Abstract
Particular major histocompatibility complex (MHC) class II alleles clearly contribute to T cell-mediated autoimmune type 1 diabetes (T1D) in both humans and nonobese diabetic (NOD) mice. However, studies in NOD mice indicate MHC class I-restricted T cell responses are also essential to T1D development. In humans, epidemiological studies have suggested that some common class I alleles, including HLA-A2.1 (A*02011), may confer increased susceptibility to T1D when expressed in conjunction with certain class II alleles. We show here that when HLA-A2.1 molecules are transgenically expressed in NOD mice, A2-restricted T cell responses arise against pancreatic β cells, leading to an earlier onset of T1D. The accelerated onset of T1D in the NOD.HLA-A2.1 transgenic mice is not due to nonspecific effects of expressing a third class I molecule, because a stock of NOD mice transgenically expressing HLA-B27 class I molecules showed no such acceleration of T1D, but rather were significantly protected from disease. These findings provide the first functional evidence that certain human MHC class I molecules can contribute to the development of T1D.
Type 1 diabetes (T1D) in both humans and nonobese diabetic (NOD) mice is the result of T cell-mediated autoimmune destruction of insulin-secreting pancreatic β cells. The immunotolerogenic defects underlying the initial development of diabetogenic T cells, as well as the events involved in their subsequent activation, are genetically controlled by multiple susceptibility (Idd) alleles both within and outside of the major histocompatibility complex (MHC) (1, 2). Although T1D is a complex disorder involving interaction of multiple susceptibility loci with unknown environmental factors, the primary genetic component of susceptibility in humans and NOD mice is provided by particular MHC haplotypes (reviewed in refs. 3–6). Within the MHC, specific combinations of HLA-DQ and -DR class II alleles provide a large component of T1D susceptibility in humans by mediating β cell autoreactive CD4 T cell responses (reviewed in refs. 3 and 4). A particularly strong class II contributor to T1D in humans is the DQ8 variant characterized by histidine and serine, rather than the more common proline and aspartic acid residues at positions 56 and 57 of the β chain. Similarly, transgenic analyses (7–11) have demonstrated that T1D development in NOD mice requires that the unusual H2-Ag7 MHC class II gene product (homologue of human DQ8) be homozygously expressed in the absence of H2-E MHC class II molecules (homologue of human DR). However, although they represent common variants shared by many strains lacking autoimmune proclivity, the Kd and Db class I molecules encoded within the H2g7 haplotype mediate autoreactive CD8 T cell responses also essential to T1D development in NOD mice. This was first demonstrated by the finding that NOD stocks made deficient in MHC class I expression and CD8 T cells by a functionally disrupted β2-microglobulin gene (NOD.β2mnull mice) were completely T1D resistant (12–15). Subsequent analyses indicated that MHC class I-dependent T cell responses are an essential component of both the initiation and most of the progression of pancreatic β cell destruction ultimately leading to T1D development in NOD mice (16).
Several studies have provided evidence that the risk of T1D development in humans is increased when certain MHC class I variants are expressed in conjunction with particular MHC class II susceptibility alleles (17–20, ‡). As in NOD mice, these putative human class I susceptibility variants include some common alleles such as HLA-A2.1 (official designation A*02011) (17, 18, 20). Unlike the case in NOD mice, it has not been possible to directly determine whether particular MHC class I genes play a role in initiating and amplifying diabetogenic T cell responses in humans. However, the functions of various human MHC class I alleles can be ascertained through their transgenic expression in mice.
Given previous studies indicating this variant might confer an increased susceptibility to T1D in humans (17, 18, 20), we assessed whether transgenic HLA-A2.1 MHC class I molecules could mediate diabetogenic T cell responses in NOD mice. Supporting this approach are other studies demonstrating that antigenic peptides presented by this particular HLA-A2.1 transgene product to murine CD8 T cells overlap those presented to human CD8 T cells by endogenously encoded HLA-A2.1 molecules (21–23). Our results demonstrate that transgenic expression of HLA-A2.1 significantly accelerates T1D onset in NOD mice, with HLA-A2.1-restricted CD8 T cells appearing in early, prediabetic insulitic lesions. These results provide functional evidence that some human class I alleles can contribute to T1D development.
Materials and Methods
Mice.
