because of its easy storage and processing, high yield, and low production cost (25). A detailed search for a number of promoters, using β-glucronidase (GUS) reporter gene, provided a choice of suitable promoters for the effective expression of transgenes in rice seeds (26). Another advantage of rice plants is that targeting to protein storage vacuoles (protein bodies) provides a greater space for the accumulation of recombinant proteins (27). A soybean glycinin A1aB1b provided one successful instance of high-level accumulation in the protein storage vacuole II (protein body II), reaching ≈5% of the total seed protein (27). Furthermore, the expression level of glycinin A1aB1b was enhanced in low storage protein mutants of rice (28). Based on the progress of molecular analysis of the expression and accumulation of transgene products, rice can be considered a potential candidate for the development of plant-derived edible vaccines.
In this study, we developed transgenic rice plants accumulating mouse T cell epitope peptides specific for pollen allergens of Cryptomeria japonica in seeds. To achieve greater accumulation, the T cell epitope peptides of Cry j I and Cry j II were expressed as a fusion protein with the soybean storage protein glycinin A1aB1b. The fusion protein (A1aB1b-Crp-1 and -2) accumulated at a level of 0.5% of the total seed protein. Oral administration of the transgenic rice seeds to mice before systemic challenge with total cedar pollen protein induced oral tolerance with the inhibition of allergen-induced allergy-associated T helper 2 (Th2) cytokine synthesis of IL-4, IL-5, and IL-13 and their supported allergen-specific IgE responses. Furthermore, it resulted in the inhibition of the pollen-induced clinical symptoms of nasal sneezing. These results demonstrate the efficacy of T cell epitope peptides expressed in transgenic rice seeds for oral delivery and induction of oral tolerance against pollen allergen-specific responses.
Methods
Plasmid Construction and Rice Transformation. Two major T cell epitopes, KQVTIRIGCKTSSS (residues 277-290 of Cry j I) and RAEVSYVHVNGAKF (residues 246-259 of Cry j II) (15, 16), named Crp-1 and -2, respectively, were inserted into variable regions in acidic and basic subunits of glycinin A1aB1b (29, 30). Fifteen amino acid residues (residues 293-307 of A1aB1b) in the acidic subunit and eight amino acid residues (residues 488-495 of A1aB1b) in the basic subunit were substituted by the Crp-1 and -2 T cell epitopes, respectively, resulting in the recombinant protein A1aB1b-Crp-1 and -2. The construction of the A1aB1b-Crp-1 and -2 gene sequence was carried out by two stages of PCR amplification. A DNA sequence coding for the acidic subunit (residues 1-292 of A1aB1b) was amplified by PCR from the pUGluBGly plasmid (27) with a set of oligonucleotides -103 and Crp1R, which added a DNA sequence coding for the Crp-1 peptide at the 3′ end of the acidic subunit of A1aB1b sequence. The other sequence coding for the basic subunit (residues 308-487 of A1aB1b) was PCR-amplified by using the primer set Crp1F and M13-RV, which provided DNA sequences coding for the Crp-1 and -2 peptides at the 5′ and 3′ end of the basic subunit of A1aB1b sequence, respectively. These two DNA fragments were then annealed and amplified by overlap PCR with -103 and M13-RV primers to generate the complete DNA fragment coding for the A1aB1b-Crp-1 and -2 protein. This product was placed under the control of the 2.3-kb GluB-1 promoter, and the plant expression cassette was then inserted into a binary vector pGPTV-35S-HPT (26). The resultant expression plasmid (Fig. 1A) was introduced into the rice genome (Oryza sativa L. cv Kitaake) by Agrobacterium tumefaciens-mediated transformation as described (26).
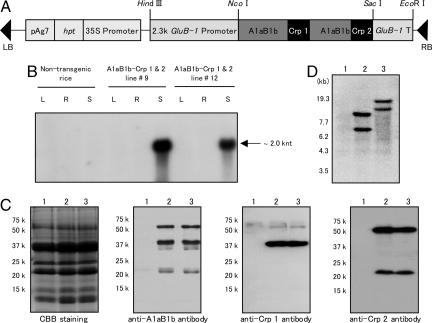
Fig. 1.
Expression of A1aB1b-Crp-1 and -2 in transgenic rice. (A) Schematic representation of the transformation plasmid. The DNA fragment coding for the A1aB1b-Crp-1 and -2 protein was placed under the control of rice seed major storage protein glutelin 2.3-kb GluB-1 promoter. The hpt gene was used for the selection of transgenic rice plants. GluB-1, rice glutelin GluB-1; 35S, cauliflower mosaic virus 35S promoter; hpt, hygromycin phosphotransferase gene; pAg7, agropine synthase polyadenylation signal sequence; RB, right border; LB, left border. (B) Northern blot analysis. Total RNA was isolated from leaves (L), roots (R), or developing seeds (S) of nontransgenic and A1aB1b-Crp-1 and -2 transgenic lines 9 and 12. (C) Western blot analysis of total protein extracted from seeds. Lane 1, nontransgenic rice; lane 2, A1aB1b-Crp-1 and -2 transgenic line 9; lane 3, A1aB1b-Crp-1 and -2 transgenic line 12. Anti-glycinin antibody, anti-Crp-1 antibody, or anti-Crp-2 antibody was used for the detection of A1aB1b-Crp-1 and -2 protein. (D) Southern blot analysis. Genomic DNA isolated from young leaves of rice plants was digested with SacI and fractionated by electrophoresis on 0.8% agarose gel. Lane 1, nontransgenic rice; lane 2, A1aB1b-Crp-1 and -2 transgenic line #9; lane 3, A1aB1b-Crp-1 and -2 transgenic line 12.
