Abstract
Repetitive stimulation potentiates contractile tension of fast-twitch skeletal muscle. We examined the role of myosin regulatory light chain (RLC) phosphorylation in this physiological response by ablating Ca2+/calmodulin-dependent skeletal muscle myosin light chain kinase (MLCK) gene expression. Western blot and quantitative-PCR showed that MLCK is expressed predominantly in fast-twitch skeletal muscle fibers with insignificant amounts in heart and smooth muscle. In contrast, smooth muscle MLCK had a more ubiquitous tissue distribution, with the greatest expression observed in smooth muscle tissue. Ablation of the MYLK2 gene in mice resulted in loss of skeletal muscle MLCK expression, with no change in smooth muscle MLCK expression. In isolated fast-twitch skeletal muscles from these knockout mice, there was no significant increase in RLC phosphorylation in response to repetitive electrical stimulation. Furthermore, isometric twitch-tension potentiation after a brief tetanus (posttetanic twitch potentiation) or low-frequency twitch potentiation (staircase) was attenuated relative to responses in muscles from wild-type mice. Interestingly, the site of phosphorylation of the small amount of monophosphorylated RLC in the knockout mice was the same site phosphorylated by MLCK, indicating a potential alternative signaling pathway affecting contractile potentiation. Loss of skeletal muscle MLCK expression had no effect on cardiac RLC phosphorylation. These results identify myosin light chain phosphorylation by the dedicated skeletal muscle Ca2+/calmodulin-dependent MLCK as a primary biochemical mechanism for tension potentiation due to repetitive stimulation in fast-twitch skeletal muscle.
Keywords: calcium, calmodulin, twitch
Skeletal muscle contraction depends on a voltage-driven conformational change in the L-type Ca2+ channel in the transverse tubule that triggers Ca2+ release from the sarcoplasmic reticulum through the intracellular ryanodine receptor (1, 2). The Ca2+ binds to troponin in thin filaments, thereby allowing myosin cross bridges to bind actin and generate muscle tension (3). However, muscle contractions involve more complex mechanisms that affect performance. Since Ranke noted in 1865 (4) that, with stimuli uniform in strength the later twitch contractions were stronger than the first, there has been considerable interest in identifying the mechanisms involved in isometric twitch potentiation during trains of stimuli at low frequency (staircase) or after a tetanus (posttetanic potentiation). Considerations have been given to changes in compliance of the series elastic elements, to activation of more fibers within a muscle, to increased Ca2+ release within a single fiber to activate fully the contractile proteins, and to changes in excitation-contraction coupling processes (5-8).
Ca2+ released during muscle contraction can also activate the dedicated protein kinase Ca2+/calmodulin-dependent skeletal muscle myosin light chain kinase (skMLCK) to initiate myosin regulatory light chain (RLC) phosphorylation (9, 10). RLC phosphorylation has no significant effect on skeletal muscle actin-activated myosin ATPase activity (9, 11). However, Manning and Stull (12) noted a temporal correlation between the extent of RLC phosphorylation and potentiation of peak isometric twitch tension. This correlation was prominent in fast-twitch, but not slow-twitch, muscles (13). Subsequent studies showed mechanical and biochemical effects in skinned fibers and myosin thick filaments, with movement of phosphorylated myosin cross bridges away from the thick filament backbone, resulting in an increase in the rate at which cross bridges enter force-producing states (9, 11, 14). Nevertheless, it is still not known how much RLC phosphorylation quantitatively contributes to potentiation of twitch tension in intact skeletal muscle fibers. Additional biochemical investigations reveal calmodulin modulation of the L-type Ca2+ channel and ryanodine receptor (1, 2, 15), raising the possibility that stimuli with repetitive Ca2+ release could affect the functions of these two proteins and thereby alter excitation-contraction coupling properties. Thus, during repetitive motor unit firing at physiological frequencies that initiate unfused tetanus, muscle force may be enhanced by multiple mechanisms involving Ca2+. In terms of RLC phosphorylation per se, there are additional complications, including the presence of smooth muscle MLCK (smMLCK) in skeletal muscle fibers (16) and the potential for RLC phosphorylation by other kinases, similar to the case found in smooth muscle and non-muscle cells (17).
To resolve these issues regarding contributions of different biochemical mechanisms involved in contraction potentiation, as well as RLC phosphorylation, we disrupted the MYLK2 gene to eliminate expression of skMLCK.
Methods
Generation of skMLCK-Deficient Mice. The homologous short arm of the skMLCK gene (MYLK2) used in the targeting vector was a 2.5-kb NotI and BamHI fragment amplified from genomic DNA from mouse embryonic stem cells (Strain 129/SvJ) by PCR. The length of 5′ terminal arm in the construct was ≈2.6 kb and included intron 4, exon 5, intron 5, and part of exon 6 (Fig. 1). The length of the 3′ terminal arm was ≈6.8 kb and included introns 7-13 and exons 8-14, followed by a herpes virus thymidine kinase cassette to permit negative selection with gancyclovir. The 1.7-kb Neo coding region replaced the targeted locus region, which included part of exon 6, intron 6, and exon 7. The targeted deletion included 660 bp that encode the ATP-binding sequence of the catalytic core. The targeting vector was subsequently linearized with NotI and transfected into 129/SvEvTac embryonic stem cells by electroporation. Clones surviving G418 and gancyclovir were isolated, and positive clones were identified by Southern blotting. Selected clones were then injected into C57BL/B6 blastocysts and implanted into pseudopregnant C57/ICR females by the Transgenic Core Facility at the University of Texas Southwestern Medical Center at Dallas. The chimeric founder mice were bred to C57BL/6 for transmission of the mutant skMLCK allele. Animals were housed on a 12-h light and 12-h dark cycle with free access to water and standard mouse diet. Animal experiments were conducted following protocols approved by the Institutional Animal Care and Use Committee.