NOD/Lt mice are maintained at The Jackson Laboratory by brother–sister mating. Currently, T1D develops in 90% of female and 63% of male NOD/Lt mice by 30 weeks of age. T and B lymphocyte-deficient NOD-scid mice (official designation NOD-Prkdcscid) are maintained at the N11 backcross generation. The previously described (16) NOD-scid.RIP-B7 mice are maintained at the N8 backcross generation. NOD.β2mnull mice transgenically expressing human β2m (huβ2m) have been described (24) and are currently maintained at the N8 backcross generation. A stock of B6 mice transgenically expressing a full-length genomic HLA-A2.1 construct (25) was provided by Victor Engelhard (University of Virginia Medical Center, Charlottesville). These mice served as progenitors for an N10 backcross of NOD.HLA-A2.1 mice, which were shown to be fixed to homozygosity for markers delineating all known Idd loci of NOD origin by using previously described methods (26). The HLA-A2.1 transgene was also subsequently transferred to the NOD-scid background and fixed to homozygosity (designated NOD-scid.HLA-A2.1 mice). Expression of transgenic HLA-A2.1 molecules is detected by flow cytometric analysis (FACS) of peripheral blood leukocytes (PBL) with the fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody CR11–351 (kindly provided by Victor Engelhard). All mice are housed under specific pathogen-free conditions and allowed free access to food (National Institutes of Health diet 31A, Purina) and acidified drinking water. All stocks of scid mice were treated on an alternating weekly basis with trimethoprim-sulfamethoxazole (Sulfatrim, Barre-National, Baltimore) in the drinking water.
Assessment of Diabetes Development.
Diabetes development was defined by glycosuric values of ≥3 as assessed with Ames Diastix (kindly supplied by Miles Diagnostics, Elkhart, IN).
Flow Cytometric Analysis of Splenic Leukocyte Populations.
Single cell suspensions of splenocytes were analyzed by multicolor FACS. Total T lymphocytes were detected by staining with a FITC-conjugated CD3ɛ specific monoclonal antibody (clone 145-2C11). Total T cells were then further characterized for CD4 expression by using the monoclonal antibody GK1.5 conjugated to the red fluorescent tag Cy3.18-OSu (Cy3, Biological Detection Systems, Pittsburgh), or for CD8 expression with the monoclonal antibody 53-6.72 conjugated to phycoerythrin (PE) whose red fluorescence intensity can easily be distinguished from that of Cy3. Total B lymphocytes were detected by staining with FITC-conjugated polyclonal anti-mouse Ig (Southern Biotechnology Associates, Birmingham, AL). Expression of murine MHC class I molecules was assessed by staining with PE-conjugated Kd-specific monoclonal antibody (clone SF1-1.1) and FITC-conjugated Db-specific antibody (clone 28-14-8).
Splenocyte Adoptive Transfer.
Aliquots of 1 × 107 splenic leukocytes isolated as described (27) from prediabetic (8-week-old) female NOD or NOD.HLA-A2.1 donors were injected intravenously into 6- to 7-week-old T and B cell-deficient NOD-scid or NOD-scid.HLA-A2.1 female recipients. Recipient mice were monitored for 17 weeks after reconstitution for the development of T1D. At diabetes onset or the end of the observation period, recipient splenocytes were analyzed by multicolor flow cytometry for proportions of T cell and B cell reconstitution.
Secondary Transfers.
Bone marrow was isolated from 7-week-old female NOD donors as described (27). Mature T lymphocytes were depleted by incubation of bone marrow with 10 μg/ml purified anti-CD4 (clone GK1.5) and anti-CD8 (clone 53-6.72) antibodies. T cell depleted bone marrow cells (5 × 106) were injected intravenously into lethally irradiated (1200 rad) 5-week-old female NOD and NOD.HLA-A2.1 recipients. Six weeks after the initial bone marrow transfer, 1 × 107 splenic leukocytes from the primary recipients were injected intravenously into 4- to 6-week-old female NOD-scid mice as described above. The secondary recipients were then monitored for T1D development through 17 weeks postrepopulation.
Isolation of HLA-A2.1 Restricted Islet Autoreactive T Lymphocytes.
Islets from (NOD-scid.RIP-B7 × NOD-scid.HLA-A2.1)F1 mice are free of endogenous lymphocytic infiltrates, but capable of both antigenic peptide presentation by HLA-A2.1 class I molecules and of providing potent B7.1-mediated T cell costimulation. Islets from these hybrids were isolated as described (28). Islets were treated with 2000 rad from a 137Cs source and plated 15–20 per well in very complete (VC)-DMEM (29) in a round-bottom 96-well plate. Two days later, 10 islets from NOD.HLA-A2.1 mice were added to each well of adherent F1 islets and overlaid with 100 μl of very complete (VC)-DMEM containing 50 units/ml IL-2, 5 ng/ml IL-7, 10 μg/ml anti-CD4 (clone GK1.5), 10 μg/ml anti-Kd (clone 31-3-4), and 10 μg/ml anti-Db (clone 28-14-8s). Occasional wells were characterized by outgrowth of T cells that were passaged on fresh irradiated F1 islets at weekly intervals, until sufficient numbers of cells were obtained for functional experiments.
Cell-Mediated Lysis Assay.
