Abstract
Fcp1 is an essential protein phosphatase that hydrolyzes phosphoserines within the C-terminal domain (CTD) of the largest subunit of RNA polymerase II (Pol II). Fcp1 plays a major role in the regulation of CTD phosphorylation and, hence, critically influences the function of Pol II throughout the transcription cycle. The basic understanding of Fcp1–CTD interaction has remained ambiguous because two different modes have been proposed: the “dockingsite” model versus the “distributive” mechanism. Here we demonstrate biochemically that Fcp1 recognizes and dephosphorylates the CTD directly, independent of the globular non-CTD part of the Pol II structure. We point out that the recognition of CTD by the phosphatase is based on random access and is not driven by Pol II conformation. Results from three different types of experiments reveal that the overall interaction between Fcp1 and Pol II is not stable but dynamic. In addition, we show that Fcp1 also interacts with a region on the polymerase distinct from the CTD. We emphasize that this non-CTD site is functionally distinct from the docking site invoked previously as essential for the CTD phosphatase activity of Fcp1. We speculate that Fcp1 interaction with the non-CTD site may mediate its stimulatory effect on transcription elongation reported previously.
Keywords: C-terminal domain dephosphorylation, C-terminal domain hyperphosphorylation
RNA polymerase II (Pol II) is distinguished from RNA polymerases I and III by the unique C-terminal domain (CTD) of its largest subunit, Rpb1 (1–3). The CTD, through its interaction with the Mediator complex, plays a central role in transcription activation (4–6), and it also plays critical roles in coupling mRNA synthesis to mRNA processing events, such as 5′ capping, splicing, and 3′ cleavage and polyadenylation (7, 8). Composed of a tandemly repeated heptapeptide of the consensus sequence Y1S2P3T4S5P6S7, the CTD undergoes reversible phosphorylation (9–11). Whereas the hypophosphorylated Pol II isoform (Pol IIA) assembles with general transcription factors into the transcription preinitiation complex (12–14), the hyperphosphorylated isoform (Pol IIO) is the dominant species found in transcription elongation complexes in vivo (15–17). The phosphorylation state of the CTD is controlled by the activities of CTD-specific kinases and phosphatases. Several transcription factor-associated CTD kinases have been identified, including CDK7/Kin28, CDK9/Bur1/Ctk1 and CDK8/Srb10 (11, 18–20). Other kinases (e.g., Cdc2 kinase and mitogen-activated protein kinase 2) can phosphorylate the CTD in vitro, but their contributions to CTD phosphorylation in vivo are uncertain (18, 21–23).
Pol IIO is believed to be dephosphorylated before it can recycle for another round of transcription initiation (13, 14, 21, 24). Fcp1 was the first CTD phosphatase discovered to fulfill such a function (25, 26). Fcp1 was reported to remove phosphates specifically from the Pol II CTD, but not from the other phosphoproteins tested (25). The human and yeast Fcp1 enzymes were stimulated by the C-terminal domain of general transcription factor TFIIF (26–29). Fcp1 was shown in yeast to be responsible for in vivo dephosphorylation of the CTD and essential for global transcription by Pol II and for cell viability (28, 30). Fcp1 orthologs are found in all known fungal and metazoan proteomes (28, 31, 32). Fcp1 is an aspartyl phosphatase containing two core domains, FCP homology and breast cancer 1 C terminus (BRCT) (28). The FCP homology domain includes the DXDX(T/V) motif of the acylphosphatase active site (30, 31). The recent crystal structure of the small-CTD phosphatase Scp1 (33), which is homologous to the FCP homology domain of Fcp1 (34), together with mutational data from Schizosaccharomyces pombe Fcp1 (35), defined the active site and a likely catalytic mechanism but did not reveal the structural basis for recognition of phosphorylated Pol II.
The model invoked for Pol IIO recognition by Fcp1 emphasized a binding site on Pol II structure distinct from the CTD. Based on the inability of human Fcp1 to function on a recombinant hyperphosphorylated CTD (CTDo) and on results obtained from a competition assay, this “docking-site” model suggests that dephosphorylation of CTD by Fcp1 requires prior binding of Fcp1 to a non-CTD site on the polymerase, after which the phosphatase acts processively on the CTD (27). An alternative “distributive” model was recently suggested for S. pombe Fcp1 based on kinetic analysis of the sequential removal of phosphates from a synthetic Ser-2 phosphopeptide consisting of four repeats of the CTD heptad (36). This model suggests a classical enzyme–substrate relationship between Fcp1 and the CTD. Data from the later work also defined a minimal requirement on the heptad sequences flanking a phosphoserine (36, 37).
Here we explore the structural requirements for CTD recognition by Fcp1 by using highly purified, crystalline quality Pol II from Saccharomyces cerevisiae and calf thymus, along with pure proteins of S. cerevisiae Fcp1, recombinant S. pombe Fcp1, GST–CTD fusion and yeast CTD-less Pol II. We found that Fcp1 phosphatase of budding or fission yeast was equally functional toward Pol II from budding yeast and calf thymus, thus arguing against the species specificity suggested in ref. 26. We have determined that the Fcp1 phosphatase activity does not require the non-CTD part of Pol II structure, thus ruling out the requirement for prior binding to a docking-site. We detected direct Fcp1 interaction with CTDo but also observed diminution of the interaction with hypophosphorylated CTD (CTDa), suggesting a relationship between the substrate discrimination by Fcp1 (CTDo vs. CTDa) and the binding preference by its BRCT domain [Pol IIO vs. Pol IIA and phosphorylated CTD peptide vs. CTD peptide, as observed for human BRCT (38)]. Therefore, the recognition of CTD by the phosphatase must be based on random diffusion instead of being driven by the Pol II conformation, and the phosphatase activity of Fcp1 cannot be processive.