Southern and Northern Blot Analysis. Genomic DNA was prepared from young leaves by using the cetyltrimethylammonium bromide (CTAB) extraction method (28). Total RNA was extracted by the phenol/chloroform extraction method (28) from frozen rice seeds, leaves, or roots. Southern and Northern blot analyses were carried out by using standard methods (28). Hybridizations were performed at 65°C by using 32P-labeled full-length A1aB1b-Crp-1 and -2 probes.
Detection of A1aB1b-Crp-1 and -2 Protein. Rice seeds were ground to a fine powder by using a Multibeads shocker (Yasui Kikai, Osaka, Japan), and total seed protein was extracted with an extraction buffer containing 4% (wt/vol) SDS, 8 M urea, 5% (wt/vol) 2-mercaptoethanol, 50 mM Tris·HCl (pH 6.8), and 20% (wt/vol) glycerol as described (28). Total seed protein was separated by using SDS/12% or 15% PAGE, and then transferred to Hybond-P poly(vinylidene difluoride) membranes (Amersham Pharmacia) for Western blot analysis. To confirm the accumulation of Crp-1 and -2 T cell epitope peptides in transgenic rice seeds, anti-Crp-1 peptide and anti-Crp-2 peptide antibodies were raised in rabbit (Qiagen, Tokyo). A rabbit anti-glycinin A1aB1b antibody had been prepared previously (27). The membranes were probed with one of the primary antibodies, and then incubated with a goat anti-rabbit IgG secondary antibody conjugated to horseradish peroxidase (Promega) for visualizing signals. Accumulation levels of A1aB1b-Crp-1 and -2 protein were determined by the quantitative dot immunoblotting with anti-glycinin A1aB1b antibodies as described (27).
Mouse Feeding Experiments. A first group of eight BALB/c male mice at 6 weeks of age (CLEA Japan, Tokyo) was orally fed with 200 mg of fine powder of rice seeds containing 70 μg of A1aB1b-Crp-1 and -2 protein suspended in 1.0 ml of PBS once a day over a period of 4 weeks. A second group of mice was fed with equal amounts of seed powder from nontransgenic rice plants. For a third group of mice, PBS was administered as a control experiment. All mice were then i.p. challenged twice at weeks 4 and 5 with 0.1 mg of total protein extracts of Japanese cedar pollen (Cosmo Bio, Tokyo) adsorbed on 5 mg of aluminum hydroxide (alum) (Cosmo Bio) in 500 μl of PBS. At the first challenge at week 4, recombinant mouse IL-4 (R & D Systems) was mixed with the allergen solution at 0.1 μg per mouse to maximize the induction of allergen-specific IgE responses. Our preliminary study demonstrated that the coadministration of IL-4 resulted in the acceleration of allergen-specific IgE responses compared with the case when IL-4 was not coadministered.
ELISA. At week 7 of the experiment, mice were bled to allow measurements of total and allergen-specific antibodies by ELISA as described (31, 32) with a slight modification. Immunoplates (Nalge Nunc) were coated with 2 μg/ml anti-mouse IgE or anti-mouse IgG antibody (Southern Biotechnology, Birmingham, AL). After washing and blocking of the plates, serial dilutions of serum were added to the wells, which were then washed. For detection of allergen-specific antibodies, total protein extracts of pollen were biotinilated according to the manufacturer's procedure (Pierce) and added to the wells as a secondary antibody. Total IgE levels in serum were measured by a sandwich ELISA as described (31). After washing the plates, streptavidin-horseradish peroxidase conjugate (Pierce) was added to the wells, and the reaction was developed with peroxidase substrate solution (Moss, Pasadena, MD). The last serum dilution yielding an OD450 value of 0.1 over the background was recorded as the endpoint titer for each sample.
T Cell Proliferation and Cytokine Assay. CD4+ T cells were purified from splenocytes at week 7 of the experiment by MACS beads separation using anti-mouse CD4 Ab-conjugated magnetic beads (Miltenyi Biotec). The cells were cultured at 1 × 105 cells per well together with gamma-ray-irradiated splenic antigen-presenting cells at 5 × 105 cells per well for 6 days at 37°C with or without 20 μg/ml total protein extracts of pollen in 96-well plates. Our preliminary study showed that a cedar pollen protein concentration of 20 μg/ml resulted in the optimal dose for the induction of maximum allergen-specific CD4+ T cell responses among the different doses tested (e.g., 4-40 μg/ml). Each well was then pulsed with 0.25 μCi of [3H]thymidine (Amersham Pharmacia; 1 Ci = 37 GBq) for the last 22 h of incubation, and the cells were harvested to allow measurement of radioactivity levels. At the same time, the other aliquots of cells were incubated under identical conditions for 5 days to assess the different Th1- and Th2-type cytokine production by ELISA as described (33).