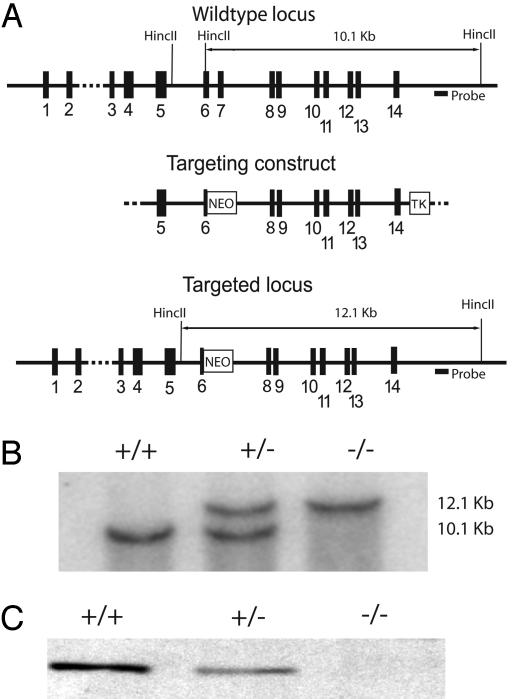
Fig. 1.
Disruption of the skMLCK gene in mice. (A) Schematic representation of the targeting vector and proposed homologous recombination in the targeted locus. The position of the probe for Southern blotting is indicated. (B) Southern blot analysis of germ-line mice. HincII-digested genomic DNA was prepared from mouse tail and hybridized with the probe as described under Methods. A new genomic fragment of 12.1 kb was observed only in skMLCK+/- and skMLCK-/- mice whereas a 10.1-kb fragment representing the wild-type gene was present in skMLCK+/+ and skMLCK+/- mice. (C) Western blot analysis of skMLCK expression in extensor digitorum longus muscles from wild-type (+/+), heterozygous (+/-), and knockout (-/-) mice.
Genotyping and Southern Analysis of Mice. DNA was extracted from mouse tail samples by proteinase K digestion, phenol extraction, and isopropanol precipitation. DNA was digested overnight with HincII restriction endonuclease and separated on an agarose gel. DNA was immobilized on a Nylon membrane by Rapid Downward Transfer Systems (Schleicher & Schuell) and probed with an α-32P-labeled 503-bp DNA probe located outside of the targeting vector (Fig. 1). The probe primers used for PCR were as follows: 5′-AGTCACGAGAGTCGAAGCATGTGG-3′ and 5′-TCTGCACACTGGTATGGTCTGGC-3′.
Western Blots. For analysis of skeletal and smooth muscle MLCK expression, tissues were collected and homogenized in SDS sample buffer (18). Lysate protein concentration was measured by bicinchoninic acid (BCA) protein assay reagent, and 40 μg of protein was loaded per well for 7.5% SDS/PAGE, followed by transfer to nitrocellulose membranes. Smooth and skeletal muscle MLCK were probed with K36, a monoclonal antibody against smMLCK (Sigma) or polyclonal goat antibody against skMLCK (31).
RLC phosphorylation was measured in isolated mouse extensor digitorum longus muscles quick-frozen in clamps prechilled in liquid nitrogen by urea/glycerol-PAGE and immunoblotting where the nonphosphorylated RLC is separated from monophosphorylated RLC (19, 20). RLC phosphorylation was measured similarly in ventricular heart samples quickly dissected and frozen in liquid nitrogen from anesthetized mice. Changes in RLC phosphorylation occur on the order of 20-30 min in heart so immediate in situ fixation is not essential to measure the extents of phosphorylation that reflect in vivo values (21). Antibodies raised in rabbits to purified skeletal or cardiac RLCs were used for the appropriate analyses. Horseradish peroxidase (HRP)-conjugated secondary antibodies (Pierce) were used with the Amersham Pharmacia ECL system, and quantitative measurements were processed on a Storm PhosphorImager and analyzed by imagequant software.
