Abstract
Primates exhibit complex social and cognitive behavior in the wild. In the laboratory, however, the expression of their behavior is usually limited. A large body of literature shows that living in an enriched environment alters dendrites and synapses in the brains of adult rodents. To date, no studies have investigated the influence of living in a complex environment on brain structure in adult primates. We assessed dendritic architecture, dendritic spines, and synaptic proteins in adult marmosets housed in either a standard laboratory cage or in one of two differentially complex habitats. A month-long stay in either complex environment enhanced the length and complexity of the dendritic tree and increased dendritic spine density and synaptic protein levels in the hippocampus and prefrontal cortex. No differences were detected between the brains of marmosets living in the two differentially complex environments. Our results show that the structure of the adult primate brain remains highly sensitive even to modest levels of experiential complexity. For adult primates, living in standard laboratory housing may induce reversible dendritic spine and synapse decreases in brain regions important for cognition.
Keywords: dendritic spine, enriched environments, hippocampus, marmoset, prefrontal cortex
Experience can change the structure of the adult mammalian brain. A large body of evidence documents that exposing laboratory rodents to complex or “enriched” settings enhances multiple aspects of brain structure, including the size and weight of brain regions, the number and size of neurons and glia, the complexity of dendritic trees, and the number of synapses (e.g., refs. 1-7). Studies suggest that dendritic spines, a primary site of excitatory synapses, are particularly sensitive to experience. Indeed, dendritic spines and synapses in the rodent brain are enhanced by living in an enriched environment (e.g., refs. 8-15). More specific experiences, such as learning, physical exercise, and experimentally induced neural activity, have also been linked to changes in the number, shape, and size of dendritic spines and synapses in rodents (16-22). In this regard, experiences that alter dendritic spines and synapses also affect the NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subtypes of the glutamate receptor (23-27).
Primates are known for their complex social and cognitive functions, but most laboratory monkeys are housed under conditions that do not allow for the expression of the full repertoire of their behaviors. Although previous work has demonstrated structural changes in the developing primate brain in animals raised under conditions of social and sensory deprivation (28-30), no studies have investigated the effects of living in “enriched” environments on any aspect of brain structure or biochemistry in the adult primate.
To investigate this issue, we examined the brains of common marmosets (Callithrix jacchus), New World monkeys, raised under standard laboratory conditions and moved as adults to a new standard laboratory cage or to one of two differentially complex environments. A month-long stay in either complex environment increased dendritic spine density, dendritic length, and dendritic complexity of neurons in the hippocampus and the prefrontal cortex (PFC), and raised the expression levels of several synaptic proteins in the same areas. Dendritic architecture and spine and synaptic measures did not differ between monkeys living in the two environments of varying size and intricacy.
Materials and Methods
Animal Care and Treatment. Adult male and female marmosets, weighing 250-500 gm, 1.5-5.5 years old, were used for these studies. All of the animals were sexually mature and would be classified as young to middle-aged adults. Animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
At the start of the experiment, male-female pairs were assigned to move to either a new standard laboratory control cage (n = 10), a complex single cage (n = 10), or a complex double cage (n = 4). One pair of animals was housed in each cage. The distribution of ages for animals in different groups did not differ statistically, with the average age of 2.6, 3.4, and 3.7 years for animals in the control and complex single- and complex double-cage conditions, respectively.
Control animals lived in cages (29 × 30 × 32 inches) without enriching objects and received food in bowls. This cage size, the smallest used in the study, is almost double the minimum National Institutes of Health mandated standard for primates of this size. Animal pairs assigned to the complex single-cage condition lived in larger cages (48 × 30 × 66 inches) equipped with branches, straw nests, vegetation, and ≈15 unique objects, including some that support foraging, such as branches with holes filled with dried fruit and live worms. Animals in the complex double-cage condition lived in two large conjoined cages, each the size of a single complex cage, containing objects similar to those described above. Thus, animals in the complex double cage had access to twice the number (≈30) and twice the variety of objects relative to monkeys in complex single cages. To enhance the level of experiential novelty and complexity, objects in the complex environments were moved and rotated out every other day for the complex single cage and every day for the complex double cage.
After 1 mo, animals were injected with an overdose of sodium pentobarbital and transcardially perfused by using 4% paraformaldehyde with 1.5% (vol/vol) picric acid (Sigma-Aldrich).
