Abstract
The signal recognition particle (SRP)-translocation pathway is conserved in all three domains of life and delivers membrane and secretory proteins to the cytoplasmic membrane or endoplasmic reticulum. We determined the requirement in the cariogenic oral pathogen Streptocococcus mutans of the three universally conserved elements of the SRP pathway: Ffh/SRP54, scRNA, and FtsY/SRα. Previously, we reported that insertional interruption of S. mutans ffh was not lethal, but resulted in acid sensitivity. To test whether S. mutans could survive extensive disruption of the SRP pathway, single and double deletions of genes encoding Ffh, scRNA, and FtsY were generated. Without environmental stressors, all mutant strains were viable, but unlike the wild-type, none could initiate growth at pH 5.0 or in 3.5% NaCl. Survival of challenge with 0.3 mM H2O2 was also diminished without ffh. Members of the YidC/Oxa1/Alb3 family are also ubiquitous, involved in the translocation and assembly of membrane proteins, and have been identified in prokaryotes/mitochondria/chloroplasts. Two genes encoding YidC homologs, YidC1 and YidC2, are present in streptococcal genomes with both expressed in S. mutans. Deletion of YidC1 demonstrated no obvious phenotype. Elimination of YidC2 resulted in a stress-sensitive phenotype similar to SRP pathway mutants. Mutants lacking both YidC2 and SRP components were severely impaired and barely able to grow, even in the absence of environmental stress. Here, we report the dispensability of the cotranslational SRP protein translocation system in a bacterium. In S. mutans, this pathway contributes to protection against rapid environmental challenge and may overlap functionally with YidC2.
Keywords: protein translocation, streptococcus, Ffh, FtsY, membrane biogenesis
The signal recognition particle (SRP) is a highly conserved ribonucleotide-protein complex involved in cotranslational membrane targeting of signal-peptide-bearing proteins (1). The SRP pathway is conserved in all domains of life and has been considered crucial for the vitality of all organisms (2-6). Saccharomyces cerevisiae represents the only known example of an organism able to endure mutations within the SRP pathway (7). Mutants are exceedingly sick and adapt physiologically by repression of protein synthesis, induction of heat shock genes, and dramatic slow-down of growth (8). Comparative phylogenetic analyses demonstrate that genomes of Eukaryotes, Archaea, and Bacteria encode at a minimum the SRP components SRP54 protein, known in Eubacteria as Ffh (54-kDa homolog), and a 4.5 or 7S small cytoplasmic RNA (scRNA) (5). The third universally conserved element of the pathway is the SRP receptor subunit (SRα), or in Escherichia coli, FtsY (9).
The bacterial SRP translocation pathway and protein secretion has been studied in E. coli and Bacillus subtilis; however, little is known in other bacteria, including the streptococci. As the major etiological agent of human dental caries (reviewed in ref. 10), Streptococcus mutans is well studied at the genetic and physiologic level. Major factors contributing to pathogenicity include acidogenicity and aciduricity (reviewed in ref. 11). The unique biological nature of S. mutans allows it to ferment a wide range of dietary carbohydrates and, within minutes after sugar consumption, lower the surrounding plaque pH to below pH 4.0. This rapid acidification is followed by expression of numerous proteins, including chaperones and membrane proteins, in a process necessary for cellular viability referred to as the acid-tolerance response (12, 13). Transposon mutagenesis in S. mutans resulted in several acid-sensitive mutants (14) and one mutation mapped to ffh within the sat (secretion and acid tolerance) operon (15). Surprisingly, interruption of ffh was not lethal (16), in contrast to what was reported for E. coli and B. subtilis (3, 17), but did render S. mutans sensitive to acidic pH.
In Eubacteria, the SRP is composed of Ffh and scRNA. This ribonucleotide-protein complex delivers incompletely translated substrates to the membrane-associated receptor protein, FtsY, for completion of translation and translocation into or through the membrane (4). The targeting path is determined as the nascent peptide chain emerges from the ribosome (18). The SRP specifically recognizes and binds to highly hydrophobic signal peptide sequences on the ribosome (19), and then the SRP/ribosome complex targets to the receptor FtsY (9, 20) via a high-affinity GTP-dependent interaction. The complex is then transferred to the translocon resulting in release of the SRP from the ribosome-nascent chain complex. Translation resumes and the polypeptide is directed through the translocon. GTP hydrolysis drives dissociation of the SRP from its receptor allowing it to initiate a new round of targeting (21). The majority of integral membrane proteins are channeled via a translocon comprised of two heterotrimeric complexes, SecYEG and SecDFYajC (reviewed in ref. 22).
Members of the YidC/Oxa1/Alb3 family have been identified in prokaryotes, mitochondria, and chloroplasts (23). These proteins are believed to serve as membrane-localized chaperones involved in translocation and assembly of a large number of membrane proteins (23, 24) and function in concert with the Sec translocon (23, 25, 26), as well as independently (27, 28). Recently, Oxa2 was identified in mitochondria of Neurospora crassa and functionally complemented the related yeast protein Cox18 (29). Domain swapping and complementation experiments using engineered variants of E. coli YidC suggest that the ribosome binding function associated with a C-terminal α-helical coiled-coil tail present in mitochondrial Oxa1 can be transferred, but appending this tail to YidC destroys it's ability to complement Oxa2/Cox18 (30). Studies suggest that Oxa2/Cox18/YidC act primarily on completely synthesized substrates, whereas Oxa1 primarily mediates cotranslational translocation of membrane proteins (30, 31). The S. mutans genome contains two copies of genes encoding YidC homologs; however, the relative function of either compared to YidC of E. coli or mitochondrial Oxa1 or Oxa2 is not yet known. Oxa1 and Oxa2 both have reported roles in the biogenesis of respiratory chain complexes and cytochrome oxidase activity (32); however, streptococci lack a cytochrome system.