Quantitative Real-Time PCR (Q-PCR). Total RNA was extracted from mouse tissues by using RNAStat 60 (Tel-Test, Friendswood, TX). Two micrograms of total RNA was DNase I (Roche) treated and reverse transcribed by using random hexamers (Roche Applied Science) and SuperScript II Reverse Transcriptase (Invitrogen). The following primers used for real-time PCR analysis were designed by using primer express 2.0 software (Applied Biosystems): mouse smMLCK, forward 5′-AGAAGTCAAGGAGGTAAAGAATGATGT-3′, reverse 5′-CGGGTCGCTTTTCATTGC-3′; mouse skMLCK, forward 5′-ACATGCTACTGAGTGGCCTCTCT-3′, reverse 5′-GGCAGACAGGACATTGTTTAAGG-3′; mouse 36B4, forward 5′-CACTGGTCTAGGACCCGAGAAG-3′, reverse 5′-GGTGCCTCTGGGAGATTTTCG-3′. Real-time PCRs were performed on an Applied Biosystems Prism 7000. Reactions contained 50 ng of reverse transcribed RNA, 10 μl of 2× SYBR green Master Mix (Applied Biosystems), and 150 nM forward and reverse primer sets in a total volume of 20 μl. Smooth and skeletal MLCK RNA expression was calculated by using the comparative CT method with 36B4 used as the normalizer. Final results are expressed as the percentage of the mean for the highest expressing tissue from wild-type mice.
Isolated Extensor Digitorum Longus Muscle Preparation. The effects of skMLCK gene ablation on the contractile and RLC phosphorylation properties were studied in isolated extensor digitorum longus muscles as described (19, 22). Briefly, the muscles were surgically isolated from 8- to 12-week-old mice and mounted on Grass FT03.C force transducers, bathed in physiological salt solution at 30°C, and gassed continuously with 98% O2-5% CO2. Muscles were stimulated with two platinum wire electrodes to establish optimal stimulus intensity and muscle length for maximal isometric twitch tension (Lo). After adjusting muscle length to Lo or 0.7 Lo (length equal to 0.7 of the maximal force development), they remained quiescent for at least 30 min before isometric twitches were elicited three times at one twitch per min to establish control tension values. Muscles were then stimulated at 150 Hz for 2 s, and the posttetanic twitch response was measured at 10 s. A separate set of muscles were quick-frozen at 12 s after tetanus for RLC phosphorylation measurements. In a different experiment, muscles were stimulated at 10 Hz for 15 s to elicit a progressive increase in isometric twitch tension (staircase), followed by muscle freezing for RLC phosphorylation measurements.
Phosphorylation Site Mapping for Skeletal Muscle RLC. Extensor digitorum longus muscles from wild-type and skMLCK knock-out mice were excised and subjected to mechanical/hypoxic stress by manually rolling the muscle between the thumb and index finger for 5 s, a handling procedure that routinely increases RLC phosphorylation. Muscles were then frozen with aluminum tongs cooled in liquid nitrogen and stored at -80°C. Muscles were homogenized on ice in 400 μl of 50 mM Tris, 1 mM EDTA, 1 mM DTT, 1% Nonidet P-40, 0.25 mM trans-epoxysuccinyl-l-leucyl-amido-(4-guanidino)-butane and 0.25 mM diisopropylfluorophosphate. One milliliter of 10% trichloroacetic acid was added to 150-μl aliquots of homogenate immediately upon completion of homogenization or after overnight incubation at room temperature to allow for protein dephosphorylation. The precipitated proteins were collected by centrifugation and solubilized in urea sample buffer for RLC phosphorylation measurements for the Western blot analysis as described above. For mass spectrometry analysis, 30-90 μg of protein was subjected to electrophoresis on the urea/glycerol gels, followed by staining with Colloidal Blue (Invitrogen) for the visualization of the monophosphorylated RLC band.
The protein band was cut into small pieces and destained with 25 mM ammonia bicarbonate solution [pH 8.0, methanol/water, 50:50 (vol/vol)] three times for 10 min each. The destained gels were cleaned in an acidic buffer (acetic acid/methanol/water, 10:50:40, vol/vol/vol) three times for 1 h each, and swollen in water two times for 20 min each. The gel pieces were dehydrated in acetonitrile before drying in a SpeedVac (Thermo Savant, New York). About 150 ng of modified porcine trypsin (Promega) at the concentration of 10 ng/μl in 50 mM ammonia bicarbonate (pH 8.0) were added to the gel pieces for overnight digestion. The resulting tryptic peptides were extracted sequentially with E1 buffer (acetonitrile/water/TFA, 50:45:5, vol/vol/vol) and E2 buffer (acetonitrile/water/TFA, 75:24:1, vol/vol/vol). The peptide extracts were combined and dried in a SpeedVac.
Tryptic digests of the protein were analyzed by MALDI-TOF mass spectrometry (Voyager DE Pro mass spectrometer, Applied Biosystems), nanospray precursor ion scan in QStar XL mass spectrometer (Applied Biosystems), and HPLC/LTQ 2-D ion trap mass spectrometer (ThermoFinnigan, San Jose, CA), coupled with Agilent 1100 nano-flow HPLC system (Agilent, Palo Alto, CA) for identification of phosphopeptide and precise localization of phosphorylation sites.
Results
Disruption of the skMLCK Gene. We ablated the functional expression of skMLCK in fast-twitch skeletal muscle of mice. Southern screening showed only a 12.1-kb DNA fragment obtained after HincII digestion in homozygous knockout mice with no expression of skMLCK protein (Fig. 1). In wild-type mice, only a 10.1-kb DNA fragment was obtained whereas heterozygous mice showed both DNA fragments, with half as much MLCK protein expressed relative to wild-type mice. No obvious phenotypes were noted for the knockout mice, including litter size, fertility, or viability relative to wild-type mice as old as 6 months.