We have obtained additional data indicating that Fcp1 also interacts with Pol II in a region separate from the CTD. We discuss the possibility that this interaction may cause a conformational change around the DNA/RNA binding cleft of the polymerase. Such a CTD-independent interaction with Pol II could offer a structural basis for the stimulatory effect that Fcp1 has on transcription elongation (39–41). We stress that Fcp1 interaction with the non-CTD site does not play the role of molecular docking that was defined as a requirement for effective processing of the CTD (27), because the globular structure of Pol II proves nonessential for Fcp1 phosphatase activity.
Experimental Procedures
Protein Expression and Purification. Purifications of the RNA polymerases and Fcp1 proteins were performed as described in Supporting Experimental Procedures, which is published as supporting information on the PNAS web site.
Preparation of Phosphorylated Substrates (Pol IIO and GST–CTDo). Substrates for the CTD phosphatase assay were generated through in vitro phosphorylation with mitogen-activated protein kinase 2. Details are given in Supporting Experimental Procedures. The resulting Pol IIO and GST-CTDo proteins were all repurified to remove free ATP and the kinase using protocols given in Supporting Experimental Procedures.
CTD Phosphatase Assay and Inhibition Assay. CTD phosphatase assays were performed according to procedures described in refs. 25, 27, and 34, except that all of the substrates had been repurified. The CTD phosphatase inhibition assay was performed essentially as described in ref. 27. See Supporting Experimental Procedures for details.
GST-Pulldown Assay. This affinity pulldown assay was performed by using purified GST–CTDa and GST–CTDo, with GST as a negative control, in a scale that permitted protein detection by Coomassie. See Supporting Experimental Procedures for details.
Gel Filtration Chromatography. For testing the stability of interaction between Pol IIO and the Fcp1 mutant, D172N, size-exclusion chromatography (SEC) was performed on a Superose-6 column (details are given in Supporting Experimental Procedures).
Heparin-Binding Assay. For quantitative binding assays, heparin columns were constructed to sizes compatible with a scale sufficient for detection by Coomassie. The scheme (see Supporting Experimental Procedures) entailed rapid passages through the columns of the loading materials containing Pol II, Fcp1, and BSA and their various mixtures.
Results
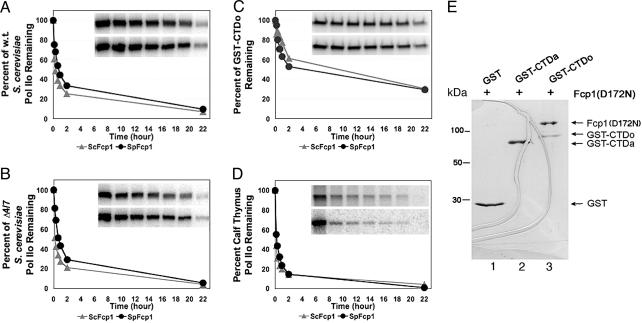
Yeast Fcp1 Phosphatase Activity Is Independent of the Globular Structure of Pol II. We performed CTD dephosphorylation experiments with highly purified yeast Pol II and either S. cerevisiae Fcp1 purified directly from the yeast or recombinant S. pombe Fcp1 purified from Escherichia coli (for protein purities see Fig. 5 A–C, which is published as supporting information on the PNAS web site). The S. pombe Fcp1 was included because of its solubility in recombinant form and functional similarity to the budding yeast and human counterparts (31, 42). First, phosphorylated and 32P-labeled Pol II substrates were generated by in vitro phosphorylation using the Ser/Thr kinase mitogen-activated protein kinase 2. Mitogen-activated protein kinase 2 phosphorylates Ser-2 and Ser-5 sites of the CTD in vitro as has been demonstrated by phosphorylated CTD-specific antibodies (43), although with preference toward Ser-5 (44). We confirmed conversion of Pol IIA to Pol IIO by a mobility change in the largest subunit (Rpb1) of Pol II in SDS/PAGE (Fig. 5D). Generation of Pol IIO was also verified by Western blotting with the phospho-Ser-5-specific H14 antibody (Fig. 5D) (19, 45, 46) before polymerase repurification. Calf thymus Pol IIO was prepared and radioactively labeled with the same procedures (Fig. 5E). The polymerases were repurified upon verification of hyperphosphorylation. Recombinant GST–CTD fusion protein carrying the yeast heptad repeats was phosphorylated, analyzed (Fig. 5F), and repurified. Activities of the phosphatases were measured by the removal of the 32P radiolabel from Rpb1 over time.
We first observed that the Rpb4 and Rpb7 subunits of Pol II were not required for Fcp1 phosphatase function in vitro. The 12-subunit (WT) and 10-subunit (Δ4/7) S. cerevisiae Pol IIO were dephosphorylated by S. cerevisiae or S. pombe Fcp1 with nearly identical kinetics (Fig. 1 A and B). This observation argues against the proposal that Rpb4 is involved in the formation of Pol II–Fcp1 complex in S. pombe (47). The discrepancy may be reconciled in terms of (i) host-dependent utilization of Rpb4, which is essential for S. pombe viability (48) but nonessential for S. cerevisiae (49) and (ii) the weakness of interaction between Rpb4 and Fcp1 of S. pombe, given that it was detected only by Western blotting of the GST-pulldown fraction (47) and that this interaction may be even weaker in S. cerevisiae. Residual Fcp1-resistant phosphorylation (15–20% of input) might be attributable to Ser-PO4 in a few nonconsensus heptads present in the yeast CTD (3) that are not efficiently recognized by Fcp1 and/or the presence of Ser-PO4 sites on Rpb1 outside the CTD.
Fig. 1.