In light of the phylogenetically ubiquitous conservation of Ffh, scRNA, and FtsY and the survival of S. mutans in the absence of a complete Ffh protein, this study was undertaken to evaluate the essentiality of the S. mutans SRP pathway. The contributions of the S. mutans YidC homologs to viability and resistance to environmental stress were also evaluated singly and in combination with SRP genes. We provide evidence that, in S. mutans, the SRP pathway is uniquely nonessential under nonstress conditions, and clearly enables the organism to contend with rapid environmental challenges. Elimination of yidC2, but not yidC1, also resulted in a stress-sensitive phenotype and simultaneous disruption of the SRP pathway and YidC2 rendered S. mutans severely impaired and barely able to grow even under nonstress conditions. Our results suggest the involvement of SRP-cotranslational translocation in S. mutans' ability to maintain a membrane composition resistant to varying environmental stressors and that YidC2 may serve at least partially redundant functions with the SRP pathway.
Materials and Methods
Bacterial Strains and Growth Conditions. Bacterial strains are listed in Table 2, which is published as supporting information on the PNAS web site. Mutants were derived from S. mutans NG8 (33). Mutations of ffh (SMU.1060), ftsY (SMU.744), or scRNA (base pairs 293148-293220 of the genomic sequence; GenBank accession no. AE014133) in S. mutans UA159 had identical phenotypes to those in NG8 (not shown). S. mutans was grown in Todd-Hewitt broth (BBL, Becton Dickson Microbiology System, Cockeysville, MD) supplemented with 0.3% (wt/vol) yeast extract (THYE) at 37°C with 5% CO2. Kanamycin (500 μg/ml plates or 100 μg/ml broth) and erythromycin (10 μg/ml) were used as antibiotics. E. coli was grown in LB broth supplemented with 300 μg/ml erythromycin when appropriate.
DNA Methods. Standard methods were used to isolate and manipulate plasmid DNA. S. mutans genomic DNA was isolated as described (14). Cloning, restriction endonuclease digestions, and DNA ligations were carried out according to standard protocols. Restriction enzymes and T4 DNA ligase were obtained from New England Biolabs. PCRs used iQ Supermix (Bio-Rad).
Construction of Mutant Strains. Nonpolar deletion mutants were constructed by PCR ligation mutagenesis (34). Genes were replaced with an antibiotic marker cassette [kan (16) or erm (35)] without introducing transcriptional stops or disrupting the translational reading frame. Sequences flanking the replaced genes were amplified by using S. mutans genomic DNA as template and appropriate primers (Table 3, which is published as supporting information on the PNAS web site). PCR-amplified fragments were digested via restriction sites engineered into the primers and ligated to the marker cassette. Ligated DNA fragments were gel-purified (Qiagen, Valencia, CA), served as templates for a second round of PCR amplification and the products used to transform S. mutans (36). Allelic replacement mutants were selected by antibiotic resistance and confirmed by PCR (Fig. 3, which is published as supporting information on the PNAS web site). Replaced genes and loci are shown schematically in Fig. 4, which is published as supporting information on the PNAS web site. For construction of double mutant strains ΔftsYΔffh and ΔscRNAΔffh, the kanr-resistant single mutant strains AH307 (ΔftsY) or AH312 (ΔscRNA) were transformed with a linear DNA amplicon containing the ffh deletion construct harboring the erythromycin resistance cassette. Double mutants were selected on erythromycin-containing agar plates and verified by PCR (Fig. 3).
Construction of yidC1 and yidC2 mutant strains was carried out as described above for the SRP mutants with Erm used as the selection marker. For yidC/SRP-pathway mutant strains, Δ(ylxM-ffh-satC)::kan and ΔftsY::kan fragments were PCR-amplified by using genomic DNA from AH316 or AH307 as templates. Resulting linear DNA fragments were used to transform AH374 and AH398. Double mutant strains were selected on THYE agar plates containing kanamycin and erythromycin and confirmed by PCR (Fig. 3).
Complementation of the ftsY Mutation. To complement the ftsY mutation, ftsY was PCR-amplified by using primers AH14F and AH1R (Table 3). Because ftsY is the fourth orf in a putative operon, genomic DNA from mutant strain AH308 Δ(orf1-orf2-orf3), which maintains the upstream promoter sequence, followed by the kan-resistance gene and ftsY structural gene, was used as the template. The PCR amplicon was ligated to the EcoRV site of the Streptococcus-E. coli shuttle vector pDCerm (37), and the resulting plasmid, (ermr and kanr), was introduced into the ftsY null mutant, AH307, and NG8 by natural transformation. Transformants were selected on erythromycin.
RNA Isolation and RT-PCR to Identify scRNA. Total RNA from 10-ml exponential phase cultures of S. mutans, lysed with the Mini Bead-Beater (Biospec Products), was isolated by using the RNeasy Mini kit (Qiagen). RNA was treated with RQ1 RNase-free DNase (Promega) and repurified through RNeasy columns.
RT-PCR was used to confirm the presence of scRNA in total RNA samples, using primer PC62R (Table 3) and SuperScript II RNase H- Reverse Transcriptase (Invitrogen) for the reverse transcription reaction and primers PC62F and PC62R and iQ Supermix (Bio-Rad) for the PCR. Genomic DNA from strain NG8 and total RNA from strains NG8 and AH312 served as control templates for PCR.
Bacterial Growth Under Acid, Osmotic, and Oxidative Stress. S. mutans was grown for 48 h on THYE-agar (pH 7.0) with antibiotic at 37°C in air with 5% CO2, then passaged into 10 ml of THYE (pH 7.0) and incubated overnight. Cultures were diluted 1:20 in THYE pH 7.0 without antibiotic and grown to O.D.600 of 0.3-0.4. A 100-well Bioscreen C plate (Labsystems, Helsinki, Finland) was filled with 350 μl of prewarmed media (THYE pH 7.0 or 5.0; pH 7.0 with 3.5% NaCl; or pH 7.0 with 0.3 mM H2O2). Twenty-microliter culture volumes were added to the wells and grown for 16 h with absorbance recorded every 20 min. Wells containing media only served as background controls. Doubling time (Td) was calculated by measuring the slope of the logarithmic growth phase (38) and the formula: Td = (t2 - t1)ln (2)/ln(OD2) - ln(OD1). Results represent an average of five replicates with standard deviation of the mean. Each experiment was performed two to four times. Statistical significance was determined by using Student's t test and a P value of <0.05.