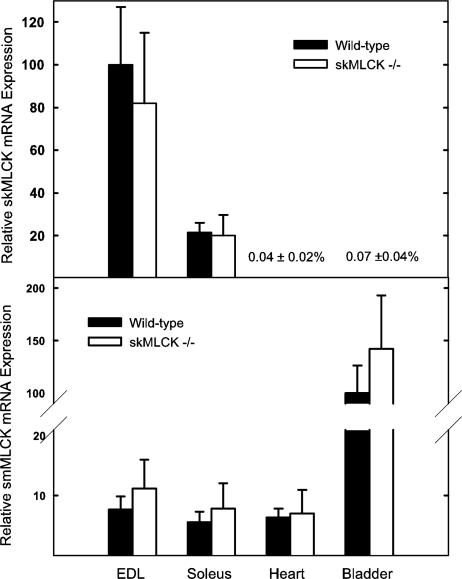
Amounts of mRNA Expression for Skeletal and Smooth Muscle MLCKs in Different Tissues. Expression levels of skeletal and smMLCK mRNAs were determined in fast-twitch extensor digitorum longus muscle, slow-twitch soleus muscle, heart (mid-left ventricle), and urinary bladder from wild-type and skMLCK-/- mice by using Q-PCR. In wild-type mice, expression of skMLCK mRNA was greatest in extensor digitorum longus muscle, where amounts were 5-fold greater than in soleus muscle (Fig. 2). Minimal amounts of skMLCK mRNA (<0.1% of extensor digitorum longus muscle) were detected in heart and bladder from wild-type mice, consistent with the Western blot data showing no significant skMLCK protein expression in these tissues (see below). To determine whether ablation of skMLCK could lead to a compensatory increased expression of smMLCK, the mRNA abundance of the latter was determined in tissues from wild-type and the skMLCK-/- mice (Fig. 2). In wild-type mice, expression of smMLCK mRNA was greatest in bladder, and amounts were not significantly altered in bladders from knockout mice. Expression of smMLCK mRNA in extensor digitorum longus, soleus, and heart tissues of wild-type mice was ≈6-8% of that observed in wild-type bladder and was not significantly altered in skMLCK-/- mice.
Fig. 2.
Expression of skeletal and smooth muscle MLCK mRNA in different types of muscles. Relative amounts of skMLCK and smMLCK mRNA were determined by Q-PCR in tissues from wild-type and skMLCK-/- mice as described under Methods. Values are reported as the means ± SEM for three mice of each genotype.
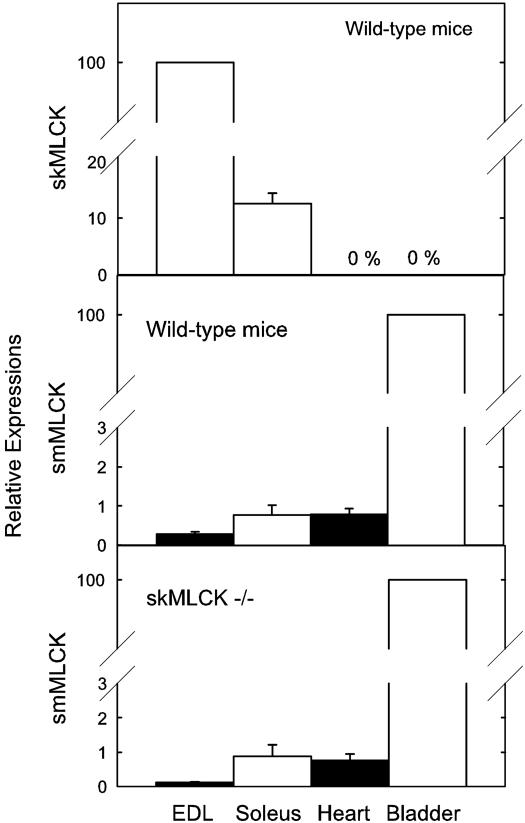
Expression of MLCKs in Tissues from Wild-Type and skMLCK Knockout Mice. Quantitative Western blotting of different tissues from wild-type mice showed abundant expression of the skMLCK in fast-twitch extensor digitorum longus muscles, with substantially less kinase expressed in slow-twitch soleus muscle (Fig. 3). The kinase was not detected in cardiac or bladder smooth muscle tissues or in nonmuscle tissues such as liver, lung, or kidney (data not shown). Considering the previous report that skMLCK was expressed in cardiac myocytes (23), we did additional probing with gels overloaded with 200 μg of protein, but still no kinase was detected. These results on protein expression of skMLCK in different tissues are consistent with results obtained for the mRNA distributions.
Fig. 3.
Expression of skeletal and smooth muscle MLCK protein in different types of muscles. Relative expression amounts of skMLCK and smMLCK protein were determined by Western blotting in tissues from wild-type and skMLCK-/- mice as described under Methods. Values are reported as the means ± SEM for at least four mice of each genotype.