Circulating Cortisol Measures. For a subset of animals, blood samples were drawn and centrifuged to collect plasma. Circulating cortisol was measured by using the solid-phase radio immunoassay system (1:10 dilution; Coat-A-Count, Diagnostic Products, Los Angeles).
Golgi Impregnation. For Golgi impregnation, 100-μm-thick unilateral coronal sections throughout the left hippocampus and PFC were cut in 3% potassium dichromate. The single-section Golgi impregnation procedure was used to process the tissue (31). All slides were coded before data collection for this and other analyses. Dendritic spines were counted on the secondary and tertiary dendrites of dentate gyrus (DG) granule cells, CA1 and PFC pyramidal cells, and striatal medium spiny neurons; for dendritic length and branching analyses, CA1 pyramidal cells (apical and basal dendritic trees) and PFC pyramidal neurons in layers II/III (basal dendritic trees) were examined (see Supporting Text, which is published as supporting information on the PNAS web site).
1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindocarbocyanine Perchlorate (DiI) Labeling. For a subset of animals, crystals of a carbocyanine dye DiI (Molecular Probes) were implanted in the corpus callosum at the level of the caudal PFC. This approach retrograde-labeled PFC pyramidal neurons in layers II/III of posterolateral cytoarchitectonic area FD (32). Dendritic spine analyses were carried out on secondary dendrites of the basal tree, as for Golgi impregnated tissue. This method of DiI insertion also anterograde-labeled varicose axons extending into the striatum; these axons may originate from the PFC (33). These axons were analyzed for the frequency of varicosities, defined as focal swellings showing at least a 50% increase in diameter relative to surrounding axon thickness (see Supporting Text).
Immunohistochemistry. For a subset of the animals, quantitative immunohistochemical analyses of staining intensity for synaptic and dendritic markers [GluR2, synaptophysin, spinophilin, NMDA-NR1, and microtubule-associated protein 2 (MAP-2)] were carried out in areas showing changes in dendritic spine density. A confocal microscope was used to conduct optical intensity analysis in the locations of dendrites that showed environment-based differences in dendritic architecture: stratum lacunosum moleculare and stratum radiatum of the CA1 region, stratum moleculare of the DG, and layer III of posterolateral cytoarchitectonic area FD of the PFC (32) (see Supporting Text).
Nissl Labeling. Forty-micrometer-thick sections throughout the granule cell layer (GCL) and PFC were stained for Nissl substance by using cresyl violet. Every 12th section throughout the right half of the entire DG and PFC, up to the appearance of the corpus callosum, was analyzed, including cytoarchitectonic areas FD, FC, and FF (32) (see Supporting Text).
Statistical Analyses. Two females in our study became pregnant during the experiment. No differences were observed between pregnant and nonpregnant females, so these animals were grouped together. Furthermore, no significant sex differences emerged in any analysis, so results were collapsed across this variable. Data were analyzed with one-way ANOVA, followed by Tukey HSD post hoc tests with Kramer correction for unequal sample sizes. Age dependence was examined by using Pearson's R statistic and, for the measures showing significant age correlation, results were confirmed by using analysis of covariance with age as a covariate.
Results
Marmosets living in complex housing readily interacted with the objects in their environments and foraged for food (Fig. 1A). Monkeys in all groups engaged in affiliative behavior and mating; no evidence of fighting among cage mates was observed. Body weight and circulating levels of cortisol did not differ in marmosets living in the three types of housing, suggesting no major difference in stress level across the environments [Table 2, which is published as supporting information on the PNAS web site; body weight, F(2,21) = 0.374, P = 0.6925; plasma cortisol, F(2,14) = 0.5977, P = 0.5635].
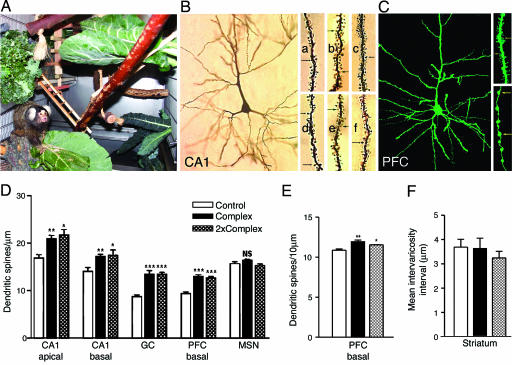
Fig. 1.