Membrane Preparation and H+/ATPase Activity. Preparation of bacterial membranes and measurement of H+/ATPase enzymatic activity were performed as described (39). ATPase-specific activities of membrane fractions prepared from cultures grown at pH 7.0 or 5.0 (acid shock) of the wild-type and mutant strains were calculated and expressed as nmol of Pi released per min per mg of total protein. The assay was performed twice with duplicate samples in each assay. Statistical significance was determined by using Student's t test, P < 0.05.
Results and Discussion
Elimination of ffh Is Not Lethal. We previously identified S. mutans mutants unable to initiate growth at acidic pH (14). One transposon (Tn917)-insertion-generated mutation mapped to the ylxM-ffh intergenic region of the sat operon (15). Mutations of individual genes within the sat operon (Fig. 4) were generated by insertional inactivation and, with the exception of the acid-sensitive ffh mutant MK4, were indistinguishable from wild-type S. mutans (16). The S. mutans UA159 genomic sequence (40) demonstrates no ffh homologs outside of the sat operon. Because MK4 possessed DNA encoding the N-terminal 209 aa of Ffh, the question remained whether the truncated protein retained residual functional activity; therefore, a mutant strain was generated in which the entire ffh gene was deleted and growth was assessed (Table 1). The cellular growth yield of AH329 (Δffh) was similar to the wild-type under nonstress conditions (not shown), but the Td of the mutant was increased relative to the parent (P < 0.001). Importantly, strain AH329 demonstrated a similar phenotype as MK4, indicating that the N-terminal region of Ffh did not contribute to MK4's viability. The effect of disruption of ffh on tolerance to several environmental stressors, including acid, salt, and hydrogen peroxide, was evaluated in parallel for mutants MK4 and AH329. As expected, growth of both was severely impaired upon acid shock at pH 5.0 (Table 1). In addition, these mutant strains were unable to initiate growth when transferred into 3.5% salt-containing medium, a defining characteristic of S. mutans. Although addition of H2O2 to the culture medium did not have as pronounced an effect on the ffh mutants as did acid or salt stress, the Td of the complete ffh deletion mutant strain AH329 was significantly increased (P < 0.05) relative to the nonstress condition. There was no effect of high (42°C) or low temperature (30°C) on growth of either mutant strain (not shown). Taken together, the results indicate that complete removal of Ffh from S. mutans is not lethal, but does render the organism less able to contend with several environmental stressors. Interestingly, mutant MK4 was shown previously to adapt to acid stress and grow when pH was allowed to drop gradually from normal metabolism, indicating that given time compensatory mechanisms are engaged upon environmental challenge in the absence of an intact SRP pathway (39).
Table 1. Summary of doubling times for S. mutans wild-type and SRP-pathway and yidC-mutant strains.
| Growth conditions
|
|||||
|---|---|---|---|---|---|
| Strain | Description | Nonstress | pH 5.0 | 3.5% NaCl | 0.3 mM H2O2 |
| NG8 | wild-type | 44.07 ± 0.74 | 79.11 ± 0.68* | 93.28 ± 2.18* | 50.17 ± 1.04 |
| MK4 | ffh::kan | 57.61 ± 1.85† | 0 ± 0*† | 0 ± 0*† | 62.91 ± 1.81‡ |
| AH329 | Δffh | 66.10 ± 1.31† | 0 ± 0*† | 0 ± 0*† | 84.04 ± 1.39§¶ |
| AH316 | Δ(ylxM-ffh-satC) | 82.34 ± 6.08† | 0 ± 0†¶ | 0 ± 0†¶ | 87.97 ± 1.94§ |
| AH307 | ΔftsY | 63.06 ± 0.80† | 0 ± 0†∥ | 0 ± 0†∥ | 70.86 ± 2.67‡ |
| AH308 | Δ(orf1orf2orf3) | 44.16 ± 0.26 | 79.27 ± 0.16∥ | 94.58 ± 5.83* | 47.89 ± 0.93 |
| AH325 | Δ(orf1orf2orf3ftsY) | 62.16 ± 1.25† | 0 ± 0†∥ | 0 ± 0†∥ | 65.11 ± 0.42‡ |
| AH351 | ΔffhΔftsY | 63.84 ± 1.24† | 0 ± 0†∥ | 0 ± 0†∥ | 76.01 ± 0.04§¶ |
| AH307(pDCerm-FtsY) | ΔftsY+pDCerm-ftsY | 46.08 ± 0.76 | 77.90 ± 0.91* | 110.82 ± 3.10*‡ | 49.33 ± 0.29 |
| AH312 | ΔscRNA | 72.91 ± 1.98† | 0 ± 0*† | 0 ± 0*† | 73.45 ± 0.91§ |
| AH341 | ΔffhΔscRNA | 76.57 ± 1.35† | 0 ± 0†∥ | 0 ± 0†∥ | 88.10 ± 0.78§¶ |
| AH374 | ΔyidC1 | 50.71 ± 0.40§ | 99.25 ± 1.00‡∥ | 116.43 ± 5.68* | 53.49 ± 2.34 |
| AH386 | ΔyidC1Δ(ylxM-ffh-satC) | 91.30 ± 1.43† | 0 ± 0§∥ | 0 ± 0†∥ | 89.41 ± 0.05§ |
| AH390 | ΔyidC1ΔftsY | 64.93 ± 1.65§ | 0 ± 0§∥ | 0 ± 0†∥ | 68.88 ± 0.86§ |
| AH394 | ΔyidC1ΔscRNA | 67.75 ± 0.82§ | 0 ± 0§∥ | 0 ± 0†∥ | 72.94 ± 0.37§¶ |
| AH398 | ΔyidC2 | 89.86 ± 0.98† | 0 ± 0§∥ | 0 ± 0†∥ | 92.81 ± 0.08† |
| AH402 | ΔyidC2Δ(ylxM-ffh-satC) | ND | NT | NT | NT |
| AH405 | ΔyidC2ΔftsY | ND | NT | NT | NT |
Results are expressed as the mean ± standard error (in minutes). Statistical significance compared to wild-type strain NG8 by using Student's t test is indicated as follows: †, P < 0.001; §, P < 0.05. Statistical significance compared to nonstress conditions by using Student's t test is indicated as follows: ∥, P < 0.001; *, P < 0.01; ¶, P < 0.05. ND, not determined. Growth was only detectable after ≥48 h of culture in liquid media. NT, not tested.