Fcp1 interacts with and dephosphorylates the CTD directly without a requirement for the globular structure of Pol II. Shown are comparisons of phosphatase activities of S. cerevisiae Fcp1 (ScFcp1) and S. pombe Fcp1 (SpFcp1) toward the different substrates. (A) The 12-subunit S. cerevisiae Pol IIO (w.t.). (B) The 10-subunit S. cerevisiae Pol IIO (Δ4/7). (C) GST-CTDo. (D) Calf thymus Pol IIO. The substrates were prepared as described in Experimental Procedures. The phosphatase activities manifested in the form of the gradually decreasing radioactivities in Rpb1 bands as a function of incubation time. (A Inset–D Inset) The raw data for ScFcp1 (upper lanes) and SpFcp1 (lower lanes), with lanes corresponding to the time points. Note that the characteristic gel mobility change was not produced here because of limited run durations. (E) A GST-pulldown assay to measure direct Fcp1–CTD interaction. Similar molar amounts of the purified GST, GST–CTDa, and GST–CTDo (repurified after in vitro phosphorylation) were coupled to glutathione-Sepharose resins, respectively. An excess amount of the inactive mutant Fcp1(D172N) of S. pombe was tested for binding. After 600-fold washing, the columns were eluted and analyzed by SDS/PAGE with Coomassie staining. The positions of GST, GST–CTDa, GST–CTDo, and the mutant Fcp1 are indicated on the right. The curved lines were due to gel cracking.
S. cerevisiae and S. pombe Fcp1 dephosphorylated GST–CTDo at rates similar (within a factor of two) to the rate of dephosphorylation of Pol IIO; at the 2-h time point, ≈45% of the total labels were removed from GST–CTDo as compared with 70% removed from Pol IIO (Fig. 1, compare A with C). We conclude that the globular structure of Pol II is not required for the phosphatase function of Fcp1. The present findings are not consistent with the docking-site model, which hypothesizes that access by Fcp1 to the CTD depends on prior binding to the non-CTD structure of Pol II. The docking-site model was formulated mainly from two observations: (i) recombinant CTDo was not a substrate of human Fcp1, and (ii) CTD-less Pol II was able to inhibit Fcp1 activity presumably by competing with Pol IIO for Fcp1 binding (to the docking-site) (27). We suggest an explanation in Discussion for the inactivity of Fcp1 toward the recombinant CTDo in that experiment.
Fungal Fcp1 Phosphatases Are Active Against Bovine Pol IIO. Consistent with our finding that yeast Fcp1 dephosphorylated CTDo without a covalently linked Pol II globular structure, we observed full phosphatase activities of Fcp1 from S. pombe and S. cerevisiae toward bovine Pol IIO. As shown in Fig. 1D, both fungal phosphatases removed the 32P labels with kinetics nearly identical to those observed in the assays using yeast Pol IIO as substrate. This observation argues against the previous claim that the yeast CTD phosphatase did not dephosphorylate Pol II of calf thymus (26) but is in agreement with the cross-species complementation of S. cerevisiae Fcp1 by its ortholog from the parasite Encephalitozoon cuniculi (37). Because S. pombe Fcp1 functioned in vitro indistinguishably from its S. cerevisiae counterpart and could be expressed in a soluble form of sufficient quantities, it continued to be used hereafter.
Fcp1 Interacts Directly with the CTD in Vitro. The preceding experiments established that the Fcp1 phosphatase acts on the CTD directly in vitro, independent of the Pol II structure beyond the CTD. Next, we performed a GST-pulldown assay to verify that Fcp1 was capable of discriminating CTDo from CTDa in the absence of Pol II structure. Initially, it was difficult to detect the interaction in the pulldown assay using WT Fcp1 (data not shown). Our interpretation was that the interaction of Fcp1 with the CTD was rather dynamic and thus undetectable by the staining method (Coomassie). Hence, we turned to an Fcp1 mutant (D172N) with a single amino acid change to the second Asp of the DxDx(T/V) motif in its FCP homology domain, which abolished its catalytic activity (31). We reasoned that without substrate turnover, the phosphatase might dwell on the CTD for longer time.
Fcp1(D172N) was pulled down by GST–CTDo but not by GST–CTDa or GST (Fig. 1E). Because Coomassie staining was used for detection, the signals were reliable and demonstrated direct interaction/recognition of CTDo by Fcp1 in the absence of the Pol II globular structure (Fig. 1E, compare lane 3 with lanes 1 and 2). A minimum of 3-fold molar excess of Fcp1(D172N) was bound to CTDo, as judged by the staining intensities. This multicopy binding could be explained by the fact that there were multiple phosphorylated heptads within the yeast GST–CTDo fusion protein that could each serve as independent Fcp1 ligands. Notably, no stable interaction was found for CTDa (Fig. 1E, lane 2). Hence, it is tempting to relate this much higher affinity of the full-length Fcp1 for CTDo to the preference of its BRCT domain for phosphorylated CTD peptide (38).
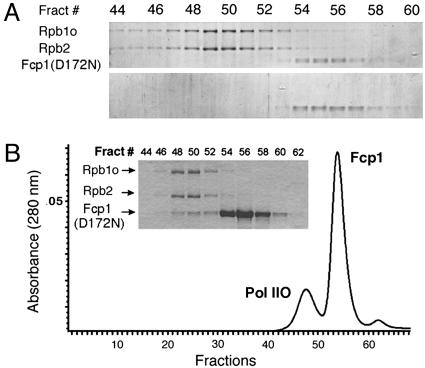
Fcp1 Interacts Dynamically with Pol II. We recently demonstrated the formation of Fcp1–Pol II complex by using an improved nondenaturing gel system (50). We realized that the stabilizing “caging” effects of gel had aided our detection of the complex. Thus, we asked whether its intrinsic stability would sustain Fcp1–Pol II complex through the continuous partitioning by gel filtration. We found that Fcp1(D172N) separated from Pol IIO during SEC (Fig. 2A). By using the inactive mutant phosphatase, we confirmed that no dephosphorylation had occurred to the CTD (data not shown). The dissociation over the sizing column agrees with our observation of the gradual loss of Fcp1 signal in GST-pulldown upon excessive washing (>600-fold). However the dissociation disagrees with a recent claim that yeast Pol IIA was capable of forming stable complex with a domain of Fcp1 (amino acids 168–606) (33), but the demonstrated gel filtration data in that work was incomplete. Only one Pol II peak fraction was shown instead of an elution profile (figure 5 of ref. 33). As proven here, the same type of SEC column clearly separated Pol IIO and the mutant Fcp1 into two peaks (Fig. 2A). However, when excess Fcp1 was loaded (Fig. 2B), as was the condition used by Kamenski et al. (33), there was an overlap between the separated peak profiles that could have been interpreted as formation of a stable complex if one fraction (e.g., Fig. 2B Inset, fraction 50) were examined in isolation.