Environmental complexity enhances dendritic spine density in the adult marmoset brain. (A) Photograph of a marmoset in a complex environment, representing ≈40% of a complex single cage, with branches, vegetation, and objects typically included in the complex environment: a straw nest, a tree stump with holes, wooden swings, a wooden ladder, and blocks. (B) Photomicrograph of a Golgi impregnated CA1 pyramidal neuron, with close-up views of representative CA1 apical (a-c) and basal (d-f) dendrites, from animals in control (a and d), and complex single (b and e) and complex double cages (c and f). Arrows point to spines. (C) Photomicrograph of a DiI-labeled PFC pyramidal neuron (green color assigned for illustration purposes), with close-up views of a representative basal dendritic segment (Upper Right) and a cortico-striatal axonal segment (Bottom Right). Arrows point to spines and varicosities, respectively. (D) Marmosets living in complex environments for 4 weeks have greater dendritic spine density on several types of Golgi-impregnated neurons in the hippocampus and the PFC, compared with marmosets living in standard laboratory environments. Error bars represent SEM; asterisks reflect statistically significant differences from control group on Tukey post hoc comparison: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (E) Marmosets living in complex environments have greater dendritic spine density on DiI-labeled neurons compared with animals living in standard laboratory conditions. (F) No differences in intervaricosity spacing on cortico-striatal axons were observed for marmosets living in standard and two types of complex housing.
Environmental Complexity Enhances Dendritic Spine Density in Hippocampus and PFC, but Not Striatum. We examined dendritic spine density on Golgi impregnated hippocampal CA1 pyramidal neurons, DG granule cells, PFC pyramidal cells in layers II/III, and medium spiny neurons in the striatum (Fig. 1 B and D). Compared with animals housed in standard conditions, those in complex housing had greater dendritic spine density on all cell types analyzed, with the exception of medium spiny neurons of the striatum [CA1 pyramidal, apical, F(2,18) = 9.547, P = 0.0015; CA1 pyramidal, basal, F(2,18) = 7.394, P = 0.0045; DG granule, F(2,19) = 21.61, P < 0.0001; PFC pyramidal, basal, F(2,14) = 31.13, P < 0.0001; medium spiny striatal neuron, F(2,18) = 1.99, P = 0.1657]. No differences were observed between animals in complex single and complex double cages (Tukey post hoc comparison after one-way ANOVA, P(Complex/2×Complex) > 0.05 in all cases).
To explore dendritic spines with a different technique, we used confocal microscopy to examine dendritic spine density on pyramidal neurons in the PFC, labeled with the lipophilic tracer DiI. Again, dendritic spine density on layer II/III pyramidal cells was greater in the marmosets living in complex housing (Fig. 1 C and E), whereas no differences were noted between animals living in the complex single and double cages [F(2,9) = 12.63, P = 0.0024; P(Complex/2×Complex) > 0.05]. Finally, no differences in dendritic spine neck length or spine head diameter were observed between animals living in different environments [Table 3, which is published as supporting information on the PNAS web site; F(2,9) = 1.601, P = 0.2543; F(2.9) = 1.795, P = 0.2208].
Although the same general relationship was observed for spine density distribution in the three groups by using the different techniques, the relative differences in dendritic spine density between control and complex housed animals were visibly smaller in the DiI-labeled than in the Golgi-impregnated neurons. Although the reason for this difference is not known, it is likely that the two methods labeled different subpopulations of pyramidal neurons. The DiI-labeled cells examined in this study were pyramidal neurons with axons in a specific part of the corpus callosum (the area where DiI crystals were implanted), whereas Golgi impregnated cells were distributed in an apparently random manner. The relatively smaller experience-based enhancement in dendritic spine density on DiI-labeled cells may reflect differential responsiveness to environmental complexity for a subpopulation of pyramidal neurons.
Age did not contribute to the observed environment-induced differences in dendritic spine density for both Golgi impregnated and DiI-labeled cells. Data were analyzed by using Pearson's correlation and for the measures that were significantly correlated with age (dendritic spine density on apical and basal dendrites in the CA1 region), and analysis of covariance was carried out with age as the covariate. We found no significant contribution of age to the enriched environment effects [Golgi impregnated cells, CA1 apical dendritic spine density, Page(ANCOVA) = 0.151; CA1 basal dendritic spine density Page(ANCOVA) = 0.135) (ANCOVA, analysis of covariance)].