Histamine Assay. To examine the levels of serum histamine, mice were challenged at week 7 with an i.p. injection of 0.1 mg of total protein extracts of pollen adsorbed on 5 mg of alum. Within 10 min after the injection, blood was taken and histamine levels were determined by using an enzyme immunoassay kit (Neogen, Lexington, KY).
Clinical Symptoms of Pollen Allergy. To examine the effect of A1aB1b-Crp-1 and -2 rice seeds induced oral tolerance for the inhibition of pollen allergen triggered clinical symptoms associating with pollinosis, other sets of mice were fed with the experimental and control rice seeds as described above. These mice were presensitized with pollen allergen via systemic route at weeks 4 and 5. At week 7 through week 8 of the experiment, these mice were then challenged once a day with 20 μl of 1 μg/ml total protein extracts of pollen dissolved in PBS via the intranasal route as described (34). Sham-challenged mice were nasally administered with 20 μl of PBS in the same manner. Nasal symptoms were evaluated by counting the number of sneezes observed in the 5 min after the last nasal challenge at week 8.
Statistics. The significance of the differences (e.g., P values) between groups was evaluated by the Mann-Whitney U test.
Results
Development of Transgenic Rice Plants Accumulating A1aB1b-Crp-1 and -2 Protein in Seeds. Thirty independent transgenic rice plants were generated, and accumulation levels of the A1aB1b-Crp-1 and -2 protein in seeds were examined by immunoblot analysis. Transgenic lines 9 and 12, which showed high levels of accumulation of A1aB1b-Crp-1 and -2 protein at the level of 7 μg per grain (≈0.5% of total seed protein), were selected and proceeded to the T3 generation by self-crossing to obtain homozygous lines.
To examine the tissue-specific expression of A1aB1b-Crp-1 and -2 gene, total RNA extracted from leaves, roots, and maturing seeds were subjected to Northern blot analysis. The transcript of the A1aB1b-Crp-1 and -2 gene was only detected in maturing seeds, whereas no band was found in the leaves or roots of transgenic lines 9 and 12 (Fig. 1B). These results indicate that the A1aB1b-Crp-1 and -2 gene is specifically expressed in seeds under the control of the 2.3-kb GluB-1 promoter.
Next, total seed protein was extracted for analysis of A1aB1b-Crp-1 and -2 protein expression by Western blot (Fig. 1C). We previously demonstrated that the glycinin A1aB1b expressed in the endosperm of transgenic rice was synthesized as a precursor form and then posttranslationally processed into two mature subunits, the acidic and basic subunits (27). As shown in Fig. 1C, three signals for the precursor, the acidic and basic subunits with molecular masses of 56, 35, and 21 kDa, respectively, were detected in A1aB1b-Crp-1 and -2 transgenic lines by using anti-glycinin A1aB1b antibody. This result indicates that the A1aB1b-Crp-1 and -2 protein was expressed and posttranslationally processed in a similar manner to the native glycinin A1aB1b (27). The accumulation of Crp-1 and -2 T cell epitope peptides in the A1aB1b-Crp-1 and -2 protein was further confirmed by immunoblot analysis using the peptide specific anti-Crp-1 and anti-Crp-2 antibodies (Fig. 1C). It was shown that the glycinin acidic subunit (35 kDa) and the precursor (56 kDa) were recognized by the anti-Crp-1 antibody, whereas the glycinin basic subunit (21 kDa) and the precursor (56 kDa) were detected by the anti-Crp-2 antibody. These results clearly indicated that the Crp-1 and -2 peptides were expressed as fusion protein with A1aB1b and processed into the acidic and basic subunits of A1aB1b, respectively.
Integration of the A1aB1b-Crp-1 and -2 gene into the rice genome was confirmed by Southern blot analysis. Because the SacI restriction enzyme cuts only once in the transformation plasmid, the number of bands indicates the number of copies of the A1aB1b-Crp-1 and -2 gene integrated into the rice genome. At least two copies of A1aB1b-Crp-1 and -2 gene were estimated to be present in transgenic rice lines 9 and 12 (Fig. 1D).
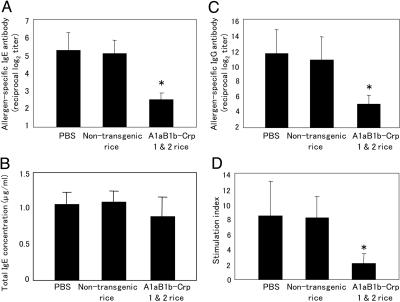
Oral Feeding of Transgenic Rice Seeds Prevents the Development of Allergen-Specific IgE and IgG Responses. In the control group of mice fed with PBS, i.p. challenge with the pollen allergen elicited significant allergen-specific IgE and IgG responses (Fig. 2 A and C). Oral feeding of nontransgenic rice seeds did not affect the high levels of allergen-specific IgE antibody response (Fig. 2A). In contrast, the level of serum allergen-specific IgE was significantly reduced in the group of mice fed with transgenic rice seeds accumulating A1aB1b-Crp-1 and -2 protein when compared to those in the control groups of mice fed with PBS or nontransgenic rice seeds (P < 0.01) (Fig. 2A). The levels of total IgE antibodies in serum were similar among the three groups of mice (Fig. 2B). In the case of allergen-specific IgG responses, the antibody titers were decreased in mice orally immunized with A1aB1b-Crp-1 and -2 rice seeds when compared to those in the control groups of mice (P < 0.01) (Fig. 2C). In addition, a dominant allergen-specific IgG1 subclass with some IgG2a and IgG2b antibodies were all decreased in the experimental group of mice (Table 1, which is published as supporting information on the PNAS web site). These results suggest that oral administration of A1aB1b-Crp-1 and -2 seeds inhibits a dominant Th2 cell-mediated antibody with some Th1-involved antibody responses to pollen allergens.