Cell-mediated lysis assays were performed essentially as described (30). Briefly, pancreatic islets were isolated from NOD-scid and NOD-scid.HLA-A2.1 mice, treated with enzyme-free cell dissociation buffer (GIBCO Invitrogen, Carlsbad, CA), and labeled with 100 μCi (1 Ci = 37 GBq) 51Cr in VC-DMEM for 1 h at 37°C. Labeled cells were washed three times to remove excess 51Cr. Labeled islet target cells were seeded at a concentration of 2.5 × 103 per well in 96-well flat-bottom plates in the presence of varying numbers of autoreactive CD8 T cells isolated from islet cultures. Control wells consisted of labeled islet target cells treated with VC-DMEM only for spontaneous cell lysis or 1% SDS for complete lysis. Supernatants were collected after 16 h and radioactive release measured. Percent specific lysis was calculated by the formula: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Statistical Analyses.
Rates of T1D development in the indicated experimental groups were assessed for statistically significant differences by Kaplan–Meier life table analysis using the STATVIEW 4.5 computer program (Abacus Concepts, Berkeley, CA).
Results
NOD.HLA-A2.1 Mice Show Accelerated Onset of Diabetes.
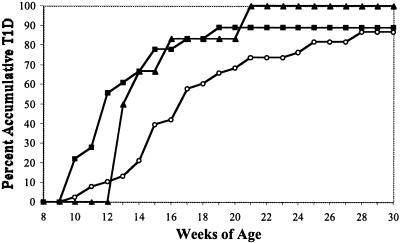
In NOD mice both homozygous and heterozygous for expression of the human A2.1 MHC class I transgene, T1D development was significantly accelerated compared with nontransgenic NOD controls (Fig. 1; P = 0.02 and 0.04, respectively). One of the potential mechanisms for explaining accelerated diabetes pathogenesis in the HLA-A2.1 transgenic NOD mice could entail alterations in CD4 and CD8 T cell development or expression levels of murine class I molecules. However, the proportions of splenic B cells, CD4 T cells, and CD8 T cells are similar between the two strains, indicating that transgenic expression of HLA-A2.1 molecules does not alter the development of any lymphocyte population (Table 1). There were also no differences in the proportions of CD4+CD25+ T cells that recent studies have suggested may have regulatory functions (reviewed in ref. 31). Similarly, as assessed by mean fluorescence intensity, Kd- and Db-specific monoclonal antibodies equivalently bound to splenocytes from each stock, indicating the HLA-A2.1 transgene had no significant impact on the expression levels of endogenous murine MHC class I molecules (Table 1). Furthermore, the HLA-A2.1 transgene did not alter expression levels of murine class I molecules on thymocytes or pancreatic β cells (data not shown). The accelerated onset of T1D in the NOD.HLA-A2.1 transgenic mice is not a nonspecific effect of expressing additional class I molecules. This specificity was demonstrated by the finding that NOD mice transgenically expressing human HLA-B27 class I molecules showed no acceleration of T1D, but rather were significantly protected from disease (5/14 diabetic females at 30 weeks of age compared with 19/20 nontransgenic controls; P = 0.003).
Figure 1.
Accelerated onset of diabetes in HLA-A2.1 transgenic NOD mice. Female NOD (open circles, n = 38) and NOD.HLA-A2.1 mice homozygous (filled squares, n = 18) or heterozygous (filled triangles, n = 6) for the transgene were monitored 30 weeks for development of diabetes. T1D development was significantly increased in NOD mice transgenically expressing HLA-A2.1 molecules in homozygous (P = 0.02) or heterozygous (P = 0.04) state.
Table 1.
Transgenic expression of HLA-A2.1 molecules does not alter splenic lymphocyte populations or expression of murine MHC class I molecules
| Total splenocytes, ×106* | B cells, % ± SEM | CD4 T cells, % ± SEM | CD8 T cells, % ± SEM | CD4+/CD25+, % ± SEM† | Kd expression, MFI ± SEM‡ | Db expression, MFI ± SEM | |
|---|---|---|---|---|---|---|---|
| NOD | 106 ± 4 | 44.4 ± 0.8 | 29.2 ± 0.8 | 15.5 ± 0.5 | 3.30 ± 0.53 | 140.2 ± 6.8 | 26.6 ± 0.9 |
| NOD.HLA-A2.1 | 109 ± 4 | 42.3 ± 0.9 | 32.3 ± 0.7 | 16.0 ± 0.5 | 3.16 ± 0.29 | 135.5 ± 4.6 | 24.0 ± 0.7 |
Data in each group represent the mean ± SEM of four female mice at 8 weeks of age.
Data represent the mean of two female mice at 10 weeks of age.
MFI is mean fluorescence intensity of antibody staining.