Environmental Complexity Enhances Dendritic Length and Branching in Hippocampus and PFC. Next, we examined dendritic length and branching on CA1 pyramidal cells (apical and basal dendritic trees) and on PFC pyramidal neurons in layers II/III (basal dendritic trees). We found that living in a complex environment enhanced both dendritic length and branching for the cell types examined [Table 1; CA1 apical, length, F(2,18) = 14.3, P = 0.0002; branching, F(2,18) = 13.24, P = 0.0003; CA1 basal, length, F(2,18) = 8.216, P = 0.0029; branching, F(2,18) = 10.8, P = 0.0008; PFC basal, length, F(2,14) = 9.538; P = 0.0033; branching, F(2,14) = 13, P = 0.001]. As observed for dendritic spine density, dendritic length and branching in the hippocampus and PFC did not differ for animals living in the two types of complex settings.
Table 1. Living in complex environments enhances dendritic length and branching in the hippocampus and the prefrontal cortex of adult marmosets.
| CA1 apical dendritic length, μm | CA1 apical dendritic branching | CA1 basal dendritic length, μm | CA1 basal dendritic branching | PFC basal dendritic length, μm | PFC basal dendritic branching | |
|---|---|---|---|---|---|---|
| Control | 808.4 ± 37.6 | 11.6 ± 1.0 | 1,174.0 ± 110.3 | 15.7 ± 1.1 | 1,176.0 ± 71.8 | 18.1 ± 0.9 |
| Complex | 1,460.0 ± 116.3*** | 17.4 ± 0.9*** | 1,968.0 ± 156.2* | 21.6 ± 0.7** | 1,594.0 ± 73.6** | 21.5 ± 0.8* |
| 2×Complex | 1,377.0 ± 192.4* | 18.8 ± 0.7** | 1,806.0 ± 325.9* | 22.4 ± 2.8** | 1,767.0 ± 70.2*** | 23.4 ± 0.4** |
Dendritic length and branching for apical and basal trees of CA1 pyramidal cells and basal trees of layer II/III PFC pyramidal cells. Asterisks reflect significant differences from controls on Tukey post hoc comparisons after one-way ANOVA, *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Environmental Complexity Does Not Influence Axonal Varicosities in the Striatum. Next, we examined varicosity spacing on axons in the striatum, where no enhancement in dendritic spine density was observed. Varicosities reflect en passant synapses on unmyelinated or thinly myelinated axons, and there is some evidence that their density and distribution can be affected by experience (34). In the striatum, each axonal varicosity usually holds one functional synapse, so the two parameters tend to covary (35). Intervaricosity spacing on striatal axons did not differ among marmosets living in three differentially complex environments [Fig. 1 F, F(2,9) = 0.5151, P = 0.614], suggesting that the effect of environmental complexity on adult primate brain structure may be region-specific.
Environmental Complexity Enhances the Expression of Spine and Synapse-Related Proteins. To further characterize the dendritic spine changes observed with living in a complex environment, we examined the levels of several proteins concentrated at the synapse: GluR2, an AMPA receptor subunit; NR1, an NMDA receptor subunit; spinophilin, a dendritic spine marker; and synaptophysin, a protein involved in synaptic vesicle trafficking (36). MAP-2, important for dendritic structure, was also analyzed. Optical intensity levels for these markers were examined by using confocal microscopy in the locations of dendrites that showed environment-induced changes in dendritic spine density, CA1 stratum lacunosum moleculare and stratum radiatum, DG stratum moleculare, and layer III of posterolateral cytoarchitectonic area FD (32). Living in a complex environment induced region-specific increases in the levels of GluR2, synaptophysin, and spinophilin, but not NMDA-NR1 and MAP-2 (Fig. 2 and Table 4, which is published as supporting information on the PNAS web site). (GluR2, CA1, F(2,9) = 13.26, P = 0.0021; DG, F(2,11) = 3.438, P = 0.0692; PFC, F(2,11) = 9.384, P = 0.0042; synaptophysin, CA1, F(2,11) = 7.158, P = 0.0102; DG, F(2,11) = 5.729, P = 0.0197; PFC, F(2,10) = 18.22, P = 0.0005; spinophilin, CA1, F(2,11) = 7.255, P = 0.0113; DG, F(2,11) = 4.035, P = 0.0457; PFC, F(2,11) = 0.8633; P = 0.4485; MAP-2, CA1, F(2,11) = 0.07843, P = 0.9251; DG, F(2,11) = 0.08984, P = 0.9147; PFC, F(2,11) = 0.1883, P = 0.8310; NMDA-NR1, CA1, F(2,11) = 2.004, P = 0.1811; DG, F(2,9) = 3.028, P = 0.0987; PFC, F(2,11) = 1.021, P = 0.3918.)
Fig. 2.
Environmental complexity enhances synaptic protein levels. (Left) Photomicrographs of hippocampal sections immunostained for spine and synapse-related proteins AMPA receptor subunit GluR2, synaptophysin, and spinophilin. (Right) Optical intensity index for GluR2 (Top), synaptophysin (Middle) and spinophilin (Bottom). Marmosets living in complex housing showed enhanced levels of GluR2 and synaptophysin in the hippocampus and the PFC, and of spinophilin in the hippocampus.
Within the CA1 region of the hippocampus, GluR2, synaptophysin, and spinophilin levels were increased by environmental complexity, and no differences were detected among animals in the two complex environment conditions. Of all the measures we examined, only spinophilin levels in the hippocampus exhibited an increase in the complex double-but not in the complex single-cage condition, compared with the standard cage. Yet even for this measure, the two groups of animals in the complex housing did not differ. In the PFC, GluR2 and synaptophysin levels were higher in the marmosets living in complex housing, compared with the animals living in the standard cage. Again, confirming the pattern observed in dendritic spine density alterations, levels of these proteins did not differ among marmosets living in the complex single and double cages.
No Effect of Environmental Complexity on Total Cell Number or Volume of the GCL and PFC. Finally, to determine whether environmental complexity, in addition to enhancing dendritic spine density and dendritic architecture, induces other larger-scale structural alterations in the adult primate brain, we compared the total number of neurons in the DG GCL and in a neuroanatomically defined portion of the PFC, cytoarchitectonic areas FD, FF, and FC (32) up to the appearance of the corpus callosum in marmosets living in the three types of environments. We found no differences in the total number of neurons or region volume among the groups [Table 5, which is published as supporting information on the PNAS web site; GCL cell number, F(2,21) = 1.083, P = 0.3569; GCL volume, F(2,21) = 0.2886, P = 0.7522; PFC cell number, F(2,21) = 0.1962, P = 0.8233; PFC volume, F(2,21) = 0.3646, P = 0.6988], suggesting that larger-scale neuroanatomical structure of the two brain regions, affected by complex experience at dendritic and possibly synaptic levels, remained the same.
Discussion
Here we have shown that environmental complexity influences the structure and biochemistry of adult nonhuman primate brains. Only 1 mo of living in a more complex environment increased dendritic spine density on DG granule cells, CA1 pyramidal cells, and PFC pyramidal cells. Because the enhancement in spine density was paralleled by increases in the length of dendrites, higher spine density implies an increase in the overall number of spines. Since dendritic spines are a primary site of excitatory synapses (37), the experience of living in a relatively complex environment probably increased the overall number of excitatory synapses in adult monkey brains, at least in the hippocampus and PFC.
In addition to increases in dendritic spines, we have detected increases in levels of the presynaptic protein synaptophysin in the DG, CA1, and PFC of marmosets in complex housing, also suggesting an increase in the number of synapses. The enhancement in levels of the AMPA receptor GluR2 in the hippocampus and PFC further supports the possibility of experience-induced formation of new excitatory synapses, although experiential stimulation may raise the number of spines, while also up-regulating the insertion of AMPA receptors into previously existing synapses. Likewise, changes in the levels of spinophilin and synaptophysin may be directly related to the formation of new synapses on new spines, or they may also reflect enhanced content of these proteins within preexisting synapses. The observations that the NMDA receptor subunit NR1 and MAP-2 were not altered by the environmental manipulations suggest that substantial global increases in the levels of all neuronal proteins did not occur.
Our findings in adult monkeys extend a vast literature on enriched environment living in the rodent. In the classical studies by Rosenzweig and colleagues, as well as those by other investigators (e.g., refs. 2, 4, 38-40), rats living in laboratory-enriched environments exhibited increases in a number of brain measures, including cortical thickness, number of glial cells, numbers of dendritic branch points, dendritic spines, and synapses. More recent studies have shown that living in enriched environments also enhances adult neurogenesis in the DG (41-43). Brain changes similar to those produced by enriched environments have been reported in rodents trained on various learning tasks (13, 20, 44, 45) or engaged in physical exercise (22, 46).
Together with our findings, this work indicates that both rodent and primate brains remain highly plastic and responsive to complex experiences in adulthood. Thus, it is clear that structural processes typically associated with brain development continue to operate in the adult brain on an ongoing basis. Although no previous studies have examined the effects of enriched environments on the anatomy of either the developing or adult primate brain, several studies have reported dramatic structural alterations in the brains of infant monkeys reared in deprived conditions. Impoverished conditions during development are known to impair cognitive abilities in rodents (40), primates (47, 48), and other mammals (49, 50). Riesen and colleagues (28, 30, 51) raised infant macaques until 6 mo of age under different levels of social, somatosensory, and motor deprivation and observed profound effects not only on their behavior but also in their brains, relative to controls. Floeter and Greenough (29) also reared infant monkeys for 6 mo with two levels of restricted social and motor experience and found behavioral pathology and decreases in the size and complexity of neurons in the cerebellum. These studies suggest that experiential deprivation during development diminishes several parameters in the primate brain.
In line with these developmental deprivation studies is the possibility that laboratory control primates are also living in deprived conditions relative to the wild. Thus, dendritic architecture, spines, and synapses may reversibly atrophy from disuse in monkeys living in standard laboratory housing, and enriched environment living restores these measures to a baseline closer to that of wild-living animals. It is possible that our laboratory control marmosets, although considerably more enriched than monkeys reared in the deprived conditions described above for the developmental studies, nevertheless remained deprived of the experiences that adult marmosets require to maintain “normal” brain structure. One reason for the apparent lack of significant differences between the brains of animals in our two complex environments may be that the brief time of the experiment (1 mo) was insufficient for subtle differences in brain measures to emerge. However, previous studies in rodents have shown that long-term living in enriched environments does not further increase enrichment effects, as measured by cortical thickness (5), dendritic branching or length (12), or numbers of synapses per neuron (15). Alternatively or in addition, our two complex environments may not have been sufficiently different. Even our more complex environment was far less intricate than natural habitats encountered in the wild. Dendritic spine and synapse differences between animals living in standard housing and those living in the wild may exceed the differences we report here, although some of the measures probably reach a maximum, after which they cannot increase further despite additional experience.
The connection between the degree of enrichment and brain measures has been examined in rodents. Data from the rodent “seminaturalistic” paradigm of Rosenzweig and colleagues (52, 53), as well as from the “superenriched” paradigm of Kuenzle and Knusel (54), in contrast to our results, suggest a somewhat graded effect of environmental complexity on some brain measures.
Altogether, our results show that dendritic spine density and, presumably, synapse number are enhanced with a brief stay in a moderately complex environment in adult marmosets. These observations attest to the plasticity and persistent responsiveness to experience of the adult primate brain. The extent to which the structural and biochemical enhancements we had observed influence behavior is yet unknown. An extensive literature links enriched environment living in developing and adult rodents with improved performance on a variety of learning tasks (e.g., refs. 4 and 55). Given the dramatic changes we had observed with a brief complex experience in the adult primate hippocampus and PFC, two brain regions important for cognition and presumably involved in foraging and navigating within complex environments, it seems likely that experiential complexity may have important behavioral consequences for adult marmosets.
Supplementary Material
Acknowledgments
We thank Mark Rosenzweig for his help, Kim Lee for technical assistance, and Benedetta Leuner and Jessica Salvatore for comments on the manuscript. This work was supported by grants from the National Institutes of Health (to E.G., C.G.G., and Y.K.).
Conflict of interest statement: No conflicts declared.
Abbreviations: DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DG, dentate gyrus; PFC, prefrontal cortex; MAP-2, microtubule-associated protein 2; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic; GCL, granule cell layer.
References
- 1.Rosenzweig, M. R., Krech, D., Bennett, E. L. & Diamond, M. C. (1962) J. Comp. Physiol. Psychol. 55, 429-437. [DOI] [PubMed] [Google Scholar]
- 2.Walsh, R. N. (1980) Int. J. Neurosci. 11, 77-89. [DOI] [PubMed] [Google Scholar]
- 3.Bhide, P. G. & Bedi, K. S. (1984) J. Anat. 138, 447-461. [PMC free article] [PubMed] [Google Scholar]
- 4.Renner, M. & Rosenzweig, M. (1987) Enriched and Impoverished Environments (Springer, New York).
- 5.Diamond, M. C. (1988) Enriching Heredity: The Impact of the Environment on the Anatomy of the Brain (Collier Macmillan, New York).
- 6.Diamond, M. C. (2001) An. Acad. Bras. Cienc. 73, 211-220. [DOI] [PubMed] [Google Scholar]
- 7.van Praag, H., Kempermann, G. & Gage, F. H. (2000) Nat. Rev. Neurosci. 1, 191-198. [DOI] [PubMed] [Google Scholar]
- 8.Holloway, R. L., Jr. (1966) Brain Res. 2, 393-396. [DOI] [PubMed] [Google Scholar]
- 9.Mollgaard, K., Diamond, M. C., Bennett, E. L., Rosenzweig, M. R. & Lindner, B. (1971) Int. J. Neurosci. 2, 113-127. [DOI] [PubMed] [Google Scholar]
- 10.Globus, A., Rosenzweig, M. R., Bennett, E. L. & Diamond, M. C. (1973) J. Comp. Physiol. Psychol. 82, 175-181. [DOI] [PubMed] [Google Scholar]
- 11.Turner, A. M. & Greenough, W. T. (1985) Brain Res. 329, 195-203. [DOI] [PubMed] [Google Scholar]
- 12.Camel, J. E., Withers, G. S. & Greenough, W. T. (1986) Behav. Neurosci. 100, 810-813. [DOI] [PubMed] [Google Scholar]
- 13.Moser, M. B., Trommald, M. & Andersen, P. (1994) Proc. Natl. Acad. Sci. USA 91, 12673-12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner, C. A., Lewis, M. H. & King, M. A. (2003) Dev. Psychobiol. 43, 20-27. [DOI] [PubMed] [Google Scholar]
- 15.Briones, T. L., Klintsova, A. Y. & Greenough, W. T. (2004) Brain Res. 1018, 130-135. [DOI] [PubMed] [Google Scholar]
- 16.Desmond, N. L. & Levy, W. B. (1986) J. Comp. Neurol. 253, 466-475. [DOI] [PubMed] [Google Scholar]
- 17.Trommald, M., Hulleberg, G. & Andersen, P. (1996) Learn. Mem. 3, 218-228. [DOI] [PubMed] [Google Scholar]
- 18.Kleim, J. A., Swain, R. A., Armstrong, K. A., Napper, R. M., Jones, T. A. & Greenough, W. T. (1998) Neurobiol. Learn. Mem. 69, 274-289. [DOI] [PubMed] [Google Scholar]
- 19.Yuste, R. & Bonhoeffer, T. (2001) Annu. Rev. Neurosci. 24, 1071-1089. [DOI] [PubMed] [Google Scholar]
- 20.Leuner, B., Falduto, J. & Shors, T. J. (2003) J. Neurosci. 23, 659-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou, Q., Homma, K. J. & Poo, M. M. (2004) Neuron 44, 749-757. [DOI] [PubMed] [Google Scholar]
- 22.Eadie, B. D., Redila, V. A. & Christie, B. R. (2005) J. Comp. Neurol. 486, 39-47. [DOI] [PubMed] [Google Scholar]
- 23.Morris, R. G., Davis, S. & Butcher, S. P. (1990) Philos. Trans. R. Soc. London B 329, 187-204. [DOI] [PubMed] [Google Scholar]
- 24.Heynen, A. J., Quinlan, E. M., Bae, D. C. & Bear, M. F. (2000) Neuron 28, 527-536. [DOI] [PubMed] [Google Scholar]
- 25.Adams, M. M., Smith, T. D., Moga, D., Gallagher, M., Wang, Y., Wolfe, B. B., Rapp, P. R. & Morrison, J. H. (2001) J. Comp. Neurol. 432, 230-243. [DOI] [PubMed] [Google Scholar]
- 26.Nagerl, U. V., Eberhorn, N., Cambridge, S. B. & Bonhoeffer, T. (2004) Neuron 44, 759-767. [DOI] [PubMed] [Google Scholar]
- 27.Naka, F., Narita, N., Okado, N. & Narita, M. (2005) Brain Dev. 27, 275-278. [DOI] [PubMed] [Google Scholar]
- 28.Struble, R. G. & Riesen, A. H. (1978) Dev. Psychobiol. 11, 479-486. [DOI] [PubMed] [Google Scholar]
- 29.Floeter, M. K. & Greenough, W. T. (1979) Science 206, 227-229. [DOI] [PubMed] [Google Scholar]
- 30.Bryan, G. K. & Riesen, A. H. (1989) J. Comp. Neurol. 286, 208-217. [DOI] [PubMed] [Google Scholar]
- 31.Gabbott, P. L. & Somogyi, J. (1984) J. Neurosci. Methods 11, 221-230. [DOI] [PubMed] [Google Scholar]
- 32.Peden, J. K. & von Bonin, G. (1974) J. Comp. Neurol. 86, 37-63. [DOI] [PubMed] [Google Scholar]
- 33.Fallon, J. H. & Ziegler, B. T. (1979) Neurosci. Lett. 15, 29-32. [DOI] [PubMed] [Google Scholar]
- 34.DeBello, W. M., Feldman, D. E. & Knudsen, E. I. (2001) J. Neurosci. 21, 3161-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kincaid, A. E., Zheng, T. & Wilson, C. J. (1998) J. Neurosci. 18, 4722-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiel, G. (1993) Brain Pathol. 3, 87-95. [DOI] [PubMed] [Google Scholar]
- 37.Sorra, K. E. & Harris, K. M. (2000) Hippocampus 10, 501-511. [DOI] [PubMed] [Google Scholar]
- 38.Bennett, E. L., Krech, D. & Rosenzweig, M. R. (1964) J. Comp. Physiol. Psychol. 57, 440-441. [DOI] [PubMed] [Google Scholar]
- 39.Greenough, W. T. (1976) in Neural Mechanisms of Learning and Memory, eds. Rosenzweig, M. R. & Bennett, E. L. (MIT Press, Cambridge, MA), pp. 255-278.
- 40.Rosenzweig, M. R. (2003) Dev. Neuropsychol. 24, 523-540. [DOI] [PubMed] [Google Scholar]
- 41.Kempermann, G., Kuhn, H. G. & Gage, F. H. (1997) Nature 386, 493-495. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson, M., Perfilieva, E., Johansson, U., Orwar, O. & Eriksson, P. S. (1999) J. Neurobiol. 39, 569-578. [DOI] [PubMed] [Google Scholar]
- 43.Kempermann, G., Gast, D. & Gage, F. H. (2002) Ann. Neurol. 52, 135-143. [DOI] [PubMed] [Google Scholar]
- 44.Greenough, W. T., Larson, J. R. & Withers, G. S. (1985) Behav. Neural Biol. 44, 301-314. [DOI] [PubMed] [Google Scholar]
- 45.Leuner, B., Mendolia-Loffredo, S., Kozorovitskiy, Y., Samburg, D., Gould, E. & Shors, T. J. (2004) J. Neurosci. 24, 7477-7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Praag, H., Christie, B. R., Sejnowski, T. J. & Gage, F. H. (1999) Proc. Natl. Acad. Sci. USA 96, 13427-13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sackett, G. P. (1972) Neurosci. Res. Prog. Bull. 10, 388-392. [PubMed] [Google Scholar]
- 48.Davenport, R. K., Rogers, C. M. & Rumbaugh, D. M. (1973) Dev. Psychol. 9, 343-347. [Google Scholar]
- 49.Wilson, M., Warren, J. M. & Abbott, L. (1965) Child Dev. 36, 843-853. [PubMed] [Google Scholar]
- 50.Fuller, J. L. & Clark, L. D. (1966) J. Comp. Physiol. Psychol. 61, 258-263. [DOI] [PubMed] [Google Scholar]
- 51.Riesen, A. H., Dickerson, G. P. & Struble, R. G. (1977) Ann. N.Y. Acad. Sci. 290, 285-294. [DOI] [PubMed] [Google Scholar]
- 52.Rosenzweig, M. R. & Bennett, E. L. (1972) J. Comp. Physiol. Psychol. 80, 304-313. [DOI] [PubMed] [Google Scholar]
- 53.Rosenzweig, M. R., Bennett, E. L., Hebert, M. & Morimoto, H. (1978) Brain Res. 153, 563-576. [DOI] [PubMed] [Google Scholar]
- 54.Kuenzle, C. C. & Knusel, A. (1974) Physiol. Behav. 13, 205-210. [DOI] [PubMed] [Google Scholar]
- 55.Rosenzweig, M. R. & Bennett, E. L. (1977) in Genetics, Environment and Intelligence, ed. Oliverio, A. (Elsevier, Amsterdam), pp. 163-196.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.