Fig. 2.
Inhibition of allergen-specific serum IgE, IgG, and CD4+ T cell responses by oral administration of A1aB1b-Crp-1 and -2 rice seeds. Levels of allergen-specific IgE (A), total IgE (B), and allergen-specific IgG (C) were examined in serum of mice fed with PBS, nontransgenic rice seeds, or A1aB1b-Crp-1 and -2 rice seeds before systemic challenge with total protein extracts of pollen. Allergen-specific splenic CD4+ T responses (D) were expressed as stimulation index calculated as the ratio of [cpm of cells cultured in the presence of allergen]/[cpm of cells cultured in the absence of allergen]. Data are expressed as mean ± SD. *, P < 0.01 for the group of mice fed with A1aB1b-Crp-1 and -2 rice seeds in comparison with the group of mice fed with PBS or nontransgenic rice seeds.
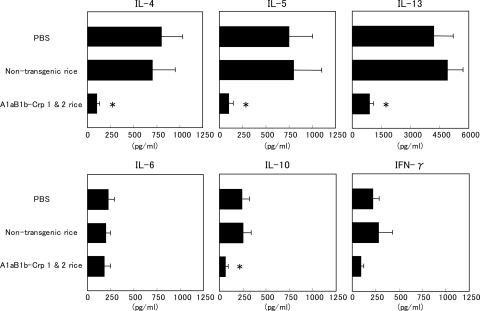
Oral Feeding of Transgenic Rice Seeds Inhibits Allergen-Specific T Cell Proliferation and IgE-Associated Th2 Cytokine Responses. To examine the effect of oral feeding of rice seeds on allergen-specific T cell responses, CD4+ T cells were isolated from the spleens of experimental and control mice and were stimulated in vitro with or without pollen allergen. Oral immunization with A1aB1b-Crp-1 and -2 rice seeds greatly suppressed the allergen-specific T cell proliferative responses when compared to those in the control mice (75% suppression, P < 0.01) (Fig. 2D). To further demonstrate the effect of oral feeding of transgenic seeds on the inhibition of allergen-specific CD4+ T cell responses, we next examined levels of Th1 and Th2 cytokine synthesis (Fig. 3). The amounts of Th1 and Th2 cytokines produced in the culture supernatants of allergen-stimulated CD4+ T cells were measured by ELISA. In the control groups of mice fed with PBS or nontransgenic rice seeds, high quantities of Th2 cytokines associating with IgE-mediated immune responses such as IL-4, IL-5, and IL-13 were produced in the culture supernatants. In the group of mice fed with A1aB1b-Crp-1 and -2 rice seeds, levels of allergic reaction-associated cytokines IL-4, IL-5, and IL-13 were significantly lower than those of control groups of mice (85%, 86%, and 78% suppression, respectively; P < 0.01) (Fig. 3). Both Th2-associated IL-10 and Th1-associated IFN-γ cytokines were not induced significantly by this allergic response-inducing system. However, their levels were also decreased in the group of mice fed with A1aB1b-Crp-1 and -2 rice seeds (75% and 59% suppression, respectively; P < 0.01) (Fig. 3). The levels of IL-6 were not drastically changed between the three groups. These findings specifically demonstrate that oral immunization of A1aB1b-Crp-1 and -2 rice seeds effectively induced the state of oral tolerance where the inhibition of IgE-associated Th2 cytokines, including IL-4, IL-5, and IL-13, was achieved at the level of allergen-specific CD4+ T cells.
Fig. 3.
Inhibition of allergen-induced Th2 cytokine production by splenic CD4+ T cells isolated from mice fed with A1aB1b-Crp-1 and -2 rice seeds. Splenic CD4+ T cells were cultured with or without total protein extracts of pollen as described earlier. Levels of Th1 and Th2 cytokines in cell-free culture supernatants of CD4+ T cells were assayed by ELISA. Data are presented as mean ± SD. *, P < 0.01 for the group of mice fed with transgenic rice seeds in comparison with the group of mice fed with PBS or nontransgenic rice seeds.
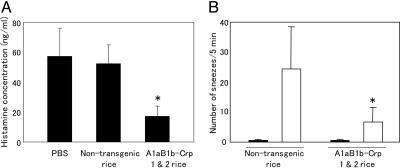
Inhibition of Levels of Histamine Released in Serum of Mice Orally Fed with A1aB1b-Crp-1 and -2 Rice Seeds. Next, we examined the levels of serum histamine release, one of the inflammatory mediators released at degranulation of mast cells associating with IgE-mediated responses (35). Mice were bled within 10 min of the challenge with pollen allergen at week 7 of the experiment. The levels of histamine released in serum were as high as ≈60 ng/ml in mice fed with PBS or nontransgenic rice seeds (Fig. 4A). On the other hand, this high level of histamine release was dramatically reduced to ≈20 ng/ml in mice orally immunized with the A1aB1b-Crp-1 and -2 rice seeds (Fig. 4A). The results show that oral administration of rice seeds containing A1aB1b-Crp-1 and -2 protein was effective in the induction of oral tolerance for the inhibition of allergy-associated immune responses including those of Th2 cell-mediated IgE response and histamine release.