Elimination of FtsY Is Similar to Elimination of Ffh. We hypothesized that elimination of FtsY, the “docking protein” for Ffh, would result in a similar phenotype as elimination of Ffh itself. A single copy of ftsY was identified in the S. mutans genome with the B. subtilis and E. coli ftsY genes as queries. In S. mutans, ftsY appears to be part of a putative four-gene operon, where it is the most downstream gene (Fig. 4b). Analysis of the deduced amino acid sequences of orfs1-3 suggests that the first protein (276 aa) is a conserved hypothetical protein, whereas the second and third (273 aa and 494 aa) have significant levels of sequence similarity (47-81%) to conserved hypothetical proteins in the haloacid dehydrogenase-like family. S. mutans FtsY demonstrates high homology (44% identity, 65% similarity) with E. coli FtsY that consists of two distinct domains; the highly conserved NG domain containing the GTP binding site and the N-terminal acidic (A) domain (41). The A domain has been reported as essential for proper targeting of membrane proteins (42); however, more recent in vivo analyses showed that, in E. coli, the N-terminal A-domain was not required for SRP-mediated protein targeting and membrane assembly (43). In contrast, in the halophilic Archaea Haloferax volcanii, the full-length FtsY is essential for the association of Ffh protein with the cell membrane (44). Therefore, the mode of action and requirement of the A domain appears to differ among species.
Similar to the Δffh mutant, the viability of the S. mutans ΔftsY mutant AH307 was in contrast to what is found in E. coli and B. subtilis (45, 46). The Td of strain AH307 was increased compared to the wild-type NG8 (P < 0.001), and a longer lag phase (not shown) was observed when AH307 was cultured without stress. As with the ffh mutant strains, AH307 failed to grow when transferred to pH 5.0, or to 3.5% NaCl in either liquid (Table 1) or solid (data not shown) media. The Td of AH307 was not significantly different in the presence of 0.3 mM H2O2 in contrast to that of the ffh mutants, and temperature had no affect on cell growth (data not shown). To establish further the dispensability of the more complete SRP pathway, the double mutant strain AH351 (ΔffhΔftsY) was generated. AH351 was viable under nonstress conditions on THYE agar (pH 7.0) and behaved in a similar fashion to both AH307 and AH329 at pH 5.0 and in the presence of 3.5% NaCl, and was unaffected by temperature (data not shown). Like ffh single mutation strain AH329, strain AH351 showed an increased Td (P < 0.05) in the presence of 0.3 mM H2O2 compared to nonstress conditions. These results show that simultaneous deletion of ffh and ftsY is not lethal and reiterates the importance of the SRP pathway in the organism's ability to overcome sudden acid or osmotic shock.
The organization of the ftsY-containing locus (Fig. 4b) suggested that ftsY and the three upstream orfs might be cotranscribed as a polycistronic message. Although not resembling known SRP-pathway components, it is possible that these orfs could encode accessory components for SRP-mediated translocation in S. mutans; therefore, two additional mutants, AH308 [Δ(orf1-orf2-orf3)] and AH325 [Δ(orf1-orf2-orf3-ftsY)], were constructed. Elimination of the orfs upstream of ftsY had no discernable effect on cell growth (Table 1). When the three upstream orfs and ftsY were both deleted in mutant AH325, the phenotype was similar to that obtained for the single ΔftsY mutant strain AH307. These results indicate that, within this putative operon, only the SRP receptor FtsY contributes to the observed mutant phenotype. To ensure that the behavior of AH307 resulted from the lack of functional FtsY, complementation was performed in trans. Introduction of the complementing plasmid, pDCerm-ftsY, into AH307 restored normal growth and tolerance to both acid and salt stress (Table 1). Introduction of the same plasmid into the wild-type had no effect on growth or stress tolerance (data not shown). We previously had complemented the insertionally inactivated ffh mutant MK4 and restored its growth phenotype (16).
Elimination of SRP-Associated scRNA Is Not Lethal. Null mutations of the scRNA component of the SRP are lethal in both E. coli and B. subtilis (47, 48). During protein synthesis, the scRNA is bound to Ffh to form the functional SRP core complex that recognizes signal peptide sequences on nascent polypeptide chains and interacts with FtsY (6, 49). The essential interaction of the scRNA with Ffh occurs in the moiety that includes domain IV of the scRNA (50). It has also been reported that, in E. coli, the scRNA is involved in protein synthesis through direct interaction with elongation factor G (51). This additional function suggests that the scRNA would be required for cell viability as well as survival under stress conditions.