We also measured smMLCK expression in different tissues from wild-type mice (Fig. 3). This MLCK was abundantly expressed in the urinary bladder smooth muscle with lower but significant amounts in the other muscle tissues. The extent of smMLCK expression in these different tissues was not significantly changed in the skeletal muscle knockout mouse, thus showing that there was not a compensatory increased expression of the smooth muscle kinase with disrupted expression of the skMLCK (Fig. 3). There were similar amounts of smMLCK expressed in soleus and cardiac muscle tissues.
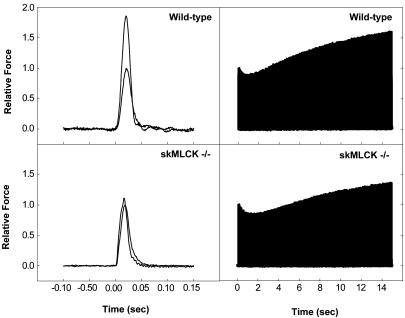
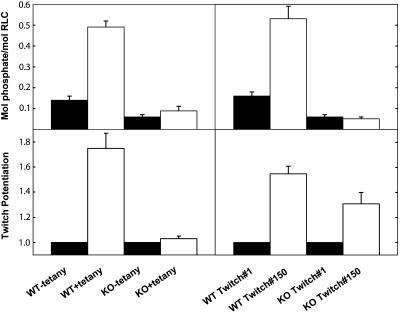
RLC Phosphorylation and Contractile Tension Potentiation in Fast-Twitch Skeletal Muscle from skMLCK Knockout Mice. The effect of repetitive stimulation on RLC phosphorylation in isolated extensor digitorum longus muscle of mice was measured with two modes of stimulation. The isometric twitch tension increased 75% at 10 s after a tetanic stimulation at 150 Hz for 2 s in muscles from wild-type mice (posttetanic potentiation) but not from skMLCK-/- mice (Figs. 4 and 5). Similarly, RLC phosphorylation increased in muscles from wild-type mice after the tetanus but not in muscles from the skMLCK-/- mice (Figs. 4 and 5). These results show that the posttetanic potentiation of the isometric twitch tension and RLC phosphorylation responses are significantly attenuated with ablation of skMLCK expression.
Fig. 4.
Effect of repetitive contractions on RLC phosphorylation and isometric twitch potentiation in isolated mouse extensor digitorum longus muscles. Shown are representative force tracings of isometric twitches showing single twitches before and after a tetanus (150 Hz, 2 s) (Left) and with continuous electrical stimulation at 10 Hz for 15 s (Right) in muscles from wild-type (Upper) and skMLCK-/- mice (Lower). The measured responses are relative to single isometric twitches before repetitive stimulation.
Fig. 5.
Effect of repetitive contractions on RLC phosphorylation and isometric twitch potentiation in isolated mouse extensor digitorum longus muscles. Average isometric twitch tension (Lower) and RLC phosphorylation (Upper) responses after a tetanus (Left) and with continuous electrical stimulation at 10 Hz for 15 s (Right) for wild-type (WT) and skMLCK-/- (KO) mice. The values represent the means ± SEM for at least six muscle responses.
The effect of repetitive stimulation at a constant low frequency on isometric twitch properties and RLC phosphorylation was also examined. Stimulation of isolated muscles from wild-type animals at 10 Hz for 15 s produced the characteristic staircase increase in isometric twitch potentiation (Figs. 4 and 5). After a transient small decline, isometric twitch tension progressively increased, reaching a maximum 55% increase by 15 s. RLC phosphorylation also increased by 15 s in response to the low frequency stimulation. In the muscles from the skMLCK-/- mice, the transient decrease in isometric tension was still present followed by a recovery phase with a 30% increase in tension. The decrease in isometric twitch potentiation response in skMLCK-/- mice was associated with attenuation of RLC phosphorylation in response to low-frequency repetitive stimulation.
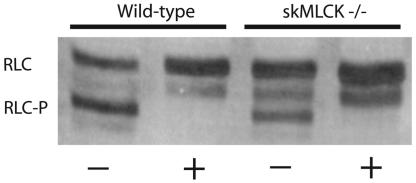
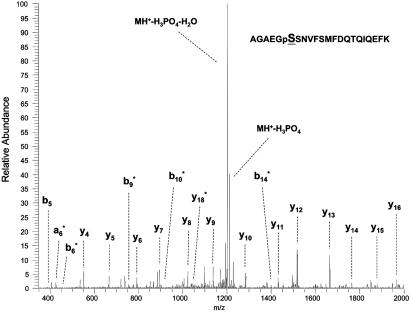
Interestingly, the extent of RLC phosphorylation in resting, isolated extensor digitorum longus muscles from the skMLCK-/- mice varied from 0.05 to 0.09 mol of phosphate per mol of RLC, not 0.00 mol of phosphate per mol of RLC. The band was identified as a monophosphorylated RLC because of the specific increase in protein migration by urea/glycerol-PAGE that is associated with monophosphorylation (Fig. 6; refs. 12 and 13). Furthermore, the monophosphorylated RLC band disappeared in a muscle homogenate prepared with a nondenaturing buffer without protein phosphatase inhibitors. The gel shown in Fig. 6 is overexposed to show clearly the monophosphorylated RLC in skMLCK-/- muscle samples. The identity of the band intermediate between the nonphosphorylated and monophosphorylated RLCs is not clear, but, in quantitative blots, it represents ≈5% or less of the total immunoreactivity and does not change with electrical stimulation. The apparent increase in density of the intermediate band with phosphatase treatment may be due to limited proteolysis of the nonphosphorylated RLC. Analysis of the purified monophosphorylated RLC from MLCK-/- mouse muscle led to detection of peptides that cover >95% sequence of the protein (Fig. 7). Collision-induced fragmentation spectrum (MS/MS) of the phosphopeptide (residues 10-31) conclusively determined the phosphorylation site at residue serine 15, adjacent to the site phosphorylated by MLCK (24). These observations suggest that another kinase that is not activated by repetitive contractions may phosphorylate skeletal muscle RLC.