Fig. 4.
Serum histamine levels (A) and the number of sneezes (B) were inhibited in the group of mice fed with A1aB1b-Crp-1 and -2 rice seeds. The number of sneezes was counted in the 5 min after the last nasal challenge at week 8 (white bars). Sham-challenged mice were nasally administered with 20 μl of PBS in the same manner (black bars). Data are expressed as mean ± SD. *, P < 0.01 for the group of mice fed with A1aB1b-Crp-1 and -2 seeds in comparison with the group of mice fed with nontransgenic rice seeds.
Inhibition of Pollen-Induced Allergic Symptoms in Mice Orally Fed with A1aB1b-Crp-1 and -2 Rice Seeds. To examine the effect of orally fed A1aB1b-Crp-1 and -2 rice seeds on the development of clinical symptoms of pollinosis, we adopted an experimental murine sneezing model (34). Mice were orally fed with nontransgenic or A1aB1b-Crp-1 and -2 rice seeds and i.p. presensitized with total protein extracts of pollen. Mice were then intranasally challenged with the pollen protein extracts. Significant nasal symptoms of sneezing developed in the group of mice fed with nontransgenic rice seeds (Fig. 4B). In contrast, the number of sneezes was reduced in the group of mice fed with A1aB1b-Crp-1 and -2 rice (P < 0.01) (Fig. 4B). Nasal challenge with PBS did not induce any nasal symptoms of sneezing (Fig. 4B). These findings demonstrated that oral administration of A1aB1b-Crp-1 and -2 rice seeds was effective in the induction of tolerance against pollen allergen leading to the inhibition for the development of allergic symptoms of sneezing in nasal tract.
Discussion
Adaptation of the concept of oral tolerance has been considered as a fundamental strategy for the development of immunotherapy for the prevention and/or treatment of allergic diseases (36). The mechanism of oral tolerance has not yet been precisely clarified; however, oral immunization of allergens is known to induce a state of systemic unresponsiveness to the administered allergens (36). To avoid unwanted anaphylactic reactions being elicited during the desensitization process using allergens, the use of T cell epitope peptides has been shown to be an attractive approach (4-8). T cell epitope peptides are incapable of binding to allergen-specific IgE antibody molecules on the surface of mast cells, so the administration of high doses of T cell epitope peptides is theoretically possible without inducing anaphylactic side effects (2). The efficacy of tolerance induction was shown to depend on the dose of allergens administered (7); thus, immunotherapy with T cell epitope peptides is expected to be both safe and effective in the treatment of allergic diseases (2). In this study, we developed transgenic rice plants expressing T cell epitope peptides in seeds and examined whether oral feeding of the transgenic rice seeds to mice could prevent the development of allergic responses against pollen allergens of Japanese cedar. Our results demonstrate that oral immunization of the transgenic rice seeds expressing A1aB1b-Crp-1 and -2 protein resulted in the generation of systemic unresponsiveness with a reduction of allergen-specific Th2-mediated IgE responses and histamine release.
It has been demonstrated that the direct production of short peptides such as T cell epitope peptides with lengths of 10-20 aa is difficult for most expression systems of eukaryotic and prokaryotic cells (37). Therefore, our efforts in this study were initially focused on the expression of Crp-1 and -2 T cell epitope peptides in transgenic rice seeds. We adopted a strategy in which Crp-1 and -2 peptides were expressed as parts of the soybean seed storage protein glycinin by inserting them into highly variable regions of acidic and basic subunits of glycinin A1aB1b (29, 30). The recombinant protein was successfully expressed in rice seeds; however, the maximum level of A1aB1b-Crp-1 and -2 accumulation (0.5% of total seed protein) was lower than that of A1aB1b (5% of total seed protein) (27). One possible explanation for this result is that the insertion of T cell epitopes into variable regions of A1aB1b potentially influences secondary structure formation or interaction between acidic and basic subunits in A1aB1b-Crp-1 and -2, which may cause the lower accumulation levels of A1aB1b-Crp-1 and -2. When expressed in transgenic rice seeds under the control of the glutelin GluB-1 promoter, glycinin A1aB1b was synthesized as a preproglycinin and posttranslationally processed into acidic and basic subunits (27). The synthesized glycinin A1aB1b was localized in protein body II, in which ≈30% of glycinin was assembled with glutelin (27). In this study, A1aB1b-Crp-1 and -2 protein was synthesized as a precursor form and then posttranslationally processed into acidic and basic subunits in a similar manner to the glycinin A1aB1b (Fig. 1C) (27). These results suggest that A1aB1b-Crp-1 and -2 protein accumulated in protein body II, although there is possibility that the insertion of T cell epitope peptides into A1aB1b may affect the intracellular localization of A1aB1b-Crp-1 and -2 protein in the endosperm cells. When anti-Crp-1 peptide antibody was used as a probe, the precursor signal of A1aB1b-Crp-1 and -2 (56 kDa) was weaker than those obtained by the anti-glycinin and anti-Crp 2 peptide antibodies (Fig. 1C). These results might be explained by the difference in binding affinity of anti-Crp-1 peptide antibody to the Crp-1 peptide accumulated in two distinct forms, the precursor and mature acidic subunit.