Fig. 2.
Fcp1 interacts dynamically with Pol II. (A) Separation of Fcp1(D172N)–Pol IIO complex over gel filtration. Equal molar amounts of Pol IIO of S. cerevisiae and the phosphatase-impaired mutant of S. pombe were incubated and resolved over a Superose-6 column. Fractions (0.3 ml each) across the two UV-absorption peaks were analyzed with SDS/PAGE and revealed by Coomassie staining. (Upper) The Pol IIO, indicated by its Rpb1o and Rpb2 subunits, peaked at fraction 50, whereas Fcp1(D172N) migrated as a separate peak at fraction 56. The elution volume for the Fcp1 in the mixed run with Pol IIO (Upper) was identical to that in the unmixed run (Lower). Therefore, continuous partitioning over the SEC column separated the complex whose formation had been demonstrated in nondenaturing gel (50). (B) Overlap between the elution profiles of Pol IIO and that of free Fcp1. (Inset) When Fcp1(D172N) was applied at a molar ratio of 20:1 over the polymerase, its amount in each fraction increased correspondingly, producing an overlap with the Pol IIO peak. Coomassie-based quantification of fraction 50 yielded a molar ratio of 0.8:1.0 for Fcp1(D712N)/Pol IIO.
To synthesize the complex formation data (GST-pulldown and native gel electrophoresis) with the dissociation data (gel filtration), we propose that Fcp1 interacts dynamically with Pol II to form a complex that can be captured in gel matrix but separated by continuous and slow partitioning.
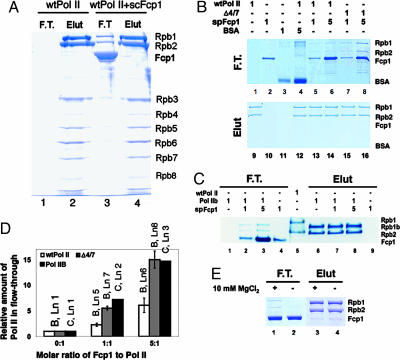
Fcp1 also Interacts with a Non-CTD Site. A rapid partitioning experiment seemed appropriate for investigating the dynamic interaction between Fcp1 and Pol II. The approach was adopted from our earlier observation of Pol II purification, during which S. cerevisiae Fcp1 was found to cause a fraction (≈6%) of the Pol II to flow through heparin Sepharose (e.g., Fig. 3A, compare lanes 3 and 1). This result was surprising because the polymerase by itself had been well known to bind stably to heparin in various Pol II purification schemes and to be eluted only by a high concentration of salt (e.g., Fig. 3A, lanes 2 and 4), and this property had been used in many in vitro assay systems when transcription inhibition was necessary. To quantitatively study the Fcp1-dependent loss of heparin binding, a carefully controlled experiment was performed by using Fcp1-free S. cerevisiae Pol IIA (both 12- and 10-subunit) and the CTD-less Pol IIB.
Fig. 3.
Fcp1 induces loss of heparin binding by Pol II in a Mg2+-dependent manner. (A) Heparin-binding behavior of S. cerevisiae 12-subunit (wtPol II) in the absence and presence of S. cerevisiae Fcp1 (scFcp1). Consistent amounts of the polymerase were loaded onto heparin Sepharose columns with or without the Fcp1, indicated respectively on the top. Consistent volumes of the flow-through (F.T.) and high-salt elution (Elut) were collected. Contents of the two fractions were analyzed by SDS/PAGE with polypeptides (marked to the right) revealed by Coomassie staining. (B) (Upper) Heparin-binding behaviors of S. cerevisiae 12-subunit and 10-subunit (Δ4/7) Pol II were characterized by measuring their amounts that flowed through the microcolumns as functions of the presence of S. pombe Fcp1 (spFcp1) and BSA. The experiments were performed in the presence of Mg2+ (5–10 mM). (Lower) Proteins bound to the column after washing were eluted with high salt. The SDS gels were all stained with Coomassie blue and quantified. Relative molar amounts of the loading materials were indicated on the top. Protein bands are identified on the right. Presence of a polymerase in a fraction is revealed by its two largest subunits (Rpb1 and Rpb2). Note the contaminating bands from the BSA sample do not match with the positions of Rpb1 and Rpb2, although they are of high molecular mass. (C) Loss of heparin binding by Pol IIB in the presence of S. Pombe Fcp1 measured with the same assay. “Rpb1b” corresponds to the largest, CTD-less subunit of S. cerevisiae Pol II. WT Pol II (wtPol II) was loaded as a marker in lane 5. (D) Quantification (in ratios) of the gels in B and C. The amount of the polymerase relative to the control (lane 1 of B) in the flow-through was a function of the Fcp1/polymerase molar ratio. For four independent experiments, intensities of Rpb1 bands in lanes 5–8 of B were normalized relative to that of WT Pol II alone in lane 1, which was low but not zero. The control for Δ4/7 alone was not included in the assay but was assumed to be identical to the WT Pol II control. Quantification of Rpb1b in the flow-through of C was done in the same manner, except that it was based on a single experiment. Labels on the top of the bars indicate the corresponding gel lanes in B or C.(E) The effect of Mg2+ ion on heparin binding by Pol II. The assay was run with an Fcp1/Pol molar ratio of 1.0. In the absence of MgCl2, the relative amount of Pol II that flowed through the heparin column dramatically reduced as judged by Coomassie staining (compare lanes 1 and 2).