The predicted 73-bp scRNA gene of S. mutans is listed in the Signal Recognition Particle Database (SRPDB, http://psyche.uthct.edu/dbs/SRPDB/SRPDB.html; ref. 52). Analysis of the sequence shows a conserved domain (domain IV) predicted to result in secondary structure similar to the E. coli and B. subtilis scRNAs (Fig. 5, which is published as supporting information on the PNAS web site). Recent crystal structure of the E. coli 4.5S scRNA bound to the M-domain of Ffh (53) showed that the Ffh M-domain interacts primarily with the internal symmetric loop (loop S). This interaction results in induction of structural fit of the asymmetric loop (loop A). The predicted secondary structure of the S. mutans scRNA contains both of these elements. In addition, conserved RNA sequences (CAGG)/(AGCA) within the S loop, as well as GGAA within the tetraloop that caps domain IV (loop T), are present in the S. mutans sequence. In B. subtilis, it has been proposed that binding of the HBsu protein to the Alu domain of the 7S scRNA causes translational arrest (54); however, this may not be the case in S. mutans. The predicted S. mutans scRNA does not contain an Alu domain, despite identification of a gene (SMU.589) that encodes a 91-aa protein in which the N-terminal 29 residues share 64% identity with B. subtilis HBsu.
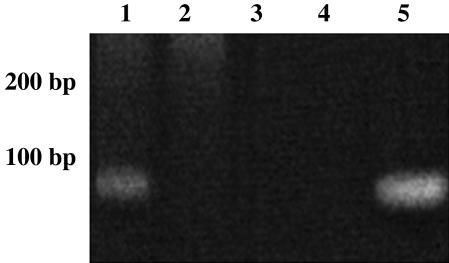
To better understand the physiological role of the S. mutans SRP scRNA, the deletion mutation strain AH312 was generated. In the absence of environmental stress, the growth rate (Table 1) and cell yield (data not shown) of AH312 were reduced, and a longer lag phase was observed compared to the wild-type. Growth rate and cell yield were severely reduced upon challenge with acid or high-salt conditions. RT-PCR confirmed the existence of the scRNA species as part of the total RNA pool in S. mutans, and that its expression was eliminated during the mutant construction. The expected 73-bp amplicon was present in the sample derived from NG8 (Fig. 1, lane 1) but absent from strain AH312 (Fig. 1, lane 2). Total RNA from each strain was used as the negative DNA control template (Fig. 1, lanes 3 and 4), and wild-type S. mutans NG8 genomic DNA was used as the positive control template (Fig. 1, lane 5).
Fig. 1.
RT-PCR analysis of scRNA in wild-type NG8 and S. mutans mutant AH312. Lanes 1, NG8 cDNA; 2, AH312 cDNA; 3, NG8 total RNA; 4, AH312 total RNA; and 5, NG8 genomic DNA.
To further confirm that the SRP itself is dispensable in S. mutans the double mutant strain AH341 (ΔffhΔscRNA) was constructed. AH341 was viable and displayed a similar phenotype as other SRP-pathway mutants under conditions of both acid and osmotic stress. In addition, AH341 was similar to mutant strains AH329 (Δffh) and AH351 (ΔffhΔftsY) in that it had a Td that was increased relative to the wild-type in the presence of 0.3 mM H202 (Table 1). This finding suggests that, although multiple components of the SRP pathway contribute to the ability of the organism to survive low pH or high-salt conditions, resistance to oxidative stress appears to be a function unique to Ffh. Again, the viability of ΔscRNA mutant strains AH312 and AH341 was surprising and demonstrates that the S. mutans scRNA cannot be an essential component of the protein synthesis machinery as has been reported in E. coli (51, 55).
H+/ATPase Activity in Wild-Type and Mutant Strains With and Without Acid Stress. Acid tolerance in S. mutans is mediated in large part by an F1F0-ATPase proton pump (56). The activity of the membrane-bound proton translocating F-ATPase is critical for establishing and maintaining a pH gradient across the cytoplasmic membrane. Expression and activity levels of H+/ATPase increase during acid stress as a protective mechanism to maintain pH homeostasis (56, 57). The S. mutans ATPase complex demonstrates substantial homology to the E. coli ATP synthase (58). The E. coli F1F0 ATP synthase complex consists of a membrane-integral F0 subunit (protein components a, b, and c) and a membrane-extrinsic F1 subunit (protein components α, β, γ, δ, and ε). The SRP and Sec translocase has been reported to mediate insertion of F0 a and b components into the membrane (59), and biochemical evidence has indicated a role for YidC in membrane insertion of the F0 subunit components a and c (60, 61). ATP synthase activity is also drastically decreased in the absence of mitochondrial Oxa1 (32). Because mutation of the SRP components resulted in acid sensitivity in S. mutans, it is possible that the inability of these mutants to contend with acid shock results from reduced integration of components of the F0 subunit into the membrane. Reduced levels of the ATPase β subunit were detected in trifluoroethanol-extracted protoplast-derived membrane fractions prepared from mutant strains lacking Ffh, scRNA, or FtsY compared to the wild-type when analyzed by two-dimensional electrophoresis (data not shown) as described (62).
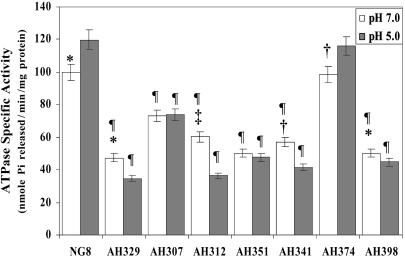
ATPase activity of membrane fractions from S. mutans mutant strains was assayed from cells cultured at pH 7.0 or pH 5.0, and levels of ATPase activity compared to the wild-type (Fig. 2). The ATPase specific activities from membrane fractions of all SRP-pathway mutant strains grown and maintained at pH 7.0 were reduced significantly, with a similar or even greater reduction observed after acid shock. As expected, the enzymatic activity of membrane fractions from acid-shocked (pH 5.0) wild-type cells was significantly increased relative to the pH 7.0-maintained culture and reflects the ability of S. mutans to rapidly adapt to this environmental challenge; this was not the case for the mutant strains in which the specific activities remained unchanged or decreased significantly in response to acid-shock. Expression of the enzymatically active F1 component was not likely affected by the ffh mutation because we showed that ATPase activity from whole decryptified cells was identical in chemostat-grown wild-type and ffh-mutant MK4 cultures (39). However, differences in activities of membrane fractions were similar to those shown here. Taken together, these results indicate that, although an enzymatically active proton ATPase can be incorporated into S. mutans membranes in the absence of a functional SRP pathway, the efficiency of the process is impaired, particularly under stress conditions, and suggest that multiple pathways are involved in membrane incorporation of ATPase subunits to maintain pH homeostasis in this highly aciduric organism.