Fig. 6.
Separation of nonphosphorylated and phosphorylated RLC by urea/glycerol-PAGE. Muscle samples were prepared as described in Methods and subjected to urea/glycerol-PAGE followed by Western blotting for RLC. Shown are results without (-) or with (+) dephosphorylation treatment.
Fig. 7.
Collision-induced fragmentation spectrum of the phosphorylated peptide from RLC. The spectrum of AGAEGpSSNVFSMFDQTQIQEFK shows that serine (15) in mouse skeletal muscle RLC (MLC2F) is phosphorylated. The b and y designate the N- and C-terminal fragments of the peptide produced by breakage at the peptide bond in LTQ, respectively. The number represents the residue number from either the N or C terminus. *, Those daughter ions with loss of 98-Da peaks.
Effect of skMLCK Ablation on Cardiac RLC Phosphorylation and Growth. Because skMLCK was reported to be significantly expressed in cardiac myocytes and proposed to be responsible for cardiac RLC phosphorylation (23), we examined the potential effects of skMLCK gene ablation on heart properties (Table 1). There were no significant differences in the extent of cardiac RLC phosphorylation in knockout animals compared with wild-type animals. Furthermore, the extents of phosphorylation were similar to previously reported values (21, 25, 26). Additionally, there were no significant differences in heart weight, body weight, and, thus, heart weight/body weight ratio in wild-type and skMLCK-/- mice. These results are consistent with the lack of detection of any significant amounts of skMLCK mRNA or protein in cardiac muscle.
Table 1. Cardiac properties of mice with ablation of skMLCK.
| Mouse | Body weight, g | Heart weight, mg | Heart/body weight ratio | RLC phosphorylation, mol P/mol RLC |
|---|---|---|---|---|
| Wild-type | 22.13 ± 1.36 | 111 ± 14.7 | 5.07 ± 0.12 | 0.44 ± 0.02 |
| skMLCK knockout | 24.21 ± 1.07 | 132 ± 9.1 | 5.45 ± 0.11 | 0.40 ± 0.01 |
Heart weight, body weight, and cardiac RLC phosphorylation were measured as described in Methods. Values are means ± SEM for at least five values. P, phosphate.
Discussion
The extents of expression of skeletal and smooth muscle MLCKs are tissue-dependent, with skMLCK showing a more restricted, tissue-specific expression. During differentiation of skeletal muscle myoblasts to myotubes, skMLCK is expressed to amounts found in adult skeletal muscles (16). Because the kinase was found only in adult skeletal muscle but not heart, smooth, or nonmuscle cells by Western blotting, it was proposed that it was the only tissue-specific MLCK. In contrast, smMLCK was found in all adult tissues, including cardiac and skeletal muscle myocytes, and thus should not be considered as a smooth muscle-specific protein. However, Davis et al. (23) cloned skMLCK from heart. With antibodies raised to the expressed kinase, they showed a substantially lesser amount of an immunoreactive band in mouse heart than in skeletal muscle by Western blotting. Because this band did not have the same migration as MLCK from mouse skeletal muscle, it may not have been skMLCK protein. Although we and others (16) were not able to detect skMLCK protein in cardiac or smooth muscle by Western blotting, Q-PCR showed skMLCK mRNA present in heart tissue in an amount substantially lower (>1,000 fold) than that observed in skeletal muscle but similar to that observed in smooth muscle tissue. Thus, skMLCK may have been cloned from a rare amount of mRNA in cardiac muscle (23). Our results from the skMLCK knockout mice also show that gene ablation had no effect on the extent of cardiac RLC phosphorylation, indicating that it is not a functional kinase in cardiac myocytes.
In agreement with the results of Herring et al. (16), we found smMLCK to be expressed in all three kinds of muscles, although the amounts measured by Western blotting and Q-PCR were much greater in smooth muscle than skeletal or cardiac muscle. Although we cannot exclude contributions from smooth muscle in blood vessels in these measurements, Herring et al. (16) reported smMLCK expression in differentiated skeletal and cardiac myocytes in culture. Perhaps smMLCK is responsible for cardiac RLC phosphorylation. This kinase phosphorylates cardiac RLC, but the catalytic efficiency is substantially less than smooth muscle RLC (27). However, mouse hearts beat at a high rate (≈600 beats per minute). Because MLCK is inactivated slowly (1 s-1; refs. 9 and 13), it is predicted that the kinase is not inactivated during the short relaxation time before the heart contracts again with increased cytoplasmic Ca2+ concentrations. Thus, in a beating heart, the kinase would be activated continuously. Additional experiments are needed to identify clearly whether smMLCK or another kinase phosphorylates cardiac RLC.
Biochemically, the skeletal muscle RLC is also not a good substrate for the smMLCK because of a Glu at the p-3 position relative to the phosphorylatable serine, which is an Arg in smooth and nonmuscle RLCs (24). Even though skeletal muscle fibers contain smMLCK in the skMLCK-/- mice, this kinase apparently does not phosphorylate the skeletal muscle RLC in response to repetitive stimulation probably because of poor substrate properties (10, 24). However, it is interesting to note that there is a small amount of monophosphorylated muscle RLC in muscles from knockout mice. Phosphorylation of the serine near the serine phosphorylated by skMLCK indicates another kinase capable of the phosphorylation. Although it will be important to identify this kinase and the signaling pathways that lead to its physiological activation, the effect on cross-bridge movement and enhanced contractile tension will be the same as phosphorylation by skMLCK.
Our results show a biochemical mechanism for the physiological potentiation of twitch contractions by repetitive stimuli in fast-twitch skeletal muscles. Ablation of the skMLCK expression results in inhibition of RLC phosphorylation in response to electrical stimulation in skeletal muscle. These results are consistent with the hypothesis that Ca2+ released during membrane excitation stimulates contraction by binding to troponin on thin filaments but also activates skMLCK by means of Ca2+ binding to calmodulin to effect skeletal muscle RLC phosphorylation. A small portion of MLCK is activated by the intracellular Ca2+ transient associated with a single twitch. However, with repetitive contractions, more activated kinase accumulates due to the slow rate (1 s-1) of inactivation induced by Ca2+/calmodulin dissociation (9). The phosphorylation of myosin RLC by the Ca2+/calmodulin-dependent MLCK increases the number of cross bridges in the strong-binding state by their displacement away from the myosin thick filament toward the actin thin filament. Although previous studies with skinned fibers provided a potential mechanism for potentiation of contractions with repetitive stimuli, it did not rule out effects of Ca2+/calmodulin on the L-type Ca2+ channel and ryanodine receptor that are manifested physiologically in other ways (1, 2). The contribution of RLC phosphorylation to tension potentiation seems to be greater in posttetanic twitch potentiation than the staircase, and previous investigations suggested a linear relationship between the extent of RLC phosphorylation and isometric twitch potentiation (9, 13). Thus, effects on Ca2+ handling may be greater with low frequency stimulation for longer durations as observed in the staircase. Prolonged repetitive Ca2+ release may affect excitation processes through calmodulin effects on the dihydropyridine and ryanodine receptors with a greater amount of Ca2+ released for contractile protein activation (1, 2). On the other hand, the twitch potentiation could involve Ca2+ independent mechanisms such as compression of skeletal muscle fibers with a decrease in the distance between thin and thick filaments. This effect would mimic the effect of RLC phosphorylation (3, 9, 11).
The kinetic properties of myosin RLC phosphorylation in skeletal muscle are suited for modulation of contraction properties because the rate of phosphorylation is reasonably fast (requiring a few seconds) whereas the dephosphorylation is slow (minutes) (12, 13, 28). Thus, a short period of contractile activity during a warm-up period results in RLC phosphorylation that will be maintained during intermittent periods of relaxation but poised for potentiation of subsequent contractions. This history-dependent phosphorylation will initially increase the rate and extent of tension development at physiological frequencies of motor nerve firing that result in unfused tetanus, thus increasing the integral of the tension-time relationship. Force enhancement for a submaximal voluntary contraction in humans may be associated with fast-twitch fibers and RLC phosphorylation (29). However, the motor nerve frequency required for force maintenance will decrease due to force potentiation by RLC phosphorylation. Thus, RLC phosphorylation will also contribute secondarily to the maintenance of force at a lower dynamic level of Ca2+, with continuous nerve firing thereby increasing muscle efficiency due to lower energy consumption for removal of cytosolic Ca2+. This general pattern of motor unit activity is commonly observed in working muscles during exercise (30) and provides a model for investigating the role of RLC phosphorylation in exercise responses (29).
Acknowledgments
We are grateful for the expert advice and assistance from Joyce Repa for Q-PCR and the expert technical assistance of Tara Billman with the mice. This work was supported by National Institutes of Health (NIH) Grants HL29043 and HL26043 and by the Moss Heart Fund. J.T.S. is the Fouad A. and Val Imm Bashour Distinguished Chair in Physiology. J.W.R. was supported as a postdoctoral fellow by NIH Training Grant T32HL007360.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MLCK, myosin light chain kinase; RLC, regulatory light chain of myosin; Q-PCR, quantitative real-time PCR; skMLCK, skeletal muscle MLCK; smMLCK, smooth muscle MLCK.