HLA-A2.1 MHC Class I Expression Promotes T1D Development Under Conditions Where Murine Class I Variants Are Nonpathogenic.
We had previously produced a NOD stock transgenically expressing human rather than a murine β2m isoform (NOD.β2mnull.huβ2m) that had a greatly reduced incidence of T1D (24). This disease resistance appears to result from murine class I molecules changing to a nondiabetogenic conformation when pairing with human β2m rather than the murine β2ma allele normally expressed by NOD (24). However, introduction of the HLA-A2.1 transgene into the normally resistant NOD.β2mnull.hβ2m stock reconstituted T1D development to a frequency not significantly different from that observed in standard NOD females (Fig. 2). These results indicate that under conditions that severely limit the pathogenic capacity of endogenous murine H2g7 class I molecules, transgenically expressed HLA-A2.1 molecules can restore full susceptibility to T1D. When splenic leukocytes were analyzed by multicolor flow cytometry, no differences in lymphocyte populations were detected between T1D-resistant NOD.β2mnull.hβ2m mice lacking the HLA-A2.1 molecule, and their T1D-susceptible, HLA-A2.1-expressing littermates.
Figure 2.
HLA-A2.1 expression restores T1D susceptibility to NOD.β2mnull.hβ2m mice. The HLA-A2.1 transgene was crossed onto the NOD.β2mnull.hβ2m stock. Female NOD mice (open circles, n = 38) and NOD.β2mnull.hβ2m mice expressing (filled squares, n = 18) or not expressing (open triangles, n = 11) HLA-A2.1 were monitored 30 weeks for development of diabetes. T1D development in NOD.β2mnull.hβ2m mice is significantly decreased compared with NOD controls (P = 0.002). Expression of the HLA-A2.1 transgene in NOD.β2mnull.hβ2m.HLA-A2.1 mice reconstitutes T1D development to an incidence not significantly different from standard NOD mice (P = 0.4).
HLA-A2.1 Molecules Contribute to T1D by Mediating Pathogenic Immune Responses.
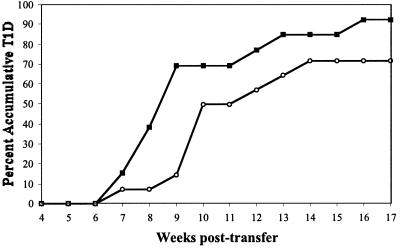
To test whether transgenic HLA-A2.1 molecules truly contribute to T1D by mediating pathogenic immune responses, splenocytes from NOD.HLA-A2.1 mice were transferred to T and B lymphocyte-deficient NOD-scid mice with and without the ability to present pancreatic β cell antigenic peptides bound to HLA-A2.1 molecules. Splenic leukocytes from young (8-week-old) prediabetic homozygous NOD.HLA-A2.1 female donors were intravenously injected into NOD-scid or NOD-scid.HLA-A2.1 recipients. A control group consisted of age-matched female NOD splenic leukocytes injected into NOD-scid recipients. NOD splenic leukocytes were not injected into NOD-scid.HLA-A2.1 recipients because of the high probability of graft versus host disease (GVHD) resulting from allogeneic recognition of the transgenic class I molecules in the recipients by donor NOD T cells. Multicolor fluorescence-activated cell sorter (FACS) analysis of splenic leukocytes demonstrated equivalent levels of B and T cell reconstitution in each recipient group (Table 2). Although T1D did develop slightly more rapidly in HLA-A2.1 positive than negative NOD-scid recipients of NOD.HLA-A2.1 splenocytes, this difference did not reach statistical significance (Fig. 3). However, a more striking finding was that T1D developed significantly faster in NOD-scid recipients repopulated with splenocytes from NOD.HLA-A2.1 than standard NOD donors (P = 0.03).
Table 2.
Lymphocyte reconstitution is equivalent in splenic transfer recipients
| Splenocyte donor | Recipient | n | B cells, % ± SEM | CD4 T cells, % ± SEM | CD8 T cells, % ± SEM |
|---|---|---|---|---|---|
| NOD | NOD-scid | 10 | 25.1 ± 1.2 | 24.8 ± 1.4 | 8.7 ± 1.0 |
| NOD.HLA-A2.1 | NOD-scid | 10 | 21.8 ± 2.0 | 28.8 ± 1.7 | 8.1 ± 0.6 |
| NOD.HLA-A2.1 | NOD-scid.HLA-A2.1 | 13 | 22.9 ± 1.3 | 30.1 ± 2.2 | 9.0 ± 0.7 |
Splenic leukocytes from the indicated 8-week-old female donors were prepared as described in Materials and Methods and transferred by i.v. injection into the indicated scid recipients. Levels of reconstitution were analyzed by FACS as mice became diabetic or at the end of the experiment.
Figure 3.
