Abstract
Leukotriene B4 (LTB4), a potent leukocyte chemoattractant derived from the 5-lipoxygenase metabolism of arachidonic acid, exerts its action by means of specific cell surface receptors, denoted BLT1 and BLT2. In this study, BLT1 receptor proteins were detected in human carotid artery atherosclerotic plaques, colocalizing with markers for macrophages, endothelial cells, and vascular smooth muscle cells (SMC). Challenge of human coronary artery SMC with either LTB4 or U75302, a partial agonist that is selective for the BLT1 receptor, induced an ≈4-fold increase of whole-cell currents by using the patch-clamp technique, indicating that these cells express functional BLT1 receptors. LTB4 induced migration and proliferation of SMC in vitro, and treatment with the BLT receptor antagonist BIIL 284 (10 mg/kg, once daily) for 14 days after carotid artery balloon injury in vivo inhibited intimal hyperplasia in rats. In the latter model, SMC derived from the intima exhibited increased levels of BLT1 receptor mRNA compared with medial SMC. BLT receptor up-regulation in the intima in vivo, as well as that induced by IL-1β in vitro, were prevented by transfection with a dominant-negative form of Iκ kinase β carried by adenovirus, indicating that BLT1 receptor expression depends on NF-κΒ. These results show that LTB4 activates functional BLT1 receptors on vascular SMC, inducing chemotaxis and proliferation, and that BLT1 receptors were up-regulated through an Iκ kinase β/NF-κB-dependent pathway. Inhibition of LTB4/BLT1 signaling during the response to vascular injury reduced intimal hyperplasia, suggesting this pathway as a possible target for therapy.
Keywords: restenosis, cell proliferation, chemotaxis, lipoxygenase, eicosanoids
Leukotrienes (LTs) are inflammatory mediators that are derived from the 5-lipoxygenase (5-LO) pathway of arachidonic acid metabolism (1). Leukotrienes exert their actions by means of membrane-bound G protein-coupled receptors; CysLT receptors, which are activated by leukotrienes C4, D4, and E4; and BLT receptors, which are activated by leukotriene B4 (LTB4) (2, 3). The last class consists of two receptor subtypes, BLT1 and BLT2, representing the high- and low-affinity receptors for LTB4, respectively (4, 5). BLT receptors are expressed on leukocytes; neutrophils, eosinophils, and T lymphocytes all migrate in response to LTB4 (6, 8-11).
A major role for the leukotriene pathway in vascular disease was suggested by studies of a congenic mouse strain demonstrating resistance to atherosclerosis linked to a locus on chromosome 6, within which the gene for 5-LO was mapped subsequently (12). Also, expression of the enzymes and receptors of the 5-LO pathway has been demonstrated in human atherosclerotic plaques (13), and genetic studies have revealed that polymorphism in the 5-LO promoter is associated with an increased carotid artery intima thickness (14) and that the gene encoding the 5-LO-activating protein (FLAP) is associated with an increased risk of stroke and myocardial infarction (15).
Although these studies implicate leukotrienes in the pathogenesis of atherosclerosis, the role of LTB4 and BLT receptors in atherogenesis is not clear. For example, treatment of atherosclerotic mice strains with the BLT1 receptor antagonist CP105696 for 35 days reduces atherosclerotic lesion area and lipid accumulation significantly (16), whereas ApoE-deficient mice crossed with BLT1 receptor-deficient mice display reduced lesion formation only during early stages of disease (4 and 8 weeks) (17). Also, studies of 5-LO-deficient atherosclerotic mouse strains are contradictory, with both a reduction of atherosclerosis (12) and no effect (18) having been reported. In the latter study, it was shown that mice lacking the gene for 5-LO are protected from aortic aneurysms (18), supporting that leukotrienes may exert their effects on vascular cells producing structural elements. Also, LTB4 is an indirectly acting vasoconstrictor in isolated guinea pig vascular preparations (19, 20), and LTB4-induced effects on rat vascular smooth muscle cells (SMC) (21, 22) have been reported, suggesting that vascular SMC may represent a target for LTB4 in some animal models. However, the function of BLT receptors on human vascular SMC has been unknown, and the aim of the present study was to map the distribution of BLT receptors within the human vascular wall; elucidate the pharmacological and physiological responses induced by LTB4; and, last, to link these responses to pathophysiological processes. Our data point to an important role for LTB4 in SMC activation, migration, and proliferation by means of NF-κB-dependent BLT1 receptors, with implications for atherosclerosis and intimal hyperplasia.
Materials and Methods
Drugs. LTB4 and U75302 were obtained from Cayman Chemical (Ann Arbor, MI). ONO 4057 was a gift from Ono Pharmaceutical. BIIL 284 was a gift from Boehringer Ingelheim.