Fig. 2.
H+/ATPase-specific activities in membrane fractions from S. mutans wild-type and SRP and YidC mutant strains grown under nonstress or pH 5.0 acid shock conditions. Statistical significance (Student's t test) compared to the pH 5.0 value for the same strain is indicated: *, P < 0.05; †, P < 0.005; ‡, P < 0.0001. Statistical significance (Student's t test) compared to wild-type at the same pH is also indicated: §, P < 0.005; ¶, P < 0.0001.
Comparison of S. mutans Homologs With Known Secretion Machinery Components; Potential Functional Overlap of the SRP Pathway with YidC2. Much of what is known about membrane biogenesis and protein secretion in Eubacteria has been gleaned from studies in E. coli and B. subtilis. That S. mutans can survive extensive genetic disruption of the SRP cotranslational protein translocation pathway indicates that important biological differences must exist between it and more widely studied organisms. The S. mutans genome contains single copies of genes encoding the SecA ATPase and the SecY, SecE, and SecG translocase components; however, no homologs encoding SecD or SecF are apparent. As expected for a Gram-positive organism, no homologs of SecB are present, nor is a reported functional substitute of SecB, CsaA, from B. subtilis (63). A single gene encoding a homolog of YajC was identified, although it is present in S. mutans in the absence of genes encoding SecD and SecF, which together with YajC comprise an operon in E. coli (22).
The genomes of S. mutans and other streptococcal species harbor two genes encoding YidC/Oxa-family homologs. They are referred to in this report as yidC1 (SMU.337) and yidC2 (SMU.1727) and are assigned by blast analysis as homologs of oxa1 and oxa2, respectively. However, as is the case between E. coli YidC and mitochondrial Oxa1 and Oxa2, the overall level of primary sequence identity between the S. mutans YidC homologs and other known YidC/Oxa1/Oxa2 proteins is low (<30%). Despite limited homology, complementation experiments indicate a degree of conserved function between the bacterial and mitochondrial proteins (30, 64), but a correlation between S. mutans YidC1 and YidC2 compared to YidC/Oxa1/Oxa2 function remains to be determined. Real-time PCR indicates that both yidC1 and yidC2 are expressed in S. mutans (data not shown). B. subtilis also harbors two YidC/Oxa-family homologs, designated SpoIIIJ and YqjG (65). SpoIIIJ, but not YqjG, is required for sporulation. Both are reportedly involved in membrane protein biogenesis and protein secretion and their simultaneous presence is required for survival.
Recent evidence suggests a previously unrecognized level of versatility of inner membrane protein biogenesis in E. coli in that its single YidC is involved in both Sec-translocase-dependent and -independent assembly (66). Mitochondria of higher eukaryotes lack the Sec machinery and an SRP pathway (67), but integrate hydrophobic proteins, including F0F1-ATPase subunits using Oxa1. Therefore, it is possible that one of the YidC homologs of S. mutans can function in cotranslational insertion of integral membrane proteins and may serve to compensate for a lack of SRP components in this organism. To evaluate this question, genes encoding YidC1 and YidC2 were deleted singly and from mutant strains lacking integral SRP components. Elimination of yidC1 did not result in a discernible phenotype compared to the wild-type under nonstress or acid, salt or oxidative stress conditions (Table 1). In addition, superimposing a yidC1 deletion onto deletions of genes encoding SRP pathway components did not result in any additional effects beyond those observed in the SRP mutants alone. On the other hand, elimination of yidC2 resulted in a stress-sensitive phenotype and increased doubling time under nonstress conditions comparable to SRP pathway mutants. As expected, similar to the SRP mutant strains, the ATPase-specific activities from the membrane fractions of the acid-sensitive yidC2 mutant strain grown and maintained at pH 7.0 and 5.0 were significantly reduced compared to the wild type (Fig. 2). This was not the case for the stress tolerant yidC1 mutant strain, in which ATPase levels were comparable to the wild-type. Growth of S. mutans was profoundly diminished in the absence of genes encoding both YidC2 and SRP pathway components even without stress. After transformation, colonies did not appear before 3 days, and when subcultured into broth, growth was not apparent for at least 48 h. Simultaneous deletion of yidC1 and yidC2 has not been achieved to date and may be lethal, although this conclusion will require evaluation using a conditional-lethal system.
Although compromised with respect to stress tolerance, the finding that S. mutans survives simultaneous elimination of multiple key components of the SRP pathway highlights the observation that at present there are “more questions than answers” in the membrane protein assembly field (reviewed in ref. 22). YidC2 also plays a role in the ability of S. mutans to contend with rapid environmental challenge and appears to function in concert with the SRP pathway in a global stress response in this organism. Overlapping and compensatory pathways clearly enable microorganisms to adapt and maintain membrane function in the face of fluctuating environmental conditions.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Dental and Craniofacial Research Grants R01 DE008007 (to L.J.B.) and R01 DE013230 (to D.G.C.), a Canada Research Chair grant (to D.G.C.), and Canadian Institutes of Health Research Grant MT-15431 (to D.G.C.).
Conflict of interest statement: No conflicts declared.
Abbreviations: SRP, signal recognition particle; scRNA, small cytoplasmic RNA; SR, SRP receptor; Td, doubling time.