Accelerated adoptive transfer of T1D by NOD.HLA-A2.1 splenocytes. Splenocytes (1 × 107) from 7- to 8-week-old female donors were transferred by i.v. injection into the indicated scid recipients. NOD.HLA-A2.1 splenocytes were injected into either NOD-scid.HLA-A2.1 (open triangles, n = 20) or NOD-scid (filled squares, n = 24) female recipients. NOD-scid recipients were also repopulated with standard NOD female splenocytes (open circles, n = 18). Recipient mice were monitored 17 weeks after repopulation for development of diabetes. T1D was transferred to NOD-scid recipients more rapidly by splenocytes from NOD.HLA-A2.1 than standard NOD donors (P = 0.03).
We reasoned there could be two explanations for the above findings. The first was that because of their more rapid rate of T1D onset, the spleens of NOD.HLA-A2.1 donors contained a wider array of diabetogenic effector cells, including those restricted to murine MHC molecules, than were present in standard age-matched NOD mice. Alternatively, in addition to mediating diabetogenic immune responses through antigen presentation, expression of the HLA-A2.1 molecules on thymic epithelium may also act to increase the positive selection of β cell autoreactive CD8 T cells whose effector function is restricted by murine Kd or Db molecules. Although positive thymic selection of T lymphocytes requires sufficient T cell receptor (TCR) interaction with MHC–peptide complexes to rescue from death due to neglect (reviewed in ref. 32), the positively selecting MHC class I molecule for a given CD8 T cell need not be the same as the MHC class I molecule necessary for effector function in the periphery (33). To distinguish between these possibilities, NOD T lymphocytes were allowed to develop in the presence or absence of HLA-A2.1 expression on thymic epithelial cells. Bone marrow from NOD female donors was used to reconstitute lethally irradiated NOD or NOD.HLA-A2.1 female recipients. Six weeks after the bone marrow transfer, splenocytes from the NOD or NOD.HLA-A2.1 recipients were found to contain equal proportions of both CD4 and CD8 T cells. Pooled splenocytes from each transfer group were then injected into female NOD-scid recipients. NOD T lymphocytes allowed to undergo positive selection in the presence of HLA-A2.1 molecules expressed on thymic epithelium actually transferred T1D to lymphocyte-deficient NOD-scid mice at a somewhat slower rate than those that matured in the absence of HLA-A2.1 molecules (P = 0.05; Fig. 4). These results indicate that expression of HLA-A2.1 molecules does not lead to greater positive selection of diabetogenic T cells restricted to murine MHC class I molecules for their effector function. They also support a likely need for HLA-A2.1 expression on the pancreatic β target to accelerate T1D development in NOD mice. Hence, it appears that the increased rate of T1D in NOD.HLA-A2.1 mice results from the addition of autoimmune responses mediated by transgenic HLA-A2.1 molecules to those mediated by the endogenous murine H2g7 variants.
Figure 4.
HLA-A2.1 expression does not increase the positive selection of diabetogenic T cells by using murine MHC class I molecules for their effector function. NOD bone marrow cells (5 × 106) were injected into lethally irradiated NOD or NOD.HLA-A2.1 recipients. Six weeks after marrow reconstitution, splenocytes from the NOD and NOD.HLA-A2.1 chimeras were found to contain equal proportions of both CD4 T cells (32.4% vs. 30.6%, respectively) and CD8 T cells (11.3% vs. 10.5%, respectively). At this time, 1 × 107 pooled splenocytes from each chimera group were transferred by i.v. injection into NOD-scid recipients. NOD-scid recipients were monitored 17 weeks after repopulation for development of diabetes. TID developed more slowly (P = 0.05) in recipients of NOD T cells that had matured in the presence (open circles, n = 14) rather than the absence (filled squares, n = 13) of HLA-A2.1 molecules on thymic epithelium.
Human A2.1 MHC Class I Molecules Mediate β Cell Autoreactive T Cell Responses.
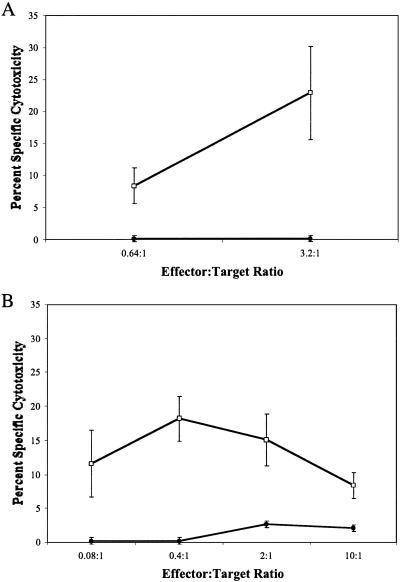
We next determined whether HLA-A2.1-restricted β cell autoreactive T cells were truly generated in the NOD.HLA-A2.1 transgenic mice. Infiltrating T cells from islets of either 10- or 6-week-old prediabetic female NOD.HLA-A2.1 mice were isolated by culture on (NOD-scid.RIP-B7 × NOD-scid.HLA-A2.1)F1 islets by using methods previously described (16). The culture medium was supplemented with anti-CD4, anti-Kd, and anti-Db monoclonal antibodies to prevent the outgrowth of unwanted populations and restrict lymphocyte expansion to only those cells expressing HLA-A2.1-restricted TCRs. The islet-derived T cells isolated from both 6- and 10-week-old NOD.HLA-A2.1 mice were able to mediate the lysis of HLA-A2.1-positive, but not -negative, β cells (Fig. 5). These results indicate that transgenically expressed human HLA-A2.1 class I molecules are able to mediate autoreactive diabetogenic T cell responses.