Two regions of pollen allergens (p277-290 of Cry j I and p246-259 of Cry j II) have been identified as major T cell epitopes in BALB/c mice (15, 16). Previously, one of the major T cell epitope peptides, p246-259 of Cry j II, was chemically synthesized and was orally administered to mice before systemic challenge with Cry j II (15). It was shown that the Cry j II-specific IgE response was significantly decreased (74% suppression) in mice orally immunized with the synthetic T cell epitope peptide. Furthermore, both Th1 and Th2 cytokine production was inhibited in the group of mice fed orally with Cry j II peptide compared to the control group of mice fed with PBS (15). In the present study, to evaluate the efficacy of newly generated transgenic rice seeds expressing Cry j I and Cry j II T cell epitope peptides for the induction of systemic unresponsiveness to pollen allergens of Japanese cedar, a group of mice was fed with the transgenic rice seeds and two other groups of mice were orally administered with nontransgenic rice seeds or PBS. We chose total protein extracts of pollen as allergen for the systemic challenge of mice to assess the effectiveness of transgenic rice seeds for taking account of future applications in the clinical treatment of pollen allergy. We further thought that this challenge method has a benefit to examine bystander tolerance effects to additional T cell epitopes. This line of detailed investigation requires further study.
It has been reported that patients with Japanese cedar pollinosis exhibit a high titer allergen-specific IgE response (38). Allergen-specific IgE antibodies have been shown to play a major biological role for the induction of pollen-associated allergic responses (38, 39). In this study, pollen allergen-specific IgE levels were significantly decreased by oral feeding of transgenic rice seeds accumulating A1aB1b-Crp-1 and -2, whereas the levels of total IgE antibodies were similar among the three groups of mice (Fig. 2 A and B). In addition, oral administration of A1aB1b-Crp-1 and -2 seeds did not affect OVA-specific CD4+ T cell proliferative responses (Fig. 5, which is published as supporting information on the PNAS web site). These results indicate that oral feeding of transgenic rice seeds induces pollen allergen-specific T cell unresponsiveness. Furthermore, it is important to note that glycinin-specific IgG and IgE antibodies were not detected in the sera of control and experimental groups of mice (data not shown).
The production of CD4+ Th2-type cell derived allergen-specific cytokines, IL-4, IL-5, and IL-13, was dramatically inhibited by oral feeding of transgenic rice seeds (Fig. 3). These Th2-type cytokines were shown to be involved in the process of IgE production. IL-4 and IL-13 stimulate and regulate Ig class switching to IgE (39-41) and IL-5 drives the proliferation and differentiation of B cells into antibody-secreting plasma cells (42, 43). The inhibition of these IgE-associated cytokine responses is one of the important factors for the control of allergen-specific IgE synthesis. Our results show that successful inhibition of these cytokine responses offers effective oral immunization by A1aB1b-Crp-1 and -2 rice seeds for the suppression of IgE-mediated hypersensitive allergic reactions. The production of IL-10 was also inhibited in the group of mice fed with transgenic rice seeds, which is consistent with a previous report describing that IL-10 is not required for induction of oral tolerance to OVA (44). On the other hand, it was recently reported that the suppression of allergic diseases in allergen immunotherapy is associated with the increased levels of IL-10 (45, 46). This conflict might be caused by different experimental designs. Further studies are required to examine this controversial result on the role of IL-10 in the induction of oral tolerance.
Allergy-associated inflammatory mediators such as histamine released by mast cells are known to cause immediate symptoms of type-I allergy (35). Allergen-IgE complex formation on the surface of mast cells triggers degranulation of mast cells leading to the histamine release (35). Thus, the reduction of allergen-specific IgE antibody levels can be an effective strategy for the suppression of histamine release by mast cells. In our study, levels of both allergen-specific IgE antibody and serum histamine release were significantly reduced in the group of mice fed orally with A1aB1b-Crp-1 and -2 rice seeds compared with the control groups (Figs. 2A and 4A). These results suggest that oral immunization of A1aB1b-Crp-1 and -2 rice seeds is effective in the suppression of allergen-specific IgE responses, which further inhibit histamine release by blocking the formation of the allergen-IgE complex. In addition, using the experimental mouse model of pollen allergy, we have shown here that oral feeding of A1aB1b-Crp-1 and -2 rice seeds inhibits the development of nasal allergic symptoms (Fig. 4B). Our findings provide further evidence of a significant potential benefit of A1aB1b-Crp-1 and -2 rice seeds for the prevention of the development of IgE-mediated allergic symptoms without any signs of the anaphylactic side effects.
The seed-expression system possesses several advantages for the production of recombinant proteins, such as simplicity of administration, low risk of contamination with animal pathogens, and low cost for production and long storage at room temperature (17-22, 25). Here, we showed that the status of systemic unresponsiveness associated with the inhibition of allergen-specific Th2-type and IgE responses was achieved by oral feeding of recombinant protein containing Crp-1 and -2 T cell epitope peptides without any purification step. Therefore, rice seeds could serve as an effective and new vehicle for the mucosal delivery of pharmatheutical molecules. Further clinical trials will be required to extend our findings for the development of rice-based edible vaccines as a peptide immunotherapy for the control of allergy.