References
- 1.Nagai, K., Oubridge, C., Kuglstatter, A., Menichelli, E., Isel, C. & Jovine, L. (2003) EMBO J. 22, 3479-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keenan, R. J., Freymann, D. M., Stroud, R. M. & Walter, P. (2001) Annu. Rev. Biochem. 70, 755-775. [DOI] [PubMed] [Google Scholar]
- 3.Phillips, G. J. & Silhavy, T. J. (1992) Nature 359, 744-746. [DOI] [PubMed] [Google Scholar]
- 4.Koch, H. G., Moser, M. & Muller, M. (2003) Rev. Physiol. Biochem. Pharmacol. 146, 55-94. [DOI] [PubMed] [Google Scholar]
- 5.Cao, T. B. & Saier, M. H., Jr. (2003) Biochim. Biophys. Acta 1609, 115-125. [DOI] [PubMed] [Google Scholar]
- 6.Herskovits, A. A., Bochkareva, E. S. & Bibi, E. (2000) Mol. Microbiol. 38, 927-939. [DOI] [PubMed] [Google Scholar]
- 7.Hann, B. C., Poritz, M. A. & Walter, P. (1989) J. Cell Biol. 109, 3223-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutka, S. C. & Walter, P. (2001) Mol. Biol. Cell 12, 577-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luirink, J., ten Hagen-Jongman, C. M., van der Weijden, C. C., Oudega, B., High, S., Dobberstein, B. & Kusters, R. (1994) EMBO J. 13, 2289-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loesche, W. J. (1986) Microbiol. Rev. 50, 353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banas, J. A. (2004) Front. Biosci. 9, 1267-1277. [DOI] [PubMed] [Google Scholar]
- 12.Svensater, G., Sjogreen, B. & Hamilton, I. R. (2000) Microbiology 146, 107-117. [DOI] [PubMed] [Google Scholar]
- 13.Quivey, R. G., Kuhnert, W. L. & Hahn, K. (2001) Crit. Rev. Oral. Biol. Med. 12, 301-314. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez, J. A., Crowley, P. J., Brown, D. P., Hillman, J. D., Youngman, P. & Bleiweis, A. S. (1996) J. Bacteriol. 178, 4166-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez, J. A., Crowley, P. J., Cvitkovitch, D. G., Brady, L. J., Hamilton, I. R., Hillman, J. D. & Bleiweis, A. S. (1999) Microbiology 145, 357-366. [DOI] [PubMed] [Google Scholar]
- 16.Kremer, B. H., van der Kraan, M., Crowley, P. J., Hamilton, I. R., Brady, L. J. & Bleiweis, A. S. (2001) J. Bacteriol. 183, 2543-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda, K., Nakamura, K., Nishiguchi, M. & Yamane, K. (1993) J. Bacteriol. 175, 4885-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buskiewicz, I., Deuerling, E., Gu, S. Q., Jockel, J., Rodnina, M. V., Bukau, B. & Wintermeyer, W. (2004) Proc. Natl. Acad. Sci. USA 101, 7902-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valent, Q. A., de Gier, J. W., von Heijne, G., Kendall, D. A., ten Hagen-Jongman, C. M., Oudega, B. & Luirink, J. (1997) Mol. Microbiol. 25, 53-64. [DOI] [PubMed] [Google Scholar]
- 20.Walter, P. & Johnson, A. E. (1994) Annu. Rev. Cell. Biol. 10, 87-119. [DOI] [PubMed] [Google Scholar]
- 21.Connolly, T., Rapiejko, P. J. & Gilmore, R. (1991) Science 252, 1171-1173. [DOI] [PubMed] [Google Scholar]
- 22.Dalbey, R. E. & Chen, M. (2004) Biochim. Biophys. Acta 1694, 37-53. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn, A., Stuart, R., Henry, R. & Dalbey, R. E. (2003) Trends Cell. Biol. 13, 510-516. [DOI] [PubMed] [Google Scholar]
- 24.Houben, E. N., ten Hagen-Jongman, C. M., Brunner, J., Oudega, B. & Luirink, J. (2004) EMBO Rep. 5, 970-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalbey, R. E. & Kuhn, A. (2004) J. Cell. Biol. 166, 769-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugsley, A. P., Francetic, O., Driessen, A. J. & de Lorenzo, V. (2004) Mol. Microbiol. 52, 3-11. [DOI] [PubMed] [Google Scholar]
- 27.Samuelson, J. C., Chen, M., Jiang, F., Moller, I., Wiedmann, M., Kuhn, A., Phillips, G. J. & Dalbey, R. E. (2000) Nature 406, 637-641. [DOI] [PubMed] [Google Scholar]
- 28.Samuelson, J. C., Jiang, F., Yi, L., Chen, M., de Gier, J. W., Kuhn, A. & Dalbey, R. E. (2001) J. Biol. Chem. 276, 34847-34852. [DOI] [PubMed] [Google Scholar]
- 29.Funes, S., Gerdes, L., Inaba, M., Soll, J. & Herrmann, J. M. (2004) FEBS Lett. 569, 89-93. [DOI] [PubMed] [Google Scholar]
- 30.Preuss, M., Ott, M., Funes, S., Luirink, J. & Herrmann, J. M. (2005) J. Biol. Chem. 280, 13004-13011. [DOI] [PubMed] [Google Scholar]
- 31.Jia, L., Dienhart, M., Schramp, M., McCauley, M., Hell, K. & Stuart, R. A. (2003) EMBO J. 22, 6438-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altamura, N., Capitanio, N., Bonnefoy, N., Papa, S. & Dujardin, G. (1996) FEBS Lett. 382, 111-115. [DOI] [PubMed] [Google Scholar]
- 33.Knox, K. W., Hardy, L. N. & Wicken, A. J. (1986) J. Gen. Microbiol. 132, 2541-2548. [DOI] [PubMed] [Google Scholar]
- 34.Lau, P. C., Sung, C. K., Lee, J. H., Morrison, D. A. & Cvitkovitch, D. G. (2002) J. Microbiol. Methods 49, 193-205. [DOI] [PubMed] [Google Scholar]
- 35.Macrina, F. L., Tobian, J. A., Jones, K. R., Evans, R. P. & Clewell, D. B. (1982) Gene 19, 345-353. [DOI] [PubMed] [Google Scholar]
- 36.Perry, D. & Kuramitsu, H. K. (1981) Infect. Immun. 32, 1295-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeng, A., Sakota, V., Li, Z., Datta, V., Beall, B. & Nizet, V. (2003) J. Bacteriol. 185, 1208-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalichi, P., Cvitkovitch, D. G. & Santerre, J. P. (2004) Biomaterials 25, 5467-5472. [DOI] [PubMed] [Google Scholar]
- 39.Crowley, P. J., Svensater, G., Snoep, J. L., Bleiweis, A. S. & Brady, L. J. (2004) FEMS Microbiol. Lett. 234, 315-324. [DOI] [PubMed] [Google Scholar]
- 40.Ajdic, D., McShan, W. M., McLaughlin, R. E., Savic, G., Chang, J., Carson, M. B., Primeaux, C., Tian, R., Kenton, S., Jia, H., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Leeuw, E., Poland, D., Mol, O., Sinning, I., ten Hagen-Jongman, C. M., Oudega, B. & Luirink, J. (1997) FEBS Lett. 416, 225-229. [DOI] [PubMed] [Google Scholar]
- 42.Young, J. C., Ursini, J., Legate, K. R., Miller, J. D., Walter, P. & Andrews, D. W. (1995) J. Biol. Chem. 270, 15650-15657. [DOI] [PubMed] [Google Scholar]
- 43.Eitan, A. & Bibi, E. (2004) J. Bacteriol. 186, 2492-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lichi, T., Ring, G. & Eichler, J. (2004) Eur. J. Biochem. 271, 1382-1390. [DOI] [PubMed] [Google Scholar]
- 45.Powers, T. & Walter, P. (1997) EMBO J. 16, 4880-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zelazny, A., Seluanov, A., Cooper, A. & Bibi, E. (1997) Proc. Natl. Acad. Sci. USA 94, 6025-6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishiguchi, M., Honda, K., Amikura, R., Nakamura, K. & Yamane, K. (1994) J. Bacteriol. 176, 157-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macfarlane, J. & Muller, M. (1995) Eur. J. Biochem. 233, 766-771. [DOI] [PubMed] [Google Scholar]
- 49.Peluso, P., Herschlag, D., Nock, S., Freymann, D. M., Johnson, A. E. & Walter, P. (2000) Science 288, 1640-1643. [DOI] [PubMed] [Google Scholar]
- 50.Zheng, N. & Gierasch, L. M. (1997) Mol. Cell 1, 79-87. [DOI] [PubMed] [Google Scholar]
- 51.Brown, S. (1987) Cell 49, 825-833. [DOI] [PubMed] [Google Scholar]
- 52.Rosenblad, M. A., Gorodkin, J., Knudsen, B., Zwieb, C. & Samuelsson, T. (2003) Nucleic Acids Res. 31, 363-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Batey, R. T., Rambo, R. P., Lucast, L., Rha, B. & Doudna, J. A. (2000) Science 287, 1232-1239. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura, K., Yahagi, S., Yamazaki, T. & Yamane, K. (1999) J. Biol. Chem. 274, 13569-13576. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura, K., Fujii, Y., Shibata, T. & Yamane, K. (1999) Eur. J. Biochem. 259, 543-550. [DOI] [PubMed] [Google Scholar]
- 56.Bender, G. R., Sutton, S. V. & Marquis, R. E. (1986) Infect. Immun. 53, 331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quivey, R. G., Jr., Kuhnert, W. L. & Hahn, K. (2000) Adv. Microb. Physiol. 42, 239-274. [DOI] [PubMed] [Google Scholar]
- 58.Smith, A. J., Quivey, R. G., Jr., & Faustoferri, R. C. (1996) Gene 183, 87-96. [DOI] [PubMed] [Google Scholar]
- 59.Yi, L., Celebi, N., Chen, M. & Dalbey, R. E. (2004) J. Biol. Chem. 279, 39260-39267. [DOI] [PubMed] [Google Scholar]
- 60.Yi, L., Jiang, F., Chen, M., Cain, B., Bolhuis, A. & Dalbey, R. E. (2003) Biochemistry 42, 10537-10544. [DOI] [PubMed] [Google Scholar]
- 61.van Bloois, E., Jan Haan, G., de Gier, J. W., Oudega, B. & Luirink, J. (2004) FEBS. Lett. 576, 97-100. [DOI] [PubMed] [Google Scholar]
- 62.Zuobi-Hasona, K., Crowley, P. J., Hasona, A., Bleiweis, A. S. & Brady, L. J. (2005) Electrophoresis 26, 1200-1205. [DOI] [PubMed] [Google Scholar]
- 63.Muller, J., Walter, F., van Dijl, J. M. & Behnke, D. (1992) Mol. Gen. Genet. 235, 89-96. [DOI] [PubMed] [Google Scholar]
- 64.van Bloois, E., Nagamori, S., Koningstein, G., Ullers, R. S., Preuss, M., Oudega, B., Harms, N., Kaback, H. R., Herrmann, J. M. & Luirink, J. (2005) J. Biol. Chem. 280, 12996-13003. [DOI] [PubMed] [Google Scholar]
- 65.Tjalsma, H., Bron, S. & van Dijl, J. M. (2003) J. Biol. Chem. 278, 15622-15632. [DOI] [PubMed] [Google Scholar]
- 66.Froderberg, L., Houben, E., Samuelson, J. C., Chen, M., Park, S. K., Phillips, G. J., Dalbey, R., Luirink, J. & De Gier, J. W. (2003) Mol. Microbiol. 47, 1015-1027. [DOI] [PubMed] [Google Scholar]
- 67.Glick, B. S. & Von Heijne, G. (1996) Protein Sci. 5, 2651-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.