Figure 5.
Lysis of NOD-scid or NOD-scid.HLA-A2.1 β cell targets by isolated autoreactive CD8 T lymphocytes. Lymphocytes cultured from islets of two 10-week-old (A) or one 6-week-old (B) NOD.HLA-A2.1 female mouse were used as effectors in a 51Cr release assay at the indicated effector:target ratios. β cell targets were isolated from lymphocyte-deficient NOD-scid (filled circles) or NOD-scid.HLA-A2.1 (open squares) mice.
Discussion
Recent epidemiological studies have suggested that, in the presence of high-risk HLA class II DR/DQ variants, the A2.1 class I allele might confer additional susceptibility to T1D in humans (17, 18, 20). In one study, high-risk individuals who also carried an HLA-A2 class I allele were more likely to develop autoantibodies and clinical T1D before 5 years of age than class II matched individuals lacking HLA-A2 (20). Although these epidemiological studies have suggested a role for certain common HLA class I alleles, including HLA-A2.1, in susceptibility to T1D in humans, it has not been possible to directly assess this contribution (17–19). Our studies using NOD mice transgenically expressing HLA-A2.1 offer functional evidence for involvement of a certain human MHC class I molecules in mediating diabetogenic immune responses. This finding is most simply demonstrated by the observation that NOD.HLA-A2.1 mice have a significantly accelerated onset of T1D. The transgenic expression of HLA-A2.1 also restores susceptibility to a NOD stock made disease resistant through replacement of murine β2m with the human isoform. This alteration in β2m induces a conformational change in murine MHC class I molecules that greatly reduces their capacity to elicit diabetogenic CD8 T cell responses (24). The ability of HLA-A2.1 expression to restore full NOD-like diabetes susceptibility on this resistant background indicates this molecule is able to efficiently mediate diabetogenic immune responses, even when contributions from endogenous murine MHC class I molecules are severely diminished. Accelerated T1D development in HLA-A2.1 transgenic mice likely results from this human MHC class I variant triggering additional β cell autoreactive CD8 T cell clonotypes to those restricted by murine H2g7 class I molecules. However, the accelerated development of T1D in the NOD.HLA-A2.1 stock is not a nonspecific effect of expressing an additional type of class I molecules, because disease onset was actually retarded rather than accelerated in NOD mice transgenically expressing the human HLA-B27 class I variant.
Significantly, we were able to confirm that transgenic HLA-A2.1 molecules could indeed elicit β cell autoreactive T cell responses in NOD mice, because such effectors could be isolated. It should be noted that we have found such T cells to be quite ephemeral in vitro, and hence difficult to expand to the large numbers needed to extensively examine their pathogenic activity in adoptive transfer studies. However, 5 × 105 T cells from a third line generated in the same way as the two others depicted in Fig. 5 induced T1D development at 6 weeks post-i.v. injection in a single NOD-scid.HLA-A2.1 recipient. The peptides presented by transgenically expressed HLA-A2.1 molecules to murine T cells overlap those presented to human T cells by endogenous HLA-A2.1 molecules (21–23). Therefore, the NOD.HLA-A2.1 stock may ultimately prove useful in identifying antigenic peptides targeted by human diabetogenic T cells.