References
- 1.Catterall, W. A. (1995) Annu. Rev. Biochem. 64, 493-531. [DOI] [PubMed] [Google Scholar]
- 2.Tang, W., Sencer, S. & Hamilton, S. L. (2002) Front. Biosci. 7, d1583-9. [DOI] [PubMed] [Google Scholar]
- 3.Gordon, A. M., Homsher, E. & Regnier, M. (2000) Physiol. Rev. 80, 853-924. [DOI] [PubMed] [Google Scholar]
- 4.Ranke, J. (1865) Tetanus: Eine Physiologische Studie (Wilhelm Engelmann, Leipzig, Germany), p. 380.
- 5.Close, R. & Hoh, J. F. (1968) J. Physiol. (London) 197, 461-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmedt, J. E. & Hainaut, K. (1968) Nature 217, 529-532. [DOI] [PubMed] [Google Scholar]
- 7.Connolly, R., Gough, W. & Winegrad, S. (1971) J. Gen. Physiol. 57, 697-709. [PubMed] [Google Scholar]
- 8.Krarup, C. (1981) J. Physiol. (London) 311, 373-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeney, H. L., Bowman, B. F. & Stull, J. T. (1993) Am. J. Physiol. 264, C1085-95. [DOI] [PubMed] [Google Scholar]
- 10.Kamm, K. E. & Stull, J. T. (2001) J. Biol. Chem. 276, 4527-4530. [DOI] [PubMed] [Google Scholar]
- 11.MacIntosh, B. R. (2003) News Physiol. Sci. 18, 222-225. [DOI] [PubMed] [Google Scholar]
- 12.Manning, D. R. & Stull, J. T. (1979) Biochem. Biophys. Res. Commun. 90, 164-170. [DOI] [PubMed] [Google Scholar]
- 13.Moore, R. L. & Stull, J. T. (1984) Am. J. Physiol. 247, C462-71. [DOI] [PubMed] [Google Scholar]
- 14.Persechini, A. & Stull, J. T. (1984) Biochemistry 23, 4144-4150. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi, N., Xu, L., Evans, K. E., Pasek, D. A. & Meissner, G. (2004) J. Biol. Chem. 279, 36433-36439. [DOI] [PubMed] [Google Scholar]
- 16.Herring, B. P., Dixon, S. & Gallagher, P. J. (2000) Am. J. Physiol. 279, C1656-C1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somlyo, A. P. & Somlyo, A. V. (2003) Physiol. Rev. 83, 1325-1358. [DOI] [PubMed] [Google Scholar]
- 18.Isotani, E., Zhi, G., Lau, K. S., Huang, J., Mizuno, Y., Persechini, A., Geguchadze, R., Kamm, K. E. & Stull, J. T. (2004) Proc. Natl. Acad. Sci. USA 101, 6279-6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer, B. M. & Moore, R. L. (1989) Am. J. Physiol. 257, C1012-C1019. [DOI] [PubMed] [Google Scholar]
- 20.Kamm, K. E., Hsu, L. C., Kubota, Y. & Stull, J. T. (1989) J. Biol. Chem. 264, 21223-21229. [PubMed] [Google Scholar]
- 21.Silver, P. J., Buja, L. M. & Stull, J. T. (1986) J. Mol. Cell. Cardiol. 18, 31-37. [DOI] [PubMed] [Google Scholar]
- 22.Lau, K. S., Grange, R. W., Isotani, E., Sarelius, I. H., Kamm, K. E., Huang, P. L. & Stull, J. T. (2000) Physiol. Genomics 2, 21-27. [DOI] [PubMed] [Google Scholar]
- 23.Davis, J. S., Hassanzadeh, S., Winitsky, S., Lin, H., Satorius, C., Vemuri, R., Aletras, A. H., Wen, H. & Epstein, N. D. (2001) Cell 107, 631-641. [DOI] [PubMed] [Google Scholar]
- 24.Stull, J. T., Nunnally, M. H. & Michnoff, C. H. (1986) in The Enzymes, eds. Krebs, E. G. & Boyer, P. D. (Academic, Orlando, FL), Vol. XVII, pp. 113-166. [Google Scholar]
- 25.High, C. W. & Stull, J. T. (1980) Am. J. Physiol. 239, H756-H764. [DOI] [PubMed] [Google Scholar]
- 26.Herring, B. P. & England, P. J. (1986) Biochem. J. 240, 205-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stull, J. T., Blumenthal, D. K., Miller, J. R. & DiSalvo, J. (1982) J. Mol. Cell. Cardiol. 14, 105-110. [DOI] [PubMed] [Google Scholar]
- 28.Manning, D. R. & Stull, J. T. (1982) Am. J. Physiol. 242, C234-C241. [DOI] [PubMed] [Google Scholar]
- 29.Oskouei, A. E. & Herzog, W. (2005) J. Appl. Physiol. 98, 2087-2095. [DOI] [PubMed] [Google Scholar]
- 30.Bigland-Ritchie, B. R., Dawson, N. J., Johansson, R. S. & Lippold, O. C. (1986) J. Physiol. (London) 379, 451-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunnally, M. H., Rybicki, S. B. & Stull, J. T. (1985) J. Biol. Chem. 260, 1020-1026. [PubMed] [Google Scholar]