Supplementary Material
Acknowledgments
We thank Dr. S. Utsumi for providing the glycinin cDNA and anti-glycinin antibody. This work was supported by a research grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (to F.T.) and CREST of the Japan Science and Technology Corporation (H. Kiyono).
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: Thn, T helper n.
See Commentary on page 17255.
References
- 1.Bousquet, J., Lockey, R. & Malling, H. J. (1998) J. Allergy Clin. Immunol. 102, 558-562. [DOI] [PubMed] [Google Scholar]
- 2.Haselden, B. M., Kay, A. B. & Larche, M. (2000) Int. Arch. Allergy Immunol. 122, 229-237. [DOI] [PubMed] [Google Scholar]
- 3.Frew, A. J. (2003) J. Allergy Clin. Immunol. 111, S712-S719. [DOI] [PubMed] [Google Scholar]
- 4.Briner, T. J., Kuo, M., Keating, K. M., Rogers, B. L. & Greenstein, J. L. (1993) Proc. Natl. Acad. Sci. USA 90, 7608-7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyne, G. F., O'Hehir, R. E., Wraith, D. C., Thomas, W. R. & Lamb, J. R. (1993) J. Exp. Med. 178, 1783-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoyne, G. F., Askonas, B. A., Hetzel, C., Thomas, W. R. & Lamb, J. R. (1996) Int. Immunol. 8, 335-342. [DOI] [PubMed] [Google Scholar]
- 7.Norman, P. S., Ohman, J. L., Jr., Long, A. A., Creticos, P. S., Gefter, M. A., Shaked, Z., Wood, R. A., Eggleston, P. A., Hafner, K. B., Rao, P., et al. (1996) Am. J. Respir. Crit. Care Med. 154, 1623-1628. [DOI] [PubMed] [Google Scholar]
- 8.Muller, U., Akdis, C. A., Fricker, M., Akdis, M., Blesken, T., Bettens, F. & Blaser, K. (1998) J. Allergy Clin. Immunol. 101, 747-754. [DOI] [PubMed] [Google Scholar]
- 9.Yasueda, H., Yui, Y., Shimizu, T. & Shida, T. (1983) J. Allergy Clin. Immunol. 71, 77-86. [DOI] [PubMed] [Google Scholar]
- 10.Sone, T., Komiyama, N., Shimizu, K., Kusakabe, T., Morikubo, K. & Kino, K. (1994) Biochem. Biophys. Res. Commun. 199, 619-625. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi, M., Inouye, S., Taniai, M., Ando, S., Usui, M. & Matuhasi, T. (1990) Allergy 45, 309-312. [DOI] [PubMed] [Google Scholar]
- 12.Namba, M., Kurose, M., Torigoe, K., Hino, K., Taniguchi, Y., Fukuda, S., Usui, M. & Kurimoto, M. (1994) FEBS Lett. 353, 124-128. [DOI] [PubMed] [Google Scholar]
- 13.Komiyama, N., Sone, T., Shimizu, K., Morikubo, K. & Kino, K. (1994) Biochem. Biophys. Res. Commun. 201, 1021-1028. [DOI] [PubMed] [Google Scholar]
- 14.Saito, S., Hirahara, K., Kawaguchi, J., Serizawa, N., Hino, K., Taniguchi, Y., Kurimoto, M., Sakaguchi, M., Inouye, S. & Shiraishi, A. (2000) Annu. Rep. Sankyo Res. Lab. 52, 49-58. [Google Scholar]
- 15.Hirahara, K., Saito, S., Serizawa, N., Sasaki, R., Sakaguchi, M., Inouye, S., Taniguchi, Y., Kaminogawa, S. & Shiraishi, A. (1998) J. Allergy Clin. Immunol. 102, 961-967. [DOI] [PubMed] [Google Scholar]
- 16.Yoshitomi, T., Hirahara, K., Kawaguchi, J., Serizawa, N., Taniguchi, Y., Saito, S., Sakaguchi, M., Inouye, S. & Shiraishi, A. (2002) Immunology 107, 517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer, R. & Emans, N. (2000) Transgenic Res. 9, 279-299. [DOI] [PubMed] [Google Scholar]
- 18.Giddings, G. (2001) Curr. Opin. Biotechnol. 12, 450-454. [DOI] [PubMed] [Google Scholar]
- 19.Daniell, H., Streatfield, S. J. & Wycoff, K. (2001) 6, 219-226. [DOI] [PMC free article] [PubMed]
- 20.Walmsley, A. M. & Arntzen, C. J. (2000) Curr. Opin. Biotechnol. 11, 126-129. [DOI] [PubMed] [Google Scholar]
- 21.Mason, H. S., Warzecha, H., Mor, T. & Arntzen, C. J. (2002) Trends Mol. Med. 8, 324-329. [DOI] [PubMed] [Google Scholar]
- 22.Streatfield, S. J. & Howard, J. A. (2003) Int. J. Parasitol. 33, 479-493. [DOI] [PubMed] [Google Scholar]
- 23.Delaney, D. E. (2002) in Plants as Factories for Protein Production, eds. Hood, E. E. & Howard, J. A. (Kluwer Academic, Dordrecht, The Netherlands), pp. 139-158.
- 24.Howard, J. A. & Hood, E. E. (2002) in Plants as Factories for Protein Production, eds. Hood, E. E. & Howard, J. A. (Kluwer Academic, Dordrecht, The Netherlands), pp. VII-X.
- 25.Stoger, E., Sack, M., Perrin, Y., Vaquero, C., Torres, E., Twyman, R. M., Christou, P. & Fischer, R. (2002) Mol. Breed. 9, 149-158. [Google Scholar]
- 26.Qu, L. Q. & Takaiwa, F. (2004) Plant Biotechnol. J. 2, 113-125. [DOI] [PubMed] [Google Scholar]
- 27.Katsube, T., Kurisaka, N., Ogawa, M., Maruyama, N., Ohtsuka, R., Utsumi, S. & Takaiwa, F. (1999) Plant Physiol. 120, 1063-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tada, Y., Utsumi, S. & Takaiwa, F. (2003) Plant Biotechnol. J. 1, 411-422. [DOI] [PubMed] [Google Scholar]
- 29.Takaiwa, F., Katsube, T., Kitagawa, S., Hisago, T., Kito, M. & Utsumi, S. (1995) Plant Sci. 111, 39-49. [Google Scholar]
- 30.Adachi, M., Takenaka, Y., Gidamis, A. B., Mikami, B. & Utsumi, S. (2001) J. Mol. Biol. 305, 291-305. [DOI] [PubMed] [Google Scholar]
- 31.Kweon, M. N., Yamamoto, M., Kajiki, M., Takahashi, I. & Kiyono, H. (2000) J. Clin. Invest. 106, 199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hino, A., Kweon, M. N., Fujihashi, K., McGhee, J. R. & Kiyono, H. (2004) Am. J. Pathol. 164, 1327-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujihashi, K., Dohi, T, Rennert, P. D., Yamamoto, M., Koga, T., Kiyono, H. & McGhee, J. R. (2001) Proc. Natl. Acad. Sci. USA 98, 3310-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwasaki, M., Saito, K., Takemura, M., Sekikawa, K., Fujii, H., Yamada, Y., Wada, H., Mizuta, K., Seishima, M., Ito, Y., et al. (2003) J. Allergy Clin. Immunol. 112, 134-140. [DOI] [PubMed] [Google Scholar]
- 35.Kinet, J. P. (1999) Annu. Rev. Immunol. 17, 931-972. [DOI] [PubMed] [Google Scholar]
- 36.Sosroseno, W. (1995) J. R. Soc. Med. 88, 14-17. [PMC free article] [PubMed] [Google Scholar]
- 37.Wirth, S., Calamante, G., Mentaberry, A., Bussmann, L., Lattanzi, M., Baranao, L. & Bravo-Almonacid, F. (2004) Mol. Breed. 13, 23-35. [Google Scholar]
- 38.Hashimoto, M., Nigi, H., Sakaguchi, M., Inoue, S., Imaoka, K., Miyazawa, H., Taniguchi, Y., Kurimoto, M., Yasueda, H. & Ogawa, T. (1995) Clin. Exp. Allergy 25, 848-852. [DOI] [PubMed] [Google Scholar]
- 39.Gould, H. J., Sutton, B. J., Beavil, A. J., Beavil, R. L., McCloskey, N., Coker, H. A., Fear, D. & Smurthwaite, L. (2003) Annu. Rev. Immunol. 21, 579-628. [DOI] [PubMed] [Google Scholar]
- 40.Robinson, D. S., Hamid, Q., Ying, S., Tsicopoulos, A., Barkans, J., Bentley, A. M., Corrigan, C., Durham, S. R. & Kay, A. B. (1992) N. Engl. J. Med. 326, 298-304. [DOI] [PubMed] [Google Scholar]
- 41.Del Prete, G. F., De Carli, M., D'Elios, M. M., Maestrelli, P., Ricci, M., Fabbri, L. & Romagnani, S. (1993) Eur. J. Immunol. 23, 1445-1449. [DOI] [PubMed] [Google Scholar]
- 42.Swain, S. L., McKenzie, D. T., Dutton, R. W., Tonkonogy, S. L. & English, M. (1988) Immunol. Rev. 102, 77-105. [DOI] [PubMed] [Google Scholar]
- 43.Takatsu, K., Tominaga, A., Harada, N., Mita, S., Matsumoto, M., Takahashi, T., Kikuchi, Y. & Yamaguchi, N. (1988) Immunol. Rev. 102, 107-135. [DOI] [PubMed] [Google Scholar]
- 44.Russo, M., Nahori, M. A., Lefort, J., Gomes, E., Keller, A. C., Rodriguez, D., Ribeiro, O. G., Adriouch, S., Gallois, V., Faria, A. M. C., et al. (2001) Am. J. Respir. Cell Mol. Biol. 24, 518-526. [DOI] [PubMed] [Google Scholar]
- 45.Zemann, B., Schwaerzler, C., Griot-Wenk, M., Nefzger, M., Mayer, P., Schneider, H., de Weck, A., Carballido, J. M. & Liehl, E. (2005) J. Allergy Clin. Immunol. 111, 1069-1075. [DOI] [PubMed] [Google Scholar]
- 46.Smart, V., Foster, P. S., Rothenberg, M. E., Higgins, T. J. V. & Hogan, S. P. (2003) J. Immunol. 171, 2116-2126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.