Pol IIA alone bound to the heparin beads tightly as expected (Fig. 3B, lanes 1 and 9). Neither Fcp1 (Fig. 3B, lanes 2 and 10) nor BSA (Fig. 3B, lanes 3 and 11) bound to heparin. Again, when Fcp1 was present, a fraction of Pol IIA migrated with the flow-through (Fig. 3B, lane 5), the operation of which was completed in 10 s. The amount of Pol IIA in the flow-through increased as the relative molar amount of Fcp1 increased (Fig. 3B, lane 6; see also Fig. 4D). The presence of a 5-fold molar excess of BSA did not change the chromatographic pattern of Pol IIA at all (Fig. 3B, lane 4), excluding the possibility of interference from nonspecific interactions. Direct comparison showed that lack of Mg2+ reduced the amount of Pol IIA in the flow-through to levels almost undetectable by Coomassie staining (Fig. 3E). Interestingly, a significantly greater fraction of 10-subunit Pol II partitioned into the flow-through than that of the 12-subunit Pol II (Fig. 3B, lanes 7 and 8; see also Fig. 4D). We did not examine the heparin-binding behavior of Pol IIO with this assay because of the limited availability of Pol IIO.
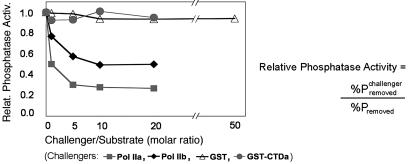
Fig. 4.
Fcp1 phosphatase activity is inhibited by Pol IIA and Pol IIB but not by GST–CTDa. The inhibition of Fcp1-dependent CTD dephosphorylation by a challenger (Pol IIA, Pol IIB, GST-CTDa, or GST) was measured by the relative (Relat.) phosphatase activity (Activ.) as a function of the challenger/substrate ratio. The relative phosphatase activity was defined as the percent phosphate removed in the presence of a challenger (at the non-zero ratios) divided by that in absence of the challenger (at the zero ratio). Percent phosphate removed was the difference between the radioactive intensities of Rpb1 in the absence and presence of a fixed amount of Fcp1. Data lines are labeled according to the different challengers.
Fcp1 caused Pol IIB to lose heparin-binding activity to a nearly identical extent as it caused Pol IIA to lose (Fig. 3 C and D), strongly arguing that the CTD was not involved in the loss of heparin binding induced by Fcp1 interaction. We therefore envision a separate region on Pol II distinct from the CTD to be the target site; interaction of Fcp1 with this non-CTD site is responsible for the loss of heparin binding by Pol II. Because we have already shown that the phosphatase activity of Fcp1 does not depend on prior binding to a docking-site, a distinction must be made here that this non-CTD site is not a candidate for a site that would mediate a docking function as defined in ref. 27. Rather, we stress that this site on the globular structure of Pol II is completely separate from the CTD phosphatase function of Fcp1.
We also observed that prior binding of Pol IIA to the heparin beads resulted in no polymerase detection in the flow-through by the same staining method (data not shown). We interpret this result in terms of interference between the two interactions and of their differential affinities: Pol II interaction with heparin polymers in its DNA/RNA cleft is thermodynamically stable and kinetically slow; Fcp1 interaction with the non-CTD site is much weaker than the former interaction. This explanation is consistent with the data that only a minor percentage (≈6%) of Pol II partitioned into the Fcp1-interacting fraction when all three components were present (heparin↔Pol II↔Fcp1), and agrees, in principle, with our SEC conclusion that Pol II↔Fcp1 interaction is dynamic. We discuss later structural implications of the Fcp1-induced loss of heparin binding and of the differential responses by the 12-subunit and 10-subunit Pol II in this interaction.
Verification of Fcp1 Binding to the Non-CTD Site by Competitive Inhibition of the Phosphatase Activity. To characterize consequences of Fcp1/non-CTD site interaction on the phosphatase activity, we again used Pol IIB (devoid of Pol IIA as judged by silver staining; see Fig. 5B) in the same phosphatase assay as described above. We asked whether the pure CTD-less Pol IIB would compete with Pol IIO for Fcp1 binding and, hence, inhibit the dephosphorylation reaction catalyzed by Fcp1. Consistent with the inhibition reported in ref. 27, the CTD-containing Pol IIA and the CTD-less Pol IIB from S. cerevisiae inhibited the phosphatase activity in dose-dependent manners (Fig. 4). Thus, the presence of CTD was not important for the inhibition; rather, it was the presence of the non-CTD structure of Pol II that had competed with Pol IIO for Fcp1 binding. Pol IIA seemed to be a somewhat better inhibitor (1.6-fold as effective) than Pol IIB, which we currently attribute to experimental variations originating mainly from measurements of protein concentrations. In a control experiment, GST–CTDa and GST were compared directly in the same assay, and neither inhibited phosphatase activity, even at a 20-fold molar excess over the substrate. We therefore interpret the inhibition as a result of the competitive binding of Fcp1 to the non-CTD site on the polymerase.
Discussion
Given the central role of reversible CTD phosphorylation in mediating effects from regulatory factors to Pol II (51), it is necessary to understand the biochemical mechanisms that control the phosphorylation status of the CTD. Although it is known that transcription-specific kinases and phosphatases are the main players (46, 52, 53), how exactly the dynamics of CTD phosphorylation are determined by their actions throughout the transcription cycle remains unclear. Actions by CTD phosphatases such as Fcp1 must be pivotal in this transaction, given the known functions (39, 46). To date, two models have been proposed to account for the substrate specificity of Fcp1. The docking-site model emphasizes prior binding to a non-CTD site for the subsequent process of CTD dephosphorylation (27, 52). The distributive model suggests a random diffusion-based mechanism in which Fcp1 acts directly on the CTD (36). The docking hypothesis seems attractive because it alludes to simple mechanisms for the regulation of CTD phosphatase activity: By interacting with the docking-site, Fcp1 may “sense” the different conformational states of unengaged and engaged polymerases, thereby eliciting its activity accordingly, or the accessibility to the docking-site may be influenced by other Pol II-associated factors (e.g., TFIIF) at various stages of transcription. Support for the dependence of Fcp1 phosphatase activity on a docking site came from the following observations. First, recombinant CTD peptides were not substrates for human Fcp1 function (27, 34, 39). Second, Fcp1 seemed specific for their cognate Pol II (26). Third, the Rpb4 subunit of Pol II was directly involved in the assembly of Pol II–Fcp1 complex in S. pombe (47). Fourth, the CTD-less Pol IIB was able to inhibit human Fcp1 activity, presumably by competing with the Pol IIO substrate for Fcp1 binding (27).
Contrary to the docking-site model, we present biochemical evidence here that Fcp1 from either budding or fission yeast exhibits phosphatase activity toward CTD in the absence of any non-CTD part of the Pol II structure. The kinetic data show nearly equal efficiency of Fcp1 toward the CTD on Pol II and the CTD fused to GST (Fig. 1 A–C). We observed no species specificity of Fcp1 from both of the yeast sources toward mammalian Pol II (Fig. 1D), contradicting the previous conclusion that yeast Fcp1 was nonfunctional on mammalian polymerase (26). An important difference between our experiments and the previous ones is our use of Pol IIO and GST–CTDo that have been repurified away from ATP and the kinase used to phosphorylate the CTD. We believe it is critical to the CTD phosphatase assay that no CTD–kinase reaction be allowed to occur. In fact, the main discrepancy between our results and those from which the docking site was proposed apparently lies in the difference between the assay protocols. For instance, in one previous report, no further purification was performed after the in vitro phosphorylation of CTD peptide (27), conceivably permitting rephosphorylation of the CTD during the subsequent dephosphorylation assay. We encountered interference in our dephosphorylation assay using GST–CTDo that had not been repurified (data not shown). Information about substrate repurification was not given in another report (39). Whereas Scp was found active against GST–CTDo, Fcp1 was claimed not to be functional on the same substrate. However, in that comparison, the Fcp1 was supplied at a concentration 10-fold lower than that of Scp (figure 2 of ref. 34, compare lane 6 of figure 2C with lane 6 of figure 2D).
To verify the activity data, we measured direct interaction between Fcp1 and the CTD using GST-pulldown. The result demonstrates that Fcp1 recognizes CTDo as the substrate much more intimately than CTDa, the product of the dephosphorylation reaction. This preference for CTDo by intact Fcp1 seems to correlate with a similar preference by its BRCT domain (38). Taken together with the results based on synthetic CTD peptides (36, 37) that defined a minimal structure of CTD to serve as Fcp1 substrate, we conclude that the CTD moiety, particularly in its phosphorylated form, is recognized directly by Fcp1 phosphatases. This finding explains the in vivo complementation of yeast Fcp1 by a parasite homolog (37).
To summarize the data concerning the phosphatase activity, we stress that the mechanism for Fcp1–CTD recognition is based on random access by Fcp1 molecules directly to the polymer of the heptads, is not mediated by sensing the globular structure of Pol II, and does not involve processivity. This realization reemphasizes the issue of what factors/conditions are responsible for rendering the CTD of elongating Pol II refractory to Fcp1 phosphatase (53). It is necessary to further characterize Fcp1 interaction with Pol II ternary complexes engaged at various stages of transcription elongation as opposed to the unengaged Pol II because conflicting results have been reported in this regard (39, 53, 54).
Aside from its CTD phosphatase function, Fcp1 induced a loss of heparin-binding affinity in Pol II. There has been no known mechanism to date that could explain this noncatalytic activity of Fcp1. But the sharply biased surface-charge distribution of Pol II (positive in the DNA/RNA cleft and negative elsewhere), which indicates that the polymerase binds heparin polymers in the cleft, prompted us to think that the loss of heparin binding may result from three possible mechanisms: (i) an occlusion caused by direct Fcp1 binding in the DNA/RNA site; (ii) a blockage of the cleft by Fcp1 binding at its opening; and (iii) a closure of the cleft by the clamp domain (55–57). The first mechanism might be discounted by the considerations that (i) the DNA/RNA site is ≈75 Å deep but only 25 Å wide, a potential topological problem for a protein as large as pombe Fcp1 (82 kDa) and that (ii) the nucleic acid cleft is occupied by the DNA template and RNA transcript, which would exclude Fcp1 from interacting with Pol II elongation complex, however Fcp1 was observed to stimulate a purified elongation complex (40). The second mode of interaction seems unlikely because the opening above the nucleic acid cleft is occupied by the upstream DNA strands reannealing in the elongation complex (58); thus, Fcp1 would be precluded from contacting the elongation complex. Again, however, Fcp1 was able to stimulate the rate of elongation of the ternary complex (40).
Assuming that the third mechanism is true, we speculate that a closure of the cleft may be caused by Fcp1 interaction with a particular surface region on Pol II. It may be considered a support for this hypothesis that, devoid of Rpb4/7, the 10-subunit Pol II responded more readily to Fcp1 influence over its heparin binding (Fig. 3 B and D). Perhaps, without the Rpb4/7 subunits nearby, Fcp1 could more readily “touch” certain structural elements on the polymerase to induce closure of the clamp domain. At present, there has been no other source of information to verify this notion of Fcp1-dependent conformational change in Pol II. Regardless of the consequence on Pol II conformation, we know that the CTD is not involved in this interaction (or loss of heparin binding) by the fact that the CTD-less Pol IIB also lost its heparin-binding ability upon Fcp1 interaction (Fig. 3C). Thus, this secondary interaction must lie outside the CTD. If so, would this interaction involve a docking site on Pol II that had been proposed to be essential for CTD dephosphorylation (27)? We think it does not, because the entire globular structure of Pol II (excluding the CTD) proved nonessential for the phosphatase function (see the discussion above). Rather, we speculate that Fcp1 interaction with the non-CTD site may play phosphatase-independent roles, such as stimulating elongation (39–41) by stabilizing the DNA/RNA hybrid in the cleft.
The overall interaction between Fcp1 and Pol II appears dynamic, as tested by gel filtration and affinity pulldown here and nondenaturing electrophoresis previously (50). It is interesting to note that the Fcp1–Pol II complex is another example of dynamicity-based interactions that mediate vital cellular processes, including signaling pathways and cell-surface recognitions (59–61).
Supplementary Material
Acknowledgments
We thank R. Kornberg and D. Bushnell for reagents. An expression construct of His6-tagged S. pombe Fcp1 from M. Kimura (RIKEN, Wako, Japan) permitted the initial experiments. This work was supported by National Institutes of Health Grant GM064651 and American Cancer Society Grant IRG-01-231-01 (both to J.F.).
Conflict of interest statement: No conflicts declared.
Abbreviations: CTD, C-terminal domain; CTDo, hyperphosphorylated CTD; CTDa, hypophosphorylated CTD; Pol II, RNA polymerase II; BRCT, breast cancer 1 C terminus; SEC, size-exclusion chromatography.
References
- 1.Allison, L. A., Moyle, M., Shales, M. & Ingles, C. J. (1985) Cell 42, 599–610. [DOI] [PubMed] [Google Scholar]
- 2.Corden, J. L., Cadena, D. L., Ahearn, J. M., Jr., & Dahmus, M. E. (1985) Proc. Natl. Acad. Sci. USA 82, 7934–7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison, L. A., Wong, J. K., Fitzpatrick, V. D., Moyle, M. & Ingles, C. J. (1988) Mol. Cell. Biol. 8, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson, C. M., Koleske, A. J., Chao, D. M. & Young, R. A. (1993) Cell 73, 1361–1375. [DOI] [PubMed] [Google Scholar]
- 5.Kim, Y. J., Bjorklund, S., Li, Y., Sayre, M. H. & Kornberg, R. D. (1994) Cell 77, 599–608. [DOI] [PubMed] [Google Scholar]
- 6.Gerber, H. P., Hagmann, M., Seipel, K., Georgiev, O., West, M. A., Litingtung, Y., Schaffner, W. & Corden, J. L. (1995) Nature 374, 660–662. [DOI] [PubMed] [Google Scholar]
- 7.Bentley, D. (2002) Curr. Opin. Cell Biol. 14, 336–342. [DOI] [PubMed] [Google Scholar]
- 8.Proudfoot, N. J., Furger, A. & Dye, M. J. (2002) Cell 108, 501–512. [DOI] [PubMed] [Google Scholar]
- 9.Dahmus, M. E. (1995) Biochim. Biophys. Acta. 1261, 171–182. [DOI] [PubMed] [Google Scholar]
- 10.Dahmus, M. E. (1996) J. Biol. Chem. 271, 19009–19012. [DOI] [PubMed] [Google Scholar]
- 11.Kobor, M. S. & Greenblatt, J. (2002) Biochim. Biophys. Acta. 1577, 261–275. [DOI] [PubMed] [Google Scholar]
- 12.Laybourn, P. J. & Dahmus, M. E. (1990) J. Biol. Chem. 265, 13165–13173. [PubMed] [Google Scholar]
- 13.Lu, H., Flores, O., Weinmann, R. & Reinberg, D. (1991) Proc. Natl. Acad. Sci. USA 88, 10004–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesnut, J. D., Stephens, J. H. & Dahmus, M. E. (1992) J. Biol. Chem. 267, 10500–10506. [PubMed] [Google Scholar]
- 15.Cadena, D. L. & Dahmus, M. E. (1987) J. Biol. Chem. 262, 12468–12474. [PubMed] [Google Scholar]
- 16.Payne, J. M., Laybourn, P. J. & Dahmus, M. E. (1989) J. Biol. Chem. 264, 19621–19629. [PubMed] [Google Scholar]
- 17.O'Brien, T., Hardin, S., Greenleaf, A. & Lis, J. T. (1994) Nature 370, 75–77. [DOI] [PubMed] [Google Scholar]
- 18.Oelgeschlager, T. (2002) J. Cell. Physiol. 190, 160–169. [DOI] [PubMed] [Google Scholar]
- 19.Jones, J. C., Phatnani, H. P., Haystead, T. A., MacDonald, J. A., Alam, S. M. & Greenleaf, A. L. (2004) J. Biol. Chem. 279, 24957–24964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinhero, R., Liaw, P., Bertens, K. & Yankulov, K. (2004) Eur. J. Biochem. 271, 1004–1014. [DOI] [PubMed] [Google Scholar]
- 21.Gebara, M. M., Sayre, M. H. & Corden, J. L. (1997) J. Cell. Biochem. 64, 390–402. [PubMed] [Google Scholar]
- 22.Patturajan, M., Schulte, R. J., Sefton, B. M., Berezney, R., Vincent, M., Bensaude, O., Warren, S. L. & Corden, J. L. (1998) J. Biol. Chem. 273, 4689–4694. [DOI] [PubMed] [Google Scholar]
- 23.Bonnet, F., Vigneron, M., Bensaude, O. & Dubois, M. F. (1999) Nucleic Acids Res. 27, 4399–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang, M. E. & Dahmus, M. E. (1993) J. Biol. Chem. 268, 25033–25040. [PubMed] [Google Scholar]
- 25.Chambers, R. S. & Dahmus, M. E. (1994) J. Biol. Chem. 269, 26243–26248. [PubMed] [Google Scholar]
- 26.Chambers, R. S. & Kane, C. M. (1996) J. Biol. Chem. 271, 24498–24504. [DOI] [PubMed] [Google Scholar]
- 27.Chambers, R. S., Wang, B. Q., Burton, Z. F. & Dahmus, M. E. (1995) J. Biol. Chem. 270, 14962–14969. [DOI] [PubMed] [Google Scholar]
- 28.Archambault, J., Chambers, R. S., Kobor, M. S., Ho, Y., Cartier, M., Bolotin, D., Andrews, B., Kane, C. M. & Greenblatt, J. (1997) Proc. Natl. Acad. Sci. USA 94, 14300–14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Archambault, J., Pan, G., Dahmus, G. K., Cartier, M., Marshall, N., Zhang, S., Dahmus, M. E. & Greenblatt, J. (1998) J. Biol. Chem. 273, 27593–27601. [DOI] [PubMed] [Google Scholar]