Immunohistochemistry. Atherosclerotic vascular tissue was collected from patients undergoing carotid endarterectomy and internal mammary arteries as controls were obtained from patients undergoing coronary artery bypass surgery. The experiments were approved by the local ethics committee. Acetone-fixed frozen sections were preincubated for 30 min with either mouse or goat serum (1:20 in PBS) before overnight incubation with primary antibody at 4°C. Mouse anti-human CD163, α-smooth muscle actin, and von Willebrand factor antibodies were obtained from DAKO. Rabbit anti-human BLT1 and BLT2 receptor antibodies were obtained from LifeSpan Biosciences (Seattle). Sections were incubated with biotinylated horse anti-mouse or goat anti-rabbit IgG, followed by avidin-biotin peroxidase complex (Vector Laboratories) and development with diaminobenzidine. Rabbit IgG staining was used as negative control.
Animal Experiments. All animal experiments were approved by the Regional Ethics Committee for Animal Research in Stockholm and performed as described in ref. 23. Briefly, 350-g male Sprague-Dawley rats (Scanbur, Sollentuna, Sweden) were subjected to balloon injury of the left common carotid artery under general anesthesia (2 mg/kg pentobarbital and 50 mg/kg fluanisone, i.p.). After balloon injury, rats were treated with either BIIL 284 (10 mg/kg, i.p.; n = 8) or vehicle (1% tylose, i.p.; n = 7) once daily for 14 days (24) (F. Kalkbrenner, personal communication). Rats were fed standard rat chow and water ad libitum. The animals were killed at day 14 after injury, and serial sections of carotid artery were stained with eosin/hematoxylin. Images were frame-grabbed, and the intimal and medial areas were analyzed blindly by using qwin software (Leica, Deerfield, IL). We analyzed 15 cross sections from each carotid artery.
Cell Culture and Adenoviral Infection. Rat SMC were isolated from the media and intima of the rat carotid artery after balloon injury as described in ref. 25 and grown in DMEM F12 containing 10% FCS and penicillin/streptomycin. Equivalent passages (between passages 5 and 9) of medial and intimal cells were compared in each set of experiments. Cells were serum-starved for 24 h before infection with two recombinant, replication-deficient, adenoviral vectors expressing Escherichia coli β-gal or the Flag-tagged dominant-negative form of IκB kinase β (dnIKKβ), which was provided by R. DeMartin (University of Vienna, Vienna) (23, 26). A multiplicity of infection from 50:1 to 200:1 was used as described in ref. 23. In some experiments, rat medial SMC were stimulated with either murine recombinant IL-1β (10 ng/ml; Genzyme) or LPS (10 mg/μl; Sigma) before RNA extraction.
Human coronary artery SMC purchased from Clonetics (Cambrex, Walkersville, MD) were cultured by using SmGM2 kit medium. Cells (between passages 5 and 8) were seeded in six-well plates (105 cells per well) 48 h before the respective treatment and serum-starved 24 h before experiments. Cells were incubated in the absence or presence of either human recombinant IL-1β (10 ng/ml; PeproTech, Rocky Hill, NJ) or LPS (10 μg/ml) and collected for either RNA or protein extraction.
Western Blot Analysis. Human coronary artery SMC were lysed in RIPA lysis buffer (150 mM NaCl/50 mM Tris, pH 8.0/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS) containing protease inhibitors (1 mM PMSF/10 μM E64/1 mM EDTA/1 μg/ml pepstatin A). Proteins (10 μg) were resolved by 10% SDS/PAGE and transferred to Hybond-P PVDF membranes. Membranes were blocked for 1 h with 5% nonfat dry milk in 0.05% PBS/Tween 20 and incubated overnight with polyclonal rabbit anti-human BLT1 antibody (1 μg/ml). For signal detection, donkey anti-rabbit IgG coupled to horseradish peroxidase and the enhanced chemiluminescence (ECL) detection system ECL Plus was used (Amersham Biosciences).
Electrophysiology. Whole-cell currents were recorded with patch-clamp technique (27) by using an EPC-10/2 patch-clamp amplifier (HEKA Electronics, Lambrecht, Germany). Borosilicate pipettes were pulled by using a DMZ puller (Zeitz Instruments, Augsburg, Germany), and they had a resistance of 3-5 MΩ in the solutions used. Whole-cell current traces were displayed with upward deflections denoting outward currents. The extracellular solution contained 138 mM NaCl, 5.6 mM KCl, 1.2 mM MgCl2, 2.6 mM CaCl2, and 5 mM Hepes/NaOH (pH 7.4); the pipette solution contained 125 mM KCl, 1 mM MgCl2, 10 mM EGTA, 25 mM KOH, and 5 Hepes/KOH (pH 7.15). In the pipette solution, we added 5.7 mM CaCl2, corresponding to a free intracellular Ca2+ concentration of ≈300 nM (maxchelator) (28). The cells were voltage-clamped at -80 mV and subsequently depolarized in +10-mV steps for 100 ms. LTB4 (1 μM) and U75302 (10 μM) were puffed by using a secondary pipette set-up 10-20 μm from the recording cell.