The mechanism by which HLA-A2.1 expression might accelerate T1D development in NOD mice was assessed in several adoptive transfer experiments. In the first of these, splenocytes from prediabetic NOD.HLA-A2.1 and standard NOD donors were transferred into T and B lymphocyte-deficient NOD-scid recipients with or without the capacity to present β cell antigenic peptides in the context of HLA-A2.1 molecules. There was a suggestion that adoptively transferred NOD.HLA-A2.1 splenocytes induced T1D development at a slightly faster rate in NOD-scid.HLA-A2.1 than standard NOD-scid recipients, but this difference did not achieve statistical significance. A more striking result was the finding that T1D developed at a significantly faster rate in NOD-scid recipients repopulated with splenocytes from NOD.HLA-A2.1 than standard NOD donors. One explanation for this finding was that given their accelerated onset of T1D, a wider array of diabetogenic effectors, including those restricted to murine MHC molecules, had accumulated in the spleens of NOD.HLA-A2.1 than age-matched standard NOD mice. Alternatively, previous studies (33) suggested it was also possible that expression of the HLA-A2.1 molecules on thymic epithelium resulted in the increased positive selection of β cell autoreactive CD8 T cells whose effector function is mediated by murine Kd or Db molecules. However, using a two step adoptive transfer system, we found that HLA-A2.1 expression on thymic epithelium did not result in the increased positive selection of diabetogenic T cells with murine MHC class I-restricted effector function. While not ruling out a requirement for expression at other sites, these results also support a likely need for HLA-A2.1 expression on the pancreatic β target to accelerate T1D development in NOD mice.
The later stages of T1D may proceed in the absence of CD8 T cells as demonstrated by the ability of purified CD4 T cells from diabetic donors to transfer disease to NOD-scid recipients (34). However, CD8 T cells are necessary for the earliest initiation and most of the progression of β cell destruction leading to T1D development (16, 27). In these studies we were able to isolate HLA-A2.1-restricted β cell autoreactive CD8 T cells from NOD.HLA-A2.1 mice at early (6 weeks) and later (10 weeks) prodromal stages of T1D development. It appears that it is the addition of such responses to those mediated by murine H2g7 class I molecules that significantly accelerates the onset of T1D in NOD.HLA-A2.1 transgenic mice. These findings provide a functional demonstration that some human MHC class I molecules can mediate diabetogenic immune responses in addition to those previously known to be elicited by particular class II alleles.
Acknowledgments
We thank Drs. S. Nathenson, T. DiLorenzo, D. Roopenian, and E. Leiter for helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health Grants DK46266, DK51090, and DK59717, and Cancer Center Support (CORE) Grant CA34196.
Abbreviations
- NOD
nonobese diabetic
- T1D
type 1 diabetes
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Honeyman, M. C., Tait, B. D., Colman, P. G., Stone, N. & Harrison, L. C. (1994) 13th International Immunology and Diabetes Workshop, p. 94, May 25–28, 1994, Chantilly, France (abstr.).
References
- 1.Serreze D V, Leiter E H. Curr Dir Autoimmun. 2001;4:31–67. doi: 10.1159/000060527. [DOI] [PubMed] [Google Scholar]
- 2.Todd J A, Wicker L S. Immunity. 2001;15:387–395. doi: 10.1016/s1074-7613(01)00202-3. [DOI] [PubMed] [Google Scholar]
- 3.Sheehy M J. Diabetes. 1992;41:123–129. doi: 10.2337/diab.41.2.123. [DOI] [PubMed] [Google Scholar]
- 4.Todd J A. Springer Semin Immunopathol. 1992;14:33–58. doi: 10.1007/BF00197131. [DOI] [PubMed] [Google Scholar]
- 5.Prochazka M, Serreze D V, Worthen S M, Leiter E H. Diabetes. 1989;38:1446–1455. doi: 10.2337/diab.38.11.1446. [DOI] [PubMed] [Google Scholar]
- 6.Wicker L S, Appel M C, Dotta F, Pressey A, Miller B J, DeLarto N H, Fischer P A, Boltz R C, Peterson L B. J Exp Med. 1992;176:67–77. doi: 10.1084/jem.176.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazaki T, Uno M, Uehira M, Kikutani H, Kishimoto T, Kimoto M, Nishimoto H, Miyazaki J, Yamamura K. Nature. 1990;345:722–724. doi: 10.1038/345722a0. [DOI] [PubMed] [Google Scholar]
- 8.Lund T, O'Reilly L, Hutchings P, Kanagawa O, Simpson E, Gravely R, Chandler P, Dyson J, Picard J K, Edwards A, et al. Nature. 1990;345:727–729. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- 9.Slattery R M, Kjer-Nielsen L, Allison J, Charlton B, Mandel T, Miller J F A P. Nature. 1990;345:724–726. doi: 10.1038/345724a0. [DOI] [PubMed] [Google Scholar]
- 10.Singer S M, Tisch R, Yang X-D, McDevitt H O. Proc Natl Acad Sci USA. 1993;90:9566–9570. doi: 10.1073/pnas.90.20.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson M S, Cetkovic-Cvrlje M, Ramiya V K, Atkinson M A, MacLaren N K, Singh B, Elliott J F, Serreze D V, Leiter E H. J Immunol. 1996;157:1279–1287. [PubMed] [Google Scholar]
- 12.Serreze D V, Leiter E H, Christianson G J, Greiner D, Roopenian D C. Diabetes. 1994;43:505–509. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 13.Katz J, Benoist C, Mathis D. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 14.Wicker L S, Leiter E H, Todd J A, Renjilian R J, Peterson E, Fischer P A, Podolin P L, Zijlstra M, Jaenisch R, Peterson L B. Diabetes. 1994;43:500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 15.Sumida T, Furukawa M, Sakamoto A, Namekawa T, Maeda T, Zijlstra M, Iwamoto I, Koike T, Yoshida S, Tomioka H, Taniguchi M. Int Immunol. 1994;6:1445–1449. doi: 10.1093/intimm/6.9.1445. [DOI] [PubMed] [Google Scholar]
- 16.DiLorenzo T P, Graser R T, Ono T, Christianson G J, Chapman H D, Roopenian D C, Nathenson S G, Serreze D V. Proc Natl Acad Sci USA. 1998;95:12538–12543. doi: 10.1073/pnas.95.21.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fennessy M, Metcalfe K, Hitman G A, Niven M, Biro P A, Tuomilehto J, Tuomilehto-Wolf E. Diabetologia. 1994;37:937–944. doi: 10.1007/BF00400951. [DOI] [PubMed] [Google Scholar]
- 18.Demaine A G, Hibberd M L, Mangles D, Millward B A. Diabetologia. 1995;38:622–628. doi: 10.1007/BF00400734. [DOI] [PubMed] [Google Scholar]
- 19.Nejentsev S, Reijonen H, Adojaan B, Kovalchuk L, Sochnevs A, Schwartz E I, Akerblom H K, Ilonen J. Diabetes. 1997;46:1888–1892. doi: 10.2337/diab.46.11.1888. [DOI] [PubMed] [Google Scholar]
- 20.Robles D T, Eisenbarth G S, Wang T, Erlich H A, Bugawan T L, Babu S R, Barriga K, Norris J M, Hoffman M, Klingensmith G, et al. Clin Immunol. 2002;102:217–224. doi: 10.1006/clim.2001.5171. [DOI] [PubMed] [Google Scholar]
- 21.Engelhard V H, Lacy E, Ridge J P. J Immunol. 1991;146:1226–1232. [PubMed] [Google Scholar]
- 22.Man S, Newberg M H, Crotzer V L, Luckey C J, Williams N S, Chen Y, Huczko E L, Ridge J P, Engelhard V H. Int Immunol. 1995;7:597–605. doi: 10.1093/intimm/7.4.597. [DOI] [PubMed] [Google Scholar]
- 23.Shirai M, Arichi T, Nishioka M, Nomura T, Ikeda K, Kawanishi K, Engelhard V H, Feinstone S M, Berzofsky J A. J Immunol. 1995;154:2733–2742. [PubMed] [Google Scholar]
- 24.Hamilton-Williams E E, Serreze D V, Charlton B, Johnson E A, Marron M P, Mullbacher A, Slattery R M. Proc Natl Acad Sci USA. 2001;98:11533–11538. doi: 10.1073/pnas.191383798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le A-X T, Bernhard E J, Holterman M J, Strub S, Parham P, Lacy E, Engelhard V H. J Immunol. 1989;142:1366–1371. [PubMed] [Google Scholar]
- 26.Serreze D V, Chapman H D, Varnum D S, Hanson M S, Reifsnyder P C, Richard S D, Fleming S A, Leiter E H, Shultz L D. J Exp Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serreze D V, Chapman H D, Varnum D S, Gerling I, Leiter E H, Shultz L D. J Immunol. 1997;158:3978–3986. [PubMed] [Google Scholar]
- 28.Gerling I C, Serreze D V, Christianson S W, Leiter E H. Diabetes. 1992;41:1672–1676. doi: 10.2337/diab.41.12.1672. [DOI] [PubMed] [Google Scholar]
- 29.Cerottini J C, Engers H D, Macdonald H R, Brunner T. J Exp Med. 1974;140:703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serreze D V, Gallichan W S, Snider D P, Croitoru K, Rosenthal K L, Leiter E H, Christianson G J, Dudley M E, Roopenian D C. Diabetes. 1996;45:902–908. doi: 10.2337/diab.45.7.902. [DOI] [PubMed] [Google Scholar]
- 31.Read S, Powrie F. Curr Opin Immunol. 2001;13:644–649. doi: 10.1016/s0952-7915(01)00273-4. [DOI] [PubMed] [Google Scholar]
- 32.Hogquist K A. Curr Opin Immunol. 2001;13:225–231. doi: 10.1016/s0952-7915(00)00208-9. [DOI] [PubMed] [Google Scholar]
- 33.Kaye J, Vasquez N J, Hedrick S M. J Immunol. 1992;148:3342–3353. [PubMed] [Google Scholar]
- 34.Christianson S W, Shultz L D, Leiter E H. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]