- 30.Kobor, M. S., Archambault, J., Lester, W., Holstege, F. C., Gileadi, O., Jansma, D. B., Jennings, E. G., Kouyoumdjian, F., Davidson, A. R., Young, R. A., et al. (1999) Mol. Cell 4, 55–62. [DOI] [PubMed] [Google Scholar]
- 31.Hausmann, S. & Shuman, S. (2002) J. Biol. Chem. 277, 21213–21220. [DOI] [PubMed] [Google Scholar]
- 32.Kimura, M. & Ishihama, A. (2004) Nucleic Acids Res. 32, 6706–6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamenski, T., Heilmeier, S., Meinhart, A. & Cramer, P. (2004) Mol. Cell 15, 399–407. [DOI] [PubMed] [Google Scholar]
- 34.Yeo, M., Lin, P. S., Dahmus, M. E. & Gill, G. N. (2003) J. Biol. Chem. 278, 26078–26085. [DOI] [PubMed] [Google Scholar]
- 35.Hausmann, S. & Shuman, S. (2003) J. Biol. Chem. 278, 13627–13632. [DOI] [PubMed] [Google Scholar]
- 36.Hausmann, S., Erdjument-Bromage, H. & Shuman, S. (2004) J. Biol. Chem. 279, 10892–10900. [DOI] [PubMed] [Google Scholar]
- 37.Hausmann, S., Schwer, B. & Shuman, S. (2004) Biochemistry 43, 7111–7120. [DOI] [PubMed] [Google Scholar]
- 38.Yu, X., Chini, C. C., He, M., Mer, G. & Chen, J. (2003) Science 302, 639–642. [DOI] [PubMed] [Google Scholar]
- 39.Cho, H., Kim, T. K., Mancebo, H., Lane, W. S., Flores, O. & Reinberg, D. (1999) Genes Dev. 13, 1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandal, S. S., Cho, H., Kim, S., Cabane, K. & Reinberg, D. (2002) Mol. Cell Biol. 22, 7543–7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedl, E. M., Lane, W. S., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. (2003) Proc. Natl. Acad. Sci. USA 100, 2328–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, Y., Flanagan, P. M., Tschochner, H. & Kornberg, R. D. (1994) Science 263, 805–807. [DOI] [PubMed] [Google Scholar]
- 43.Lin, P. S., Dubois, M. F. & Dahmus, M. E. (2002) J. Biol. Chem. 277, 45949–45956. [DOI] [PubMed] [Google Scholar]
- 44.Trigon, S., Serizawa, H., Conaway, J. W., Conaway, R. C., Jackson, S. P. & Morange, M. (1998) J. Biol. Chem. 273, 6769–6775. [DOI] [PubMed] [Google Scholar]
- 45.Bregman, D. B., Du, L., van der Zee, S. & Warren, S. L. (1995) J. Cell Biol. 129, 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho, E. J., Kobor, M. S., Kim, M., Greenblatt, J. & Buratowski, S. (2001) Genes Dev. 15, 3319–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura, M., Suzuki, H. & Ishihama, A. (2002) Mol. Cell Biol. 22, 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakurai, H., Mitsuzawa, H., Kimura, M. & Ishihama, A. (1999) Mol. Cell Biol. 19, 7511–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woychik, N. A. & Young, R. A. (1989) Mol. Cell Biol. 9, 2854–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suh, M. H., Ye, P., Datta, A. B., Zhang, M. & Fu, J. (2005) Anal. Biochem. 343, 166–175. [DOI] [PubMed] [Google Scholar]
- 51.Riedl, T. & Egly, J. M. (2000) Gene Expr. 9, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin, P. S., Tremeau-Bravard, A. & Dahmus, M. E. (2003) Chem. Rec. 3, 235–245. [DOI] [PubMed] [Google Scholar]
- 53.Kong, S. E., Kobor, M. S., Krogan, N. J., Somesh, B. P., Sogaard, T. M., Greenblatt, J. F. & Svejstrup, J. Q. (2005) J. Biol. Chem. 280, 4299–4306. [DOI] [PubMed] [Google Scholar]
- 54.Lehman, A. L. & Dahmus, M. E. (2000) J. Biol. Chem. 275, 14923–14932. [DOI] [PubMed] [Google Scholar]
- 55.Fu, J., Gnatt, A. L., Bushnell, D. A., Jensen, G. J., Thompson, N. E., Burgess, R. R., David, P. R. & Kornberg, R. D. (1999) Cell 98, 799–810. [DOI] [PubMed] [Google Scholar]
- 56.Cramer, P., Bushnell, D. A., Fu, J., Gnatt, A. L., Maier-Davis, B., Thompson, N. E., Burgess, R. R., Edwards, A. M., David, P. R. & Kornberg, R. D. (2000) Science 288, 640–649. [DOI] [PubMed] [Google Scholar]
- 57.Gnatt, A. L., Cramer, P., Fu, J., Bushnell, D. A. & Kornberg, R. D. (2001) Science 292, 1876–1882. [DOI] [PubMed] [Google Scholar]
- 58.Gnatt, A. (2002) Biochim. Biophys. Acta. 1577, 175–190. [DOI] [PubMed] [Google Scholar]
- 59.Dalgarno, D. C., Botfield, M. C. & Rickles, R. J. (1997) Biopolymers 43, 383–400. [DOI] [PubMed] [Google Scholar]
- 60.Davis, S. J., Ikemizu, S., Evans, E. J., Fugger, L., Bakker, T. R. & van der Merwe, P. A. (2003) Nat. Immunol. 4, 217–224. [DOI] [PubMed] [Google Scholar]
- 61.Vaynberg, J., Fukuda, T., Chen, K., Vinogradova, O., Velyvis, A., Tu, Y., Ng, L., Wu, C. & Qin, J. (2005) Mol. Cell 17, 513–523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.