Cell Migration. Aliquots of 10,000 cells in 0.1 ml of DMEM were seeded in 12-well chemotaxis chambers with a gelatin-coated membrane with 8-μm pores (Corning). Some samples were preincubated with either ONO 4057 (10 μM) or U 75302 (1 μM) 30 min before addition to the upper chamber. LTB4 (1-1,000 nM) was added to the lower chamber. After 6 h, cells that had migrated to the lower membrane surface were fixated and stained by eosin/hematoxylin. Five randomly chosen visual fields per membrane were counted blindly, and the mean value was used as a measure of SMC migration. Recombinant platelet-derived growth factor (PDGF) BB-homodimer (PeproTech) was used as a positive control (29), and results are expressed as the percentage of the cell migration that was induced by PDGF (10 ng/ml). One chamber in each experiment contained medium without LTB4 in the lower chamber as a negative control. Adding LTB4 (100 nM) in both the upper and the lower chambers resulted in a similar amount of migrated cells as the negative control (data not shown).
Cell Proliferation. Human coronary artery SMC were cultured in 96-well plates (5,000 cells per well). After 48 h of serum starvation, cells were treated with LTB4 (0.1-100 nM), U75302 (1 μM), or PDGF (10 ng/ml) in SmGM2 medium for 72 h. Cell proliferation was quantified by using WST-1 reagent (Chemicon) according to the manufacturer's instructions.
RNA Extraction, cDNA Synthesis, and TaqMan Real-Time PCR. Total RNA was extracted by using RNeasy Mini kit (Qiagen, Hilden, Germany) with an on-column DNase digestion step. RNA quantity and quality were assessed by a Bioanalyzer capillary electrophoresis system (Agilent Technologies, Palo Alto, CA). cDNA was synthesized by using Superscript II (Invitrogen). Quantitative Taq-Man PCR on 2.5 μl of cDNA was performed with the Prism 7700 (Applied Biosystems) sequence detector with primer/probe pairs obtained as Assay-on-Demand from Applied Biosystems for human (Hs00609525_m1) and rodent (Rn00572209_s1) BLT1 receptors, respectively. Levels of BLT1 receptor mRNA were normalized to Cyclophilin A mRNA (Hs99999904_m1 and Rn00690933_m1).
Data Analysis. All results are expressed as mean ± SEM. Statistically significant differences were determined either by Student's t test or one-way ANOVA, followed by either Dunnett's (vs. control) or Tukey's (all pairwise) test, as appropriate. P < 0.05 was considered significant.
Results
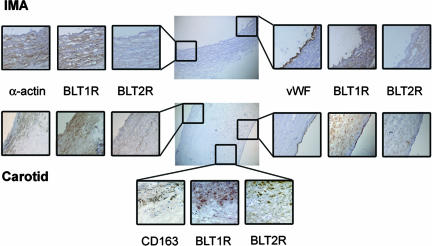
Immunohistochemical staining showed that the BLT1 receptor protein colocalized with markers for SMC (α-smooth muscle actin), endothelial cells (von Willebrand factor), and macrophages (CD163) in human atherosclerotic lesions, whereas BLT2 receptor protein was found only in macrophage areas (Fig. 1). In nonatherosclerotic internal mammary arteries, BLT1 receptor protein was expressed by SMC but not by endothelial cells (Fig. 1).
Fig. 1.
Representative immunohistochemical staining of human internal mammary artery (IMA; Top) and atherosclerotic plaques from carotid artery (Middle and Bottom). The BLT1 receptor protein (BLT1R) colocalized with α-smooth muscle actin-positive vascular SMC in both IMA and carotid artery atherosclerotic plaques, and with von Willebrand factor (vWF)-positive endothelial cells in atherosclerotic plaques, but not in nonatherosclerotic arteries. In carotid artery plaques, the BLT1 and BLT2 (BLT2R) receptor protein expression colocalized with CD163-positive monocytes/macrophages.
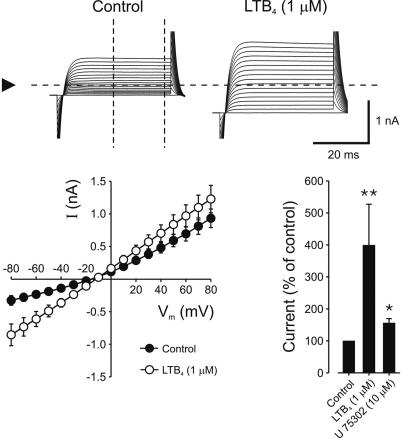
To evaluate whether the BLT receptors expressed on vascular SMC were functional LTB4 receptors, whole-cell currents in human coronary artery SMC were studied by using the patch-clamp technique. Cells were voltage-clamped at -80 mV and subsequently depolarized for 100 ms in +10-mV steps (Fig. 2). At -80 mV, the whole-cell currents were -328 ± 73 nA under control conditions and increased to -855 ± 166 nA after addition of LTB4 (1 μM; n = 9; P < 0.01). At +80 mV, whole-cell currents were 932 ± 143 and 1,227 ± 210 nA, in the absence and presence of LTB4 (1 μM), respectively (nonsignificant). The reversal potential for whole-cell currents was not altered significantly in the presence of LTB4 (control, -19 ± 7 mV; LTB4, -16 ± 10 mV; Fig. 2). When normalized to control conditions, LTB4 (1 μM) increased the whole-cell current ≈4-fold (3.9 ± 1.3; P < 0.01), whereas the selective BLT1 receptor partial agonist U75302 (10 μM) increased the current 1.5-fold (1.6 ± 0.13; P < 0.05; Fig. 2). Both the LTB4- and U75302-induced effects on whole-cell currents were fully reversible, and addition of LTB4 to the same cell for a second time did not increase the whole-cell currents significantly (data not shown). In isolated membrane patches (i.e., inside-out configuration), application of LTB4 (1 μM) did not affect membrane currents (data not shown).
Fig. 2.
Whole-cell recordings from human coronary artery SMC. (Upper) Cells were voltage-clamped by using whole-cell configuration at -80 mV and subsequently depolarized with +10 mV for 100 ms. Arrowhead, zero-current line. Cells were subsequently exposed to either LTB4 (1 μM) or the selective BLT1 receptor partial agonist U75302 (10 μM) by using a puffer system. (Lower) Current-voltage relationship of membrane current in the presence of control solution and after addition of LTB4 (Left), and normalized currents after LTB4 and U75302 (Right). *, P < 0.05; **, P < 0.01 vs. control.
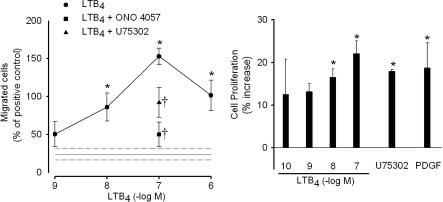
LTB4 (1-1,000 nM) induced migration of human coronary artery SMC in a chemotaxis chamber, with a maximum response at 100 nM (Fig. 3). At this concentration, LTB4 induced migration of ≈50% more cells as compared with the positive control, PDGF (10 ng/ml). Preincubation of cells with the BLT receptor antagonist ONO 4057 (10 μM) significantly inhibited SMC migration toward an LTB4 concentration of 100 nM (Fig. 3). Also, either LTB4 (0.1-100 nM) or the BLT1 receptor agonist U75302 (1 μM) increased the proliferation of human coronary artery SMC (Fig. 3). LTB4 (100 nM) increased the SMC proliferation by ≈20%, which was similar to the effect of PDGF (Fig. 3).
Fig. 3.
LTB4-induced migration and proliferation of SMC. (Left) Migration of human coronary artery SMC through an 8-μm pore filter. Results (mean ± SEM) are expressed as percentage of the migration induced by the positive control PDGF. At 10 ng/ml, recombinant PDGF induced migration of 13 ± 4 (n = 4) cells per visual field, whereas medium alone (negative control) resulted in 3 ± 0.25 (n = 4) cells per field. *, P < 0.05 vs. negative control, which is indicated by reference lines (mean ± SEM). The triangle and square indicate the inhibited migration toward LTB4 (100 nM) after pretreatment with the BLT receptor antagonists U75302 (1 μM) and ONO 4057 (10 μM; †, P < 0.05 vs. 100 nM LTB4). (Right) Proliferation of human coronary artery SMC in SmGM2 medium containing LTB4 (0.1-100 nM), U75302 (10 μM), or PDGF (10 ng/ml). Results (mean ± SEM, n = 5) are expressed as percentage of increase compared with cells incubated in medium alone (negative control). *, P < 0.05 vs. negative control.
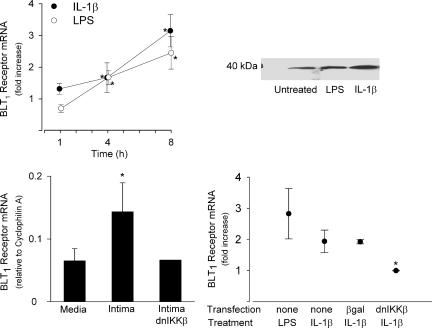
In human coronary artery SMC, BLT1 receptor mRNA was increased significantly after treatment with either IL-1β (10 ng/ml) or LPS (10 μg/ml), with continuously increasing mRNA levels over an 8-h period of incubation (Fig. 4). The 37.5-kDa BLT1 receptor protein was detected by Western blot analysis, with increasing levels after LPS or IL-1β treatment (Fig. 4). To assess the relationship between SMC phenotype and BLT1 receptor expression, mRNA levels were studied in SMC derived from the intimal thickening of rodent carotid artery subjected to balloon angioplasty. There were significantly greater amounts of BLT1 receptor mRNA in intimal cells compared with cells derived from the normal media (Fig. 4).
Fig. 4.
BLT1 receptor expression in SMC. (Upper Left) Real-time quantitative RT-PCR of BLT1 receptor mRNA in human coronary artery SMC incubated in the absence and presence of either IL-1β (10 ng/ml) or LPS (10 μg/ml) for 1, 4, and 8 h. Results (mean ± SEM) are expressed as fold increase compared with untreated cells (n = 3-5). *, P < 0.05 vs. time-matched control. (Upper Right) Western blot analysis of the 37.5-kDa BLT1 receptor protein in human coronary artery SMC after 48 h incubation in the absence (Untreated) or presence of either LPS or IL-1β.(Lower Left) BLT1 receptor mRNA in rat vascular SMC derived from either the normal media or the initimal thickening after balloon injury. Results are expressed relative to the housekeeping gene cyclophilin A (n = 3-5). *, P < 0.05 vs. media. Some intimal SMC were transfected with adenoviral vector expressing dnIKKβ (n = 2). (Lower Right) BLT1 receptor mRNA in rat medial SMC incubated with either LPS or IL-1β for 6 h. Cells were transfected with adenoviral vector expressing either β-gal or dnIKKβ. Results are expressed as fold increase compared with untreated cells (n = 3-5). *, P < 0.05 vs. IL-1β-stimulated control and β-gal-transfected cells (Tukey's test).
BLT1 receptor expression was up-regulated by treatment of rat medial SMC with inducers of nuclear factor κB (NF-κB) activation (i.e., either 10 ng/ml IL-1β or 10 μg/ml LPS; Fig. 4). To identify the signal transduction pathway involved in cytokine-induced BLT1 receptor expression, medial SMC were transfected with adenovirus carrying a dnIKKβ construct, which abrogates NF-κB signaling (23). After such transfection, IL-1β did not affect BLT1 receptor mRNA levels, although this cytokine caused an ≈2-fold increase in BLT1 receptor mRNA levels in control SMC as well as in cells transfected with adenovirus vector expressing β-gal as control (Fig. 4). In intimal SMC, the elevated BLT1 receptor mRNA was reduced to levels comparable with those in medial SMC by transfection with the dominant-negative IKΚβ (n = 2; Fig. 4).
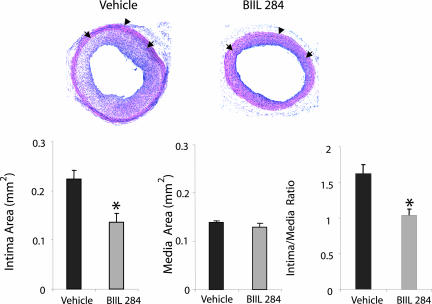
Last, the pathophysiological role of BLT receptor ligation was explored by evaluating the effects of BLT receptor antagonism on the development of intimal hyperplasia after angioplasty in vivo. Rats treated with the BLT receptor antagonist BIIL 284 (10 mg/kg, once daily) for 14 days after carotid artery balloon injury, displayed a marked reduction of the intimal hyperplasia compared with rats treated with vehicle. Representative sections of carotid arteries after angioplasty are shown in Fig. 5. The intima area was reduced to almost half of that in vehicle controls, whereas the medial area was unchanged (Fig. 5), resulting in an increase of lumen area from 0.13 ± 0.025 mm2 in vehicle-treated to 0.23 ± 0.022 mm2 in rats treated with BIIL 284 (P < 0.05).
Fig. 5.
Effects of BLT receptor antagonism on the development of intimal hyperplasia after vascular injury in vivo. (Upper) Representative sections of rat carotid artery 14 days after balloon injury, from rats treated with either vehicle (1% tylose; n = 7) or the BLT receptor antagonist BIIL 284 (10 mg/kg, i.p. once daily; n = 8). Arrows, most inner layer of internal elastic lamina; arrowheads, external elastic lamina, defining the borders of intima and the media. (Lower) Quantification of intima and media area. The ratio of intima to media is shown. *, P < 0.05 vs. vehicle.
Discussion
These findings point to a role of LTB4 in atherosclerosis and intimal hyperplasia, by identifying the vascular SMC as targets for this potent chemotactic molecule. The expression of the human BLT1 receptor on vascular SMC was demonstrated by immunohistochemical stainings of arterial samples, as well as in cultured human coronary SMC by Western blotting and RT-PCR. Together, these findings provide evidence that human vascular SMC express BLT1 receptors in vivo as well as in vitro, and they suggest that these cells may represent an additional target for LTB4.
Patch-clamp analysis and functional studies of SMC clarified that BLT1 receptors transduce a signal that leads to important functional responses in human vascular SMC. Membrane currents in human coronary artery SMC were increased significantly in the presence of either LTB4 or the selective BLT1 receptor partial agonist U75302. Also, another characteristic pharmacological feature of the BLT1 receptor (namely, its rapid desensitization by an agonist; refs. 4, 19, 20, and 30), was observed in human coronary artery SMC because a second addition of LTB4 did not significantly increase whole-cell currents. The latter notion is supported further by the spontaneous reversibility of the LTB4- and U75302-induced currents, which is similar to results of previous pharmacological studies of BLT1 receptors in other preparations (19, 20). In sensory neurons, LTB4 has been implicated in direct activation of ligandgated cation channels (31). However, no effects were observed in the present study, when isolated membrane patches were exposed to LTB4; thus, direct effects of LTB4 on ion channels in the SMC plasma membrane are excluded. Rather, the findings support the notion that human coronary artery SMC express functional, membrane-bound BLT1 receptors that, after stimulation with LTB4, activate ion channels by means of second-messenger signaling pathways.
This study also showed that LTB4 induces two important physiological responses in human coronary artery SMC (namely, chemotaxis and proliferation). The migration of human coronary SMC toward LTB4 was inhibited by the BLT receptor antagonists ONO 4057 and U75302. The latter compound selectively binds to BLT1 receptors (5) and has been reported (19) to inhibit LTB4-induced BLT1 receptor activation at the concentration used. At higher concentrations, U75302 is a partial agonist at the BLT1 receptor, as shown by its stimulation of whole-cell currents and SMC proliferation in the present study. Together, these observations support the presence of functional BLT1 receptors on vascular SMC. By identifying BLT1 as the signaling receptor of LTB4 in vascular SMC, they provide a molecular explanation for the previously reported chemotactic action of LTB4 on rat vascular SMC (21).
Studies have indicated that induction of BLT receptor expression represents an essential mechanism in the regulation of LTB4-induced effects both in vitro (20, 32) and in vivo (33). For example, all-trans retinoic acid (4, 20, 32) and glucocorticoids (32) have been reported to induce BLT receptor expression, as well as to promote functional responses. The sequence of the human BLT1 receptor promoter suggests that it contains elements responsive to the transcription factor NF-κB (41). Importantly, NF-κB is activated in human atherosclerotic plaques (26, 34) and controls SMC activation (23, 35). In the present study, LPS and IL-1β induced up-regulation of BLT1 receptor mRNA in both human and rodent SMC. Abrogated NF-κB signaling by transfection with dnIKKβ, prevented the IL-1β-induced up-regulation of the BLT1 receptor. In contrast, the responses to IL-1β in cells transfected with β-gal were not altered compared with control cells, arguing against any unspecific effect introduced by the vector and hence confirming the role of NF-κB signaling in the cytokine-induced BLT1 receptor up-regulation.
Vascular SMC are involved in functional and structural alterations of the arterial wall in diseases such as hypertension, atherosclerosis, and aneurysm formation, which are related to SMC contractility, secretion of extracellular matrix proteins, proliferation, and migration (29, 36). Interestingly, aortic aneurysm formation was recently linked to the 5-LO pathway, with a suggested mechanism of leukotriene-induced stimulation of chemokines (18). The results of this study suggest that LTB4 acting on vascular smooth muscle BLT1 receptors may constitute an additional mechanism linking the 5-LO pathway to vascular pathology.
The migration of SMC from the arterial media into the intima, leading to intimal hyperplasia, is a key feature of atheromas (37) and of restenotic lesions after angioplasty (38). Although the use of stents reduces restenosis after angioplasty, this complication remains a substantial clinical problem, with intimal hyperplasia being the predominate mechanism of restenosis after stenting (38). To explore the pathophysiological role of BLT receptors on SMC, a rodent model was used in which damage to the vascular wall by balloon angioplasty induces intimal hyperplasia. In this model, two observations strongly support a role of LTB4 in restenosis. First, SMC isolated from the intimal thickening expressed higher levels of BLT1 receptor mRNA compared with medial SMC, and second, rats treated with the BLT receptor antagonist BIIL 284 for 14 days after balloon injury had a substantially reduced intimal hyperplasia.
The increased BLT1 receptor expression in intimal SMC suggests that LTB4 signaling via BLT1 receptors may be important in intimal hyperplasia. In further support of a pivotal role of the NF-κB pathway in BLT1 receptor transcription, intimal SMC transfected with dnIKKβ did not display increased levels of BLT receptor mRNA. The latter results also suggest that the NF-κB pathway may be the main inducer of BLT1 receptor up-regulation in the intima.
The BLT receptor antagonist BIIL 284 is a prodrug, which is metabolized by esterases into its active metabolites that have high affinity for BLT receptors (24). In rats treated with BIIL 284, the intima area after angioplasty represented only 50% of that observed in the vehicle-treated group, resulting in an ≈2-fold-greater lumen size. Together, these observations open up therapeutic possibilities for the treatment of restenosis after angioplasty, by targeting the 5-LO/LTB4/BLT1 pathway.
In human atherosclerotic lesions, BLT receptor expression co-localized not only with SMC, but with several cell types, suggesting that LTB4 may play multiple roles in atherosclerosis. The notion of endothelial BLT1 receptors was recently supported by an LTB4-induced endothelium-dependent formation of histamine and thromboxane A2, a possible mechanism linking LTB4 to atherosclerosis and thrombus formation (20). In the present study, endothelial cells stained positive for the BLT1 receptor in atherosclerotic but not in normal vessels, suggesting an up-regulation of endothelial BLT1 receptors during atherogenesis. A third cell type expressing BLT receptors were CD163-positive monocytes/macrophages, which is in line with LTB4 being a potent trigger of monocyte adhesion (39) and production of MCP-1 (40). Interestingly, these cells stained positive for both the BLT1 and BLT2 receptor, implicating that also the low-affinity BLT2 receptor may be involved in vascular pathologies induced by LTB4. For example, it has been reported that macrophages from BLT1-/- mice show chemotaxis toward LTB4 by activation of a BLT2 receptor (17).
This study shows that vascular SMC express functional BLT1 receptors and that these receptors induce SMC migration and proliferation. Also, we demonstrate that an up-regulation of BLT1 receptors in intimal SMC and in medial SMC by proinflammatory cytokines occurs through the NF-κB pathway. Last, we show that BLT1 receptors are expressed in atherosclerotic lesions in man and promote intimal hyperplasia after angioplastic injury in the rat. In conclusion, targeting BLT1 receptors on SMC may represent a therapeutic strategy in the treatment of atherosclerosis and the prevention of restenosis after coronary angioplasty.
Note. After the submission of this manuscript, Heller et al. (7) reported that hypercholesterolemic ApoE-/- mice that are homozygous for a targeted BLT1 receptor gene (ApoE-/- × BLT1-/-) developed smaller atherosclerotic lesions with less SMC content than their BLT1+/+ counterparts, and they showed that LTB4 stimulated migration and proliferation of murine vascular SMC.
Acknowledgments
This work was supported by the Swedish Heart Lung Foundation, the Swedish Research Council (Project 6816), Stiftelsen Serafimerlasarettet, and European Commission FP6 funding (LSHM-CT-2004-0050333).
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 5-LO, 5-lipoxygenase; dnIKKβ, dominant-negative form of IkB kinase β; LTB4, leukotriene B4; SMC, smooth muscle cells; PDGF, platelet-derived growth factor.
References
- 1.Funk, C. D. (2001) Science 294, 1871-1875. [DOI] [PubMed] [Google Scholar]
- 2.Bäck, M. (2002) Life Sci. 71, 611-622. [DOI] [PubMed] [Google Scholar]
- 3.Brink, C., Dahlen, S. E., Drazen, J., Evans, J. F., Hay, D. W., Nicosia, S., Serhan, C. N., Shimizu, T. & Yokomizo, T. (2003) Pharmacol. Rev. 55, 195-227. [DOI] [PubMed] [Google Scholar]
- 4.Yokomizo, T., Izumi, T., Chang, K., Takuwa, Y. & Shimizu, T. (1997) Nature 387, 620-624. [DOI] [PubMed] [Google Scholar]
- 5.Yokomizo, T., Kato, K., Terawaki, K., Izumi, T. & Shimizu, T. (2000) J. Exp. Med. 192, 421-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlén, S. E., Björk, J., Hedqvist, P., Arfors, K. E., Hammarström, S., Lindgren, J. A. & Samuelsson, B. (1981) Proc. Natl. Acad. Sci. USA 78, 3887-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heller, E. A., Liu, E., Tager, A. M., Sinha, S., Roberts, J. D., Koehn, S. L., Libby, P., Aikawa, E. R., Chen, J. Q., Huang, P., et al. (2005) Circulation 112, 578-586. [DOI] [PubMed] [Google Scholar]
- 8.Goodarzi, K., Goodarzi, M., Tager, A. M., Luster, A. D. & von Andrian, U. H. (2003) Nat. Immunol. 4, 965-973. [DOI] [PubMed] [Google Scholar]
- 9.Huang, W. W., Garcia-Zepeda, E. A., Sauty, A., Oettgen, H. C., Rothenberg, M. E. & Luster, A. D. (1998) J. Exp. Med. 188, 1063-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ott, V. L., Cambier, J. C., Kappler, J., Marrack, P. & Swanson, B. J. (2003) Nat. Immunol. 4, 974-981. [DOI] [PubMed] [Google Scholar]
- 11.Tager, A. M., Bromley, S. K., Medoff, B. D., Islam, S. A., Bercury, S. D., Friedrich, E. B., Carafone, A. D., Gerszten, R. E. & Luster, A. D. (2003) Nat. Immunol. 4, 982-990. [DOI] [PubMed] [Google Scholar]
- 12.Mehrabian, M., Allayee, H., Wong, J., Shi, W., Wang, X. P., Shaposhnik, Z., Funk, C. D. & Lusis, A. J. (2002) Circ. Res. 91, 120-126. [DOI] [PubMed] [Google Scholar]
- 13.Spanbroek, R., Gräbner, R., Lötzer, K., Hildner, M., Urbach, A., Rühling, K., Moos, M. P., Kaiser, B., Cohnert, T. U., Wahlers, T., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 1238-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwyer, J. H., Allayee, H., Dwyer, K. M., Fan, J., Wu, H., Mar, R., Lusis, A. J. & Mehrabian, M. (2004) N. Engl. J. Med. 350, 29-37. [DOI] [PubMed] [Google Scholar]
- 15.Helgadottir, A., Manolescu, A., Thorleifsson, G., Gretarsdottir, S., Jonsdottir, H., Thorsteinsdottir, U., Samani, N. J., Gudmundsson, G., Grant, S. F., Thorgeirsson, G., et al. (2004) Nat. Genet. 36, 233-239. [DOI] [PubMed] [Google Scholar]
- 16.Aiello, R. J., Bourassa, P. A., Lindsey, S., Weng, W., Freeman, A. & Showell, H. J. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 443-449. [DOI] [PubMed] [Google Scholar]
- 17.Subbarao, K., Jala, V. R., Mathis, S., Suttles, J., Zacharias, W., Ahamed, J., Ali, H., Tseng, M. T. & Haribabu, B. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 369-375. [DOI] [PubMed] [Google Scholar]
- 18.Zhao, L., Moos, M. P., Grabner, R., Pedrono, F., Fan, J., Kaiser, B., John, N., Schmidt, S., Spanbroek, R., Lotzer, K., et al. (2004) Nat. Med. 10, 966-973. [DOI] [PubMed] [Google Scholar]
- 19.Sakata, K., Dahlén, S. E. & Bäck, M. (2004) Br. J. Pharmacol. 141, 449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bäck, M., Qui, H., Haeggström, J. Z. & Sakata, K. (2004) Am. J. Physiol. 287, H419-H424. [DOI] [PubMed] [Google Scholar]
- 21.Nomoto, A., Mutoh, S., Hagihara, H. & Yamaguchi, I. (1988) Atherosclerosis 72, 213-219. [DOI] [PubMed] [Google Scholar]
- 22.Kondo, K., Umemura, K., Ohmura, T., Hashimoto, H. & Nakashima, M. (1998) Thromb. Haemost. 79, 635-639. [PubMed] [Google Scholar]
- 23.Bu, D. X., Erl, W., de Martin, R., Hansson, G. K. & Yan, Z. Q. (2005) FASEB J. 19, 1293-1295. [DOI] [PubMed] [Google Scholar]
- 24.Birke, F. W., Meade, C. J., Anderskewitz, R., Speck, G. A. & Jennewein, H. M. (2001) J. Pharmacol. Exp. Ther. 297, 458-466. [PubMed] [Google Scholar]
- 25.Yan, Z. Q. & Hansson, G. K. (1998) Circ. Res. 82, 21-29. [DOI] [PubMed] [Google Scholar]
- 26.Monaco, C., Andreakos, E., Kiriakidis, S., Mauri, C., Bicknell, C., Foxwell, B., Cheshire, N., Paleolog, E. & Feldmann, M. (2004) Proc. Natl. Acad. Sci. USA 101, 5634-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. (1981) Pflügers Arch. 391, 85-100. [DOI] [PubMed] [Google Scholar]
- 28.Patton, C., Thompson, S. & Epel, D. (2004) Cell Calcium 35, 427-431. [DOI] [PubMed] [Google Scholar]
- 29.Abedi, H. & Zachary, I. (1995) Cardiovasc. Res. 30, 544-556. [PubMed] [Google Scholar]
- 30.Gaudreau, R., Le Gouill, C., Venne, M. H., Stankova, J. & Rola-Pleszczynski, M. (2002) J. Biol. Chem. 277, 31567-31576. [DOI] [PubMed] [Google Scholar]
- 31.Hwang, S. W., Cho, H., Kwak, J., Lee, S. Y., Kang, C. J., Jung, J., Cho, S., Min, K. H., Suh, Y. G., Kim, D. & Oh, U. (2000) Proc. Natl. Acad. Sci. USA 97, 6155-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obinata, H., Yokomizu, T., Shimizu, T. & Izumi, T. (2003) Biochem. Biophys. Res. Commun. 309, 114-119. [DOI] [PubMed] [Google Scholar]
- 33.Chiang, N., Gronert, K., Clish, C. B., O'Brien, J. A., Freeman, M. W. & Serhan, C. N. (1999) J. Clin. Invest. 104, 309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valen, G., Yan, Z. Q. & Hansson, G. K. (2001) J. Am. Coll. Cardiol. 38, 307-314. [DOI] [PubMed] [Google Scholar]
- 35.Staels, B., Koenig, W., Habib, A., Merval, R., Lebret, M., Torra, I. P., Delerive, P., Fadel, A., Chinetti, G., Fruchart, J. C., et al. (1998) Nature 393, 790-793. [DOI] [PubMed] [Google Scholar]
- 36.Michel, J. B., De Roux, N., Plissonnier, D., Anidjar, S., Salzmann, J. L. & Levy, B. (1990) J. Cardiovasc. Pharmacol. 16, S4-S11. [PubMed] [Google Scholar]
- 37.Hansson, G. K. (2005) N. Engl. J. Med. 352, 1685-1695. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann, R. & Mintz, G. S. (2000) Eur. Heart J. 21, 1739-1749. [DOI] [PubMed] [Google Scholar]
- 39.Friedrich, E. B., Tager, A. M., Liu, E., Pettersson, A., Owman, C., Munn, L., Luster, A. D. & Gerszten, R. E. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 1761-1767. [DOI] [PubMed] [Google Scholar]
- 40.Huang, L., Zhao, A., Wong, F., Ayala, J. M., Struthers, M., Ujjainwalla, F., Wright, S. D., Springer, M. S., Evans, J. & Cui, J. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 1-7. [DOI] [PubMed] [Google Scholar]
- 41.Kato, K., Yokomizo, T., Izumi, T. & Shimizu, T. (2000) J. Exp. Med. 192, 413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]