Abstract
Interleukin (IL)-2 is a type I four-α-helical bundle cytokine that plays vital roles in antigen-mediated proliferation of peripheral blood T cells and also is critical for activation-induced cell death. We now demonstrate that IL-2 potently decreases expression of IL-7 receptor α chain (IL-7Rα) mRNA and protein. The fact that IL-7Rα is a component of the receptors for both IL-7 and thymic stromal lymphopoietin (TSLP) suggests that IL-2 can negatively regulate signals by each of these cytokines. Previously it was known that the IL-2 and IL-7 receptors shared the common cytokine receptor γ chain, γc, which suggested a possible competition between these cytokines for a receptor component. Our findings now suggest a previously unknown type of cross-talk between IL-2 and IL-7 signaling by showing that IL-2 signaling can diminish IL-7Rα expression via a phosphatidylinositol 3-kinase/Akt-dependent mechanism.
Interleukin (IL)-2 is a member of a subfamily of type I cytokines including IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, all of which share the common cytokine receptor γ chain, γc, which is mutated in X-linked severe combined immunodeficiency (1, 2). IL-2 is produced exclusively by activated T cells and is the major T cell growth factor. Production of IL-2 is induced rapidly and potently after antigen presentation to resting T cells. The IL-2 receptor (IL-2R) comprises three polypeptide subunits: IL-2 receptor α chain (Rα), IL-2 receptor β chain (Rβ), and γc. IL-2Rβ and γc are critical for signal transduction, whereas IL-2Rα augments binding affinity but is not believed to contribute to IL-2 signaling (2). In addition to its vital roles in T cell proliferation and augmentation of the cytolytic activity of natural killer cells (2, 3), IL-2 is also important for the elimination of autoreactive T cells, as is indicated by the severe lymphoproliferation and autoimmune diseases in mice lacking IL-2, IL-2Rα, or IL-2Rβ (4–7).
Several major IL-2-signaling pathways have been described including the Janus tyrosine kinase (Jak)/signal transducer and activator of transcription (STAT), the Ras/mitogen-activated protein (MAP) kinase, and the phosphoinositol 3-kinase (PI3-kinase)/Akt/p70 S6 kinase pathways (8). IL-2 mediates the activation of Jak1 and Jak3, and these kinases in turn mediate the phosphorylation of IL-2Rβ and the activation of Stat3, Stat5a, and Stat5b. Stat5a and Stat5b are recruited to IL-2Rβ via phosphotyrosine docking sites at Tyr-392 and Tyr-510 (9, 10). IL-2 also induces the phosphorylation of Tyr-338 of IL-2Rβ, which allows the binding of Shc via its phosphotyrosine-binding domain (PTB), which in turn is believed to mediate activation of the Ras/MAP kinase (MAPK) pathway (9, 11). PI3-kinase can be recruited to the receptor as well, and both Jak1 and Src-family kinases have been suggested to be involved in its recruitment and activation (12, 13).
To study IL-2 signaling further, we sought to identify IL-2-regulated genes by using a microarray methodology. One of the genes regulated by IL-2 was the IL-7 receptor α chain (IL-7Rα). IL-7Rα mRNA and protein expression is diminished potently after stimulation with IL-2. We show that the decrease in IL-7Rα expression depends on activation of the PI3-kinase/Akt pathway. The down-regulation of IL-7Rα by IL-2 suggests an important cross-talk between IL-2- and IL-7Rα-dependent signaling.
Materials and Methods
Affymetrix GeneChip Analysis Using Human Peripheral Blood Mononuclear Cells.
Peripheral blood mononuclear cells were isolated from healthy volunteers by using density-gradient centrifugation (Ficoll), stimulated for 18 h with phytohemagglutinin (2 μg/ml), and then grown for 2 weeks in complete medium (RPMI medium 1640/10% FBS/100 units/ml penicillin and streptomycin/2 mM glutamine) supplemented with phytohemagglutinin (500 ng/ml) and human IL-2 (1 nM, Hoffmann–La Roche). The cells were evaluated by flow-cytometric analysis for CD3 and CD25 expression, and only samples with >95% T cells were studied. The cells were rested for 3 days by culturing without phytohemagglutinin and IL-2 and then treated or not treated with IL-2 (2 nM) for 4 h. mRNA was isolated by using the RNeasy and Oligotex mRNA midi kits (Qiagen, Valencia, CA). cRNA probes were generated as recommended (Affymetrix, Santa Clara, CA) and hybridized to U95A GeneChips (Affymetrix). GeneChips were washed and scanned (Hewlett–Packard, GeneArray scanner G2500A) as recommended (Affymetrix). Scanned files were analyzed for differences in gene expression by using MICROARRAY SUITE software (Affymetrix).
Isolation and Culture of Mouse Splenocytes.
Stat5a−/− and Stat5b−/− mice were provided by Lothar Hennighausen (National Institute of Diabetes and Digestive and Kidney Diseases) and Helen Davey (AgResearch, New Zealand), respectively. All experiments were performed under protocols approved by the National Institutes of Health Animal Use and Care Committee and followed the National Institutes of Health Guidelines “Using Animals in Intramural Research.” Splenocytes were isolated from 8–12-week-old wild-type C57BL/6 or Stat5a−/− or Stat5b−/− mice and suspended in complete medium containing 10 mM Hepes (pH 7.0). T cells were enriched by depleting adherent cells and cells expressing Fc receptors by panning in dishes coated with 100 μg/ml goat anti-mouse IgG. The resulting cells then were resuspended in complete medium containing 1 μg/ml anti-CD28 (PharMingen) and 1 nM IL-2 and activated by culturing for 2 days in dishes precoated with anti-CD3 (1 μg/ml in PBS, 37°C, 1.5 h). The cells were expanded in complete medium containing 1 nM IL-2 and 50 μM 2-mercaptoethanol. Where indicated, cells were rested for 12–16 h by culturing in the absence of IL-2 and then restimulated with IL-2. More than 70% of the cells from wild-type mice were viable at the end of the 12- to 16-h resting period.
To isolate CD4+ T cells, splenocytes were incubated with CD4 microbeads on ice and selected with MS magnetic columns (Miltenyi Biotec, Auburn, CA). These CD4+ T cells were activated and expanded as described above.
RNA Extraction and Northern Blotting.
Total RNA was extracted with Trizol reagent (Invitrogen) and was run on formaldehyde gels (20 μg per lane) and transferred onto nylon filters. The probes (an ≈900-bp EcoRI–StuI murine IL-7Rα cDNA fragment and an ≈300-bp PstI fragment from the pHe7 housekeeping gene cDNA) were labeled by Prime-It RmT Random primer-labeling kit (Stratagene) and [α-32P]dCTP. Signals were visualized by autoradiography and quantified by using a PhosphorImager and IMAGEQUANT software (Molecular Dynamics).
Constructs.
Dominant negative Stat5b (ΔStat5b) was made by inserting a stop codon after Tyr-754 (14) using the QuickChange site-directed mutagenesis kit (Stratagene). Both wild-type Stat5b and ΔStat5b as well as wild-type Akt/protein kinase B and its constitutively active form, myristylated Akt (myr-Akt, provided by Thomas F. Franke, Beth Israel Hospital, Harvard Medical School, Boston), were subcloned into pGFP-RV (a gift from Ken Murphy, Washington University, St. Louis).
Flow-Cytometric Analyses.
Splenocytes were stained and analyzed on a FACSort (Becton Dickinson) by using CELLQUEST software. Anti-IL-7Rα phycoerythrin (PC61), anti-CD4 Cy-chrome (L3T4), anti-CD8 allophycocyanin (Ly2), and isotype-matched control antibodies (PharMingen) were used.
Retroviral Transduction of Splenocytes.
Retroviruses were packaged in 293T cells by cotransfection of pGFP-RV constructs and pCLeco, the retroviral ecotropic packaging vector (a gift from Inder Verma, La Jolla, CA). For each infection, 2 ml of filtered retroviral supernatant was supplemented with 4 μg/ml polybrene and added to 106 splenic T cells. The cells were centrifuged at 1,000 × g at 30°C for 45 min. The supernatant was removed, and the cells were cultured in complete medium supplemented with 0.5 μg/ml anti-CD3, 1 μg/ml anti-CD28, and 1 nM IL-2. The retroviral infection was repeated 24 h later, and the cells were maintained in complete medium containing 1 nM IL-2 for 3–10 days before flow-cytometric analysis or RNA extraction.
Immunoprecipitation and Western Blotting.
Splenocytes were either not treated or treated for 30 min with wortmannin or PP2 or PP3 and then stimulated with IL-2 for 30 min. The cells were washed in ice-cold PBS, suspended in lysis buffer [50 mM Tris⋅HCl, pH 7.5/150 mM NaCl/0.5% Nonidet P-40/1 mM Na3VO4/10 μg/ml aprotinin/10 μg/ml leupeptin/1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride], and incubated on ice with occasional shaking for 45 min. Cell lysates were collected by centrifugation at 4°C for 15 min. One milligram of lysate protein was mixed with 1 μg of anti-Jak1 polyclonal antibody (Santa Cruz Biotechnology) and 20 μl of protein A Sepharose CL-4B (Amersham Pharmacia) at 4°C for 2 h. The immunoprecipitated protein was washed four times in lysis buffer and resolved by 8% SDS/PAGE. After transfer to poly(vinylidene difluoride) membranes, the immunoprecipitated Jak1 was detected with antiphosphotyrosine mAb PY99 (Santa Cruz Biotechnology). Lysate protein (20 μg) was also resolved by 8–16% SDS/PAGE and immunoblotted with antibodies to phospho-Akt (Ser-473) or Akt (Cell Signaling Technology, Beverly, MA).
To measure Akt activity, 293T cells were transfected by calcium phosphate precipitation with an expression vector encoding myr-Akt, and Akt activity was assayed by using a nonradioactive assay kit that measures glycogen synthase kinase (GSK)-3 phosphorylation (Cell Signaling Technology).
Results
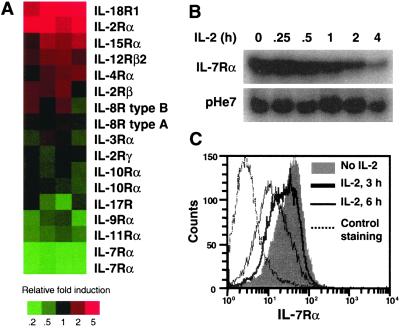
We used Affymetrix human GeneChip arrays containing oligonucleotides corresponding to ≈12,000 human DNA sequences and identified genes either induced or repressed by IL-2. A complete list of these genes will be presented elsewhere. Among cytokine receptors on the chip, IL-7Rα expression was the most potently repressed (≈4–5 fold; Fig. 1A). We confirmed the decrease in IL-7Rα mRNA (Fig. 1B). Correspondingly, IL-7Rα cell-surface expression declined after treatment with IL-2 (Fig. 1C).
Figure 1.
IL-2-induced decrease in IL-7Rα mRNA and protein. (A) Effect of IL-2 on the expression of cytokine receptors. cRNA probes derived from IL-2-stimulated peripheral blood mononuclear cells were hybridized to human U95A GeneChip arrays. Results from four independent experiments are shown according to relative induction (red) or repression (green, see scale). (B) Preactivated splenocytes were rested for 16 h and then stimulated with IL-2 for 0–4 h. RNA was isolated and Northern-blotted with probes for IL-7Rα or pHe7. (C) Splenocytes were rested and then stimulated with IL-2 for 0, 3, or 6 h. Flow-cytometric analysis then was performed by using an anti-IL-7Rα mAb.
To explore the mechanism by which IL-7Rα mRNA diminished after treatment with IL-2, we used two inhibitors of translation, cycloheximide and puromycin (Fig. 2). Whereas cycloheximide blocks the translocation reaction on ribosomes that is required for nascent chain elongation, puromycin promotes polysome dissociation. Down-regulation of IL-7Rα mRNA by IL-2 was inhibited by both cycloheximide and puromycin (Fig. 2A, compare lanes 4–7 with lanes 2 and 3, and Fig. 2B), suggesting that the IL-2-induced decrease in IL-7Rα mRNA at least in part depends on de novo protein synthesis. Pretreatment of cells with actinomycin D, an inhibitor of transcription that would be expected to block IL-7Rα transcription, resulted in diminished IL-7Rα expression at 4 h. Interestingly, it prevented the IL-2-induced decrease in IL-7Rα (Fig. 2A, lanes 10 and 11 vs. lanes 2 and 3, and Fig. 2C), suggesting that the optimal effect of IL-2 required transcription and translation of an unknown protein(s).
Figure 2.
Effect of translation and transcription inhibitors on IL-2-mediated down-regulation of IL-7Rα mRNA. (A) Rested splenocytes were preincubated with 10 μg/ml actinomycin D (Act D) for 5 min, 20 μg/ml cycloheximide (CHX), or 100 μg/ml puromycin (Puro) for 30 min before stimulation with IL-2 (2 nM). Total RNA was extracted and subjected to Northern blot analysis. (B and C) Densitometric analyses of the data shown in A; included are additional earlier time points not shown in A.
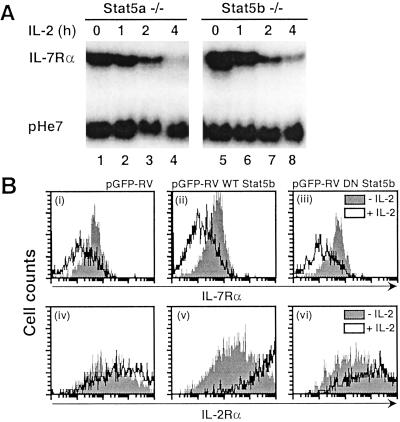
We next investigated which IL-2-signaling pathway(s) contributed to the decrease in IL-7Rα expression. The major signaling cascades known to be activated by IL-2 include Jak/STAT, PI3-kinase/Akt/p70 S6 kinase, and Ras/Raf/MAPK pathways (reviewed in ref. 8). Src-family tyrosine kinases such as p56 lck, p59 fyn, and p53/p56 lyn also are activated by IL-2, but the significance of such activation is unclear (8). We first examined whether the activation of Stat5a and Stat5b by IL-2 is involved in the down-regulation of IL-7Rα. Neither of these proteins alone was essential given that IL-2 could down-regulate IL-7Rα expression in either Stat5a- or Stat5b-deficient splenocytes (Fig. 3A). Presumably because splenocytes from Stat5a/Stat5b double-knockout mice have a severe defect in proliferation (ref. 15 and our unpublished data), we were unable to obtain enough of these cells to perform Northern blot analyses. As an alternative approach to eliminate Stat5a and Stat5b activation simultaneously, we introduced a dominant negative form of Stat5b into Stat5a-deficient splenocytes by retroviral transduction using pGFP-RV. pGFP-RV is a bicistronic retroviral vector in which GFP is under control of the internal ribosomal entry site, whereas the cloned inserts are under control of the retroviral long-terminal repeat. Thus, GFP expression was used to confirm successful retroviral transduction. Because IL-7Rα protein expression correlated with IL-7Rα mRNA levels (Fig. 1 B and C), we examined cell-surface IL-7Rα expression on transduced Stat5a−/− splenocytes by gating on GFP-positive cells. The normal decrease in cell-surface IL-7Rα expression (Fig. 3Bi) in response to IL-2 was still evident, whether wild-type (Bii) or dominant negative Stat5b (Biii) was transduced. Note that the wild-type and dominant negative Stat5b constructs were functional as demonstrated by enhanced IL-2Rα induction with wild-type Stat5b (Bv vs. Biv) and a blunted effect with the dominant negative Stat5 (Bvi vs. Biv). Thus, Stat5 activation is not required for IL-2-induced IL-7Rα down-regulation.
Figure 3.
(A) IL-2-mediated down-regulation of IL-7Rα mRNA in Stat5a−/− and Stat5b−/− splenocytes. Rested Stat5a−/− or Stat5b−/− splenocytes were stimulated with IL-2 for indicated periods and harvested for RNA extraction and Northern blotting. (B) Stat5a−/− splenocytes were preactivated and retrovirally transduced with the empty vector virus (pGFP-RV, i and iv) or with those encoding wild-type (WT, ii and v) or dominant negative (DN, iii and vi) Stat5b. Cells then were not treated or treated with IL-2, and IL-7Rα (i–iii) or IL-2Rα (iv–vi) expression was determined. For i–iii, the fold IL-7Rα suppression was 0.5, 0.4, and 0.4, and for iv–vi the fold IL-2Rα induction was 3.8, 7.2, and 2.7, respectively.
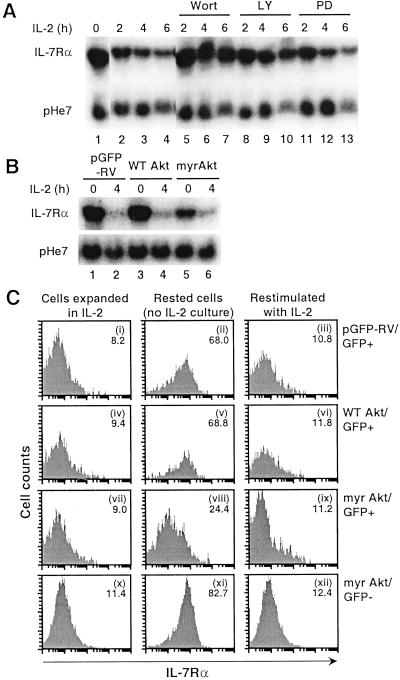
We next tested the effect of wortmannin (16) and LY294002 (17), two inhibitors of PI3-kinase. Both wortmannin (50 nM) and LY294002 (25 μM) blocked the normal decrease of IL-7Rα mRNA by IL-2 (Fig. 4A, lanes 5–10 vs. 2–4). In dose-response experiments, maximal inhibition by wortmannin was seen at 25 nM, and maximum inhibition by LY294002 was seen at 25 μM (data not shown). Because PI3-kinase links IL-2 signaling to Akt/protein kinase B and p70 S6 kinase in T lymphocytes (18), we investigated the role of Akt by using retroviral transduction of wild-type Akt or a constitutively active myristylated mutant form of Akt. We initially examined the effect of these constructs on IL-7Rα mRNA in CD4+ splenocytes given their very high transduction efficiency of ≈70–80% (7), so that purification of the transduced cells would not be required. After resting overnight, the level of IL-7Rα in wild-type Akt-transduced cells (Fig. 4B, lane 3) was similar to that seen in cells transduced with the empty vector (lane 1). In contrast, the level seen in cells expressing myr-Akt was much lower (lane 5). In all cells, the addition of IL-2 for 4 h lowered IL-7Rα levels (lanes 2, 4, and 6). These results indicated that constitutively active Akt can substitute, at least in part, for IL-2 in down-regulating expression of IL-7Rα mRNA. Given that the retroviral transduction efficiency was necessarily less than 100%, the effect of the myr-Akt may have been underestimated.
Figure 4.
IL-2-mediated down-regulation of IL-7Rα depends on PI3-kinase and Akt. (A) Ability of PI3-kinase inhibitors (wortmannin and LY294002) and a MAPK kinase inhibitor, PD98059, to decrease IL-2-induced down-regulation of IL-7Rα mRNA. Rested splenocytes were incubated for 0, 2, 4, or 6 h with IL-2 alone (lanes 1–4) or in the presence of 50 nM wortmannin (lanes 5–7), 25 μM LY294002 (lanes 8–10), or 50 μM PD98059 (lanes 11–13), RNA was isolated, and Northern blotting was performed with IL-7Rα or pHe7 probes. (B) CD4+ splenocytes were transduced with retroviruses derived from the empty vector (pGFP-RV, lanes 1 and 2) or those encoding wild-type Akt (lanes 3 and 4) or a constitutively activated myr-Akt (lanes 5 and 6). Cells were treated with IL-2 for 0 or 4 h, and RNA was isolated and Northern-blotted. (C) As described for B except total splenocytes were used. Cell-surface expression of IL-7Rα was examined on cells rested overnight and not stimulated (ii, v, and viii) or stimulated (iii, vi, and ix) with IL-2. Mean fluorescent intensities of IL-7Rα expression are shown in the upper-right corner of each panel. Gating was on GFP-positive (i–ix) or GFP-negative (x–xii) populations.
We next examined the effect of retroviral transduction of Akt constructs on cell-surface IL-7Rα expression. We transduced total splenocytes and evaluated transduced vs. nontransduced cells by gating on GFP-positive vs. negative populations. After an overnight rest, cell-surface IL-7Rα expression was restored to a high level in cells transduced with pGFP-RV or pGFP-RV/wild-type Akt (Fig. 4C, ii and v vs. i and iv). In contrast, IL-7Rα levels in myr-Akt-transduced cells remained relatively low (viii vs. vii), supporting the idea that Akt at least partially mediates the ability of IL-2 to down-regulate IL-7Rα expression. This effect was not seen in the GFP-negative nontransduced cells (xi). When cells were stimulated with IL-2, IL-7Rα expression in myr-Akt-transduced cells decreased to an even lower level (ix), which suggests either that IL-2 stimulation provided a stronger Akt signal (perhaps due to some cells expressing only low levels of transduced myr-Akt) or that pathway(s) besides Akt are involved in IL-7Rα down-regulation.
We also investigated the potential contribution of p70 S6 kinase, another important signaling molecule downstream of PI3-kinase, to the down-regulation of IL-7Rα expression using rapamycin, an inhibitor of p70 S6 kinase activation. By Northern blot analysis, 200 nM rapamycin only had a minimal effect (data not shown), thus arguing against a significant role for p70 S6 kinase in the down-regulation of IL-7Rα mRNA. Protein kinase Cζ also has been suggested to be important for IL-2-mediated proliferation of a mouse T cell line (19), and this enzyme has been shown to be regulated by PI3-kinase (20). However, IL-7Rα expression was not affected by retroviral transduction of either wild-type or constitutively active protein kinase Cζ (data not shown). Thus, the PI3-kinase/Akt pathway seems to be the major IL-2-signaling pathway involved in IL-7Rα down-regulation, although other pathways potentially could contribute.
To explore the possible role of IL-2-regulated PI3-kinase-independent pathways, we first used the MAPK kinase inhibitor PD98059. PD98059 had little if any effect (Fig. 4A, lanes 11–13 vs. 2–4), minimizing the likelihood of a role for the MAPK kinase/MAPK pathway. Although IL-2 has been reported to activate Src-family kinases including Lck, Fyn, and Lyn, the role of these kinases in IL-2 signaling is unclear (21). We evaluated the role of Src-family kinases using a potent inhibitor, PP2, which can bind in a deep, hydrophobic pocket adjacent to the ATP-binding site of Lck (22, 23). Using in vitro kinase assays, we confirmed that PP2 but not its inactive variant PP3 inhibited Lck-mediated phosphorylation of a substrate peptide and autophosphorylation (data not shown). PP2 at 10 μM partially inhibited the IL-2-induced decrease in IL-7Rα mRNA (Fig. 5A, lanes 4 and 5 vs. 2 and 3), whereas PP3 had no effect at the same concentration (lanes 6 and 7). PP2 had a similar effect at 5 μM, whereas 2.5 μM PP2 had a more modest effect (data not shown). Interestingly, the effect of PP2 was similar to that seen with a suboptimal concentration of wortmannin (12.5 nM; lanes 8 and 9), and a combination of 10 μM PP2 and 12.5 nM wortmannin (lanes 12 and 13) achieved a stronger inhibition. As expected, the inactive reagent PP3 did not alter the effect seen with suboptimal wortmannin (lanes 14 and 15).
Figure 5.
Inhibition of IL-2-induced down-regulation of IL-7Rα mRNA by PP2 and wortmannin. (A) Cells were incubated with combinations of wortmannin with PP2 or PP3 as indicated and stimulated with IL-2 for indicated periods before RNA isolation, Northern blotting, and hybridization to IL-7Rα or pHe7 probes. (B) Cells were pretreated with 50 nM wortmannin, 10 μM PP2, or 10 μM PP3 and treated with IL-2 as indicated. Cell lysate protein (1 mg) was immunoprecipitated (IP) with anti-Jak1 antibody and Western-blotted with antiphosphotyrosine antibody PY99. An additional 20 μg of protein was resolved directly on SDS/PAGE and blotted with antibodies to phospho-Ser-473 Akt or Akt. (C) 293T cells were transfected with a myr-Akt expression construct. Two days after transfection, cell lysate was prepared and immunoprecipitated with immobilized Akt antibody. The precipitated protein was pretreated with wortmannin, PP2, or PP3 for 15 min before incubation with ATP and a phosphorylation substrate, GSK-3/paramyosin fusion protein. Phosphorylation of GSK-3 substrate was detected by an antibody to phospho-GSK-3α/β (Ser-21/9).
To establish further the specific inhibition by PP2 of Src-family tyrosine kinases and signals downstream of these kinases, we treated splenocytes with wortmannin, PP2, or PP3. IL-2 induced tyrosine phosphorylation of Jak1, and this phosphorylation was not affected by wortmannin, PP2, or PP3 (Fig. 5B Top). In contrast, IL-2-induced phosphorylation of Ser-473 in Akt, which is important for full activation of Akt kinase, was inhibited by pretreatment with wortmannin or PP2 (Middle and Bottom, lanes 3 and 4 vs. 2). We also attempted to evaluate whether PP2 can inhibit Akt kinase activity. Cell lysates from myr-Akt-transfected 293T cells were immunoprecipitated with immobilized Akt antibody, and the precipitated Akt was preincubated with either wortmannin, PP2, or PP3 before ATP and a GSK fusion protein substrate were added. Phosphorylation of the substrate was detected by using anti-phospho-GSK-3α/β antibody. As shown in Fig. 5C, neither wortmannin nor PP2 affected Akt catalytic activity in vitro. These results suggest that both Src-family tyrosine kinases and PI3-kinase act through Akt to down-regulate IL-7Rα expression.
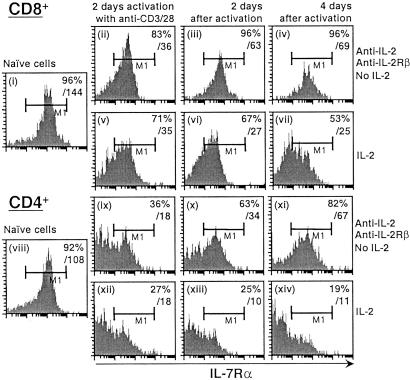
IL-7Rα expression is high on naive T cells but low to absent on activated T cells (24). We therefore investigated the ability of IL-2 to down-regulate IL-7Rα expression during T cell activation. Naive splenocytes were activated with plate-bound anti-CD3 and anti-CD28 and cultured in the presence or absence of IL-2 (with neutralizing antibodies to IL-2 and IL-2Rβ being added in the latter case to block IL-2 signaling completely). IL-7Rα was highly expressed on both naive CD8+ (Fig. 6i) and CD4+ (viii) T cells, but both the percent of IL-7Rα-expressing cells and mean fluorescent intensity declined significantly after 2 days of activation whether IL-2 was present or absent (ii, v, ix, and xii). The effect of T cell antigen receptor (TCR) stimulation on IL-7Rα expression seems IL-2-independent, because treatment with anti-CD3 + anti-CD28 for as short as 2–4 h could decrease IL-7Rα mRNA levels but interestingly was wortmannin-insensitive (data not shown). After TCR stimulation, when the cells were cultured in the presence of IL-2 for 4–6 days, IL-7Rα expression further declined in both CD8+ and CD4+ T cells (vi and vii, and xiii and xiv, respectively). When IL-2 signaling was blocked, most cells underwent apoptosis, whereas viable cells showed an increased expression of IL-7Rα (iii and iv, and x and xi, respectively). These results suggest that TCR activation may be the initial signal to down-regulate IL-7Rα and that IL-2 plays an important role in maintaining the down-regulation, although the effects of TCR and IL-2 on IL-7Rα expression may temporally overlap given the rapid production of IL-2 after TCR engagement.
Figure 6.
IL-7Rα expression in the presence or absence of IL-2 after anti-CD3/CD28 activation. Naive CD8+ or CD4+ splenocytes (i and viii) were isolated from wild-type mice and activated in anti-CD3-bound plates in the presence of anti-CD28-, anti-IL-2-, and anti-IL-2Rβ-neutralizing antibodies (both at 20 μg/ml; ii and ix, with antibodies and no IL-2) or in the presence of anti-CD28 and IL-2 for 2 days (v and xii, with IL-2). Cells then were washed, and the neutralizing antibodies were refreshed. The cells were cultured for another 2 (iii, vi, x, and xiii) or 4 days (iv, vii, xi, and xiv). Cell-surface IL-7Rα expression was determined by flow cytometry. The left boundary of the M1 region was set based on isotype-matched control antibody. Values in the upper right corners are the percentages of cells expressing high levels of IL-7Rα (M1 gate) and overall fluorescence intensity of cell-surface IL-7Rα.
Discussion
IL-7 is produced predominantly by stromal tissues (25) as well as by dendritic cells (26). The fact that Il7−/− and Il7r−/− mice show greatly diminished lymphoid development with total T cell numbers decreased 10–20-fold and an absence of B cells (27, 28) underscores the essential role of IL-7 in lymphopoiesis. Similar to Il7r−/− mice, humans with mutations in the IL7R gene exhibit greatly diminished T cell development, but in contrast to the mice, humans have normal B cell development, resulting in T−B+NK+ severe combined immunodeficiency (29). In the periphery, IL-7 is critical for the survival and “homeostatic” proliferation of naive T cells as a means of maintaining stable T cell numbers (24, 30). Along with IL-15, IL-7 also can influence CD8+ memory T cell homeostasis (31). Comparatively little is known about the biological role of thymic stromal lymphopoietin, which in addition to its own unique thymic stromal lymphopoietin receptor subunit shares IL-7Rα as a receptor component (32, 33); however, this cytokine seems to play a role in early B cell development and also can costimulate thymocytes and mature T cells (34, 35).
Members of the IL-2 subfamily of type I cytokines (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21) share γc in addition to each cytokine having its own more distinctive receptor subunit(s) (1, 2). Thus, these cytokines potentially can compete with each other for the recruitment of γc. After T cell activation by foreign antigen, expression of both IL-2 and IL-2Rα is induced (2). The induction of IL-2Rα allows the conversion of IL-2Rβ/γc intermediate affinity receptors into high-affinity IL-2 receptors, which can bind the relatively low concentrations of IL-2 that are produced physiologically (8). IL-2 potently induces the expression of IL-2Rα, facilitating responsiveness of the activated T cells to IL-2. IL-2 not only drives clonal expansion of antigen-specific T cells, but it also can subsequently promote cell death, facilitating the subsequent contraction of the immune response. In addition to increased IL-2Rα expression in response to IL-2, we now show that this same stimulus also potently suppresses IL-7Rα expression, providing an example in which expression of the receptor for one cytokine (IL-2) is induced, whereas that for another cytokine (IL-7) is diminished. We hypothesize that the ability of IL-2 to decrease IL-7Rα expression is more likely to be physiologically important in the periphery than in early thymic development given the lack of functional IL-2 receptors in the early developing thymus.
It is noteworthy that the expression of receptor subunits for certain other IL-2-family cytokines is modulated by stimulation with IL-2 as well. Similar to IL-7Rα, expression of IL-9Rα also is down-regulated by IL-2, albeit to a lesser extent; in contrast, the expression of IL-15Rα and IL-4Rα is moderately increased after stimulation with IL-2 (Fig. 1A). As noted above, each of these receptor subunits forms a receptor complex with γc for its respective ligand. Thus, in addition to the sharing of γc representing a mechanism for influencing cross-talk between IL-2 and other cytokines, it seems that IL-2 also can influence the actions of other cytokines by regulating the expression levels of other cytokine-specific receptor components.
After IL-2-stimulated cells are changed to IL-2-free medium, the viable effector T cells rapidly restore high levels of IL-7Rα expression. Thus, the cells that survive growth-factor withdrawal can up-regulate IL-7Rα to facilitate responsiveness to IL-7, which itself is a survival factor. The up-regulation of IL-7Rα in a cell therefore might help to promote its becoming a memory cell after contraction of the primary immune response. Consistent with this idea, Il7r−/− T cells can respond well to a viral infection but are ineffective at producing CD8+ memory T cells (24). Interestingly, the expression pattern of IL-7Rα parallels that of Bcl-2, an antiapoptotic protein, which is induced when thymocytes mature, decreases during T cell activation and again is increased in memory T cells (36). Indeed, IL-7 augments Bcl-2 expression, and Bcl-2 can rescue T lymphopoiesis partially in IL-7Rα-deficient or γc-deficient mice (37–39). Moreover, prevention of apoptosis by IL-7 in vitro is accompanied by an increase in Bcl-2 (40). Thus, the synchronized up-regulation of Bcl-2 and IL-7Rα might provide a survival signal for memory cells.
We identified the PI3-kinase/Akt pathway as critical for IL-2-mediated IL-7Rα down-regulation. In addition, an Src-family tyrosine kinase inhibitor, PP2, but not its inactive analogue, PP3, diminished and delayed IL-7Rα down-regulation. Both Jak1 and Src-family kinases have been suggested to be involved in IL-2-mediated activation of PI3-kinase (12, 13, 41). Indeed, PP2 diminished the phosphorylation of Ser-473 in Akt, which is essential for Akt activation. Thus, Src-family kinases may act upstream of Akt activation as suggested previously (12, 13). Given the cooperative effect of PP2 and wortmannin in diminishing IL-7Rα down-regulation, it is possible also that Src-family kinases and PI3-kinase can function in parallel to modulate the activity of downstream molecule(s). However, because PP2 completely inhibited Akt activation but only partially inhibited IL-2-induced IL-7Rα down-regulation, there may be other downstream signal(s) besides Akt that contribute to the decrease in IL-7Rα expression.
Because IL-15 and IL-2 share IL-2Rβ and γc as receptor components, we hypothesized that IL-15 also might diminish IL-7Rα expression and confirmed it by microarray, Northern blotting, and flow-cytometric analyses. Interestingly, the effect of IL-15 was less potent than that of IL-2 (data not shown); thus, of these two related cytokines, the effect of IL-2 on IL-7Rα expression seems to be the more significant.
In summary, IL-2 rapidly represses IL-7Rα expression at the same time that it induces IL-2Rα expression. Whereas IL-2Rα induction occurs by a mechanism that depends on Stat5a and Stat5b (42), we now show that repression of IL-7Rα depends on a PI3-kinase/Akt-dependent mechanism. Because IL-7Rα is a component of the receptors for both IL-7 and thymic stromal lymphopoietin, our data suggest that IL-2 may affect signaling by each of these cytokines. Under the proper circumstances, IL-2 thus may block IL-7Rα-dependent “survival-factor” effects, perhaps predisposing cells to IL-2-dependent antigen-induced cell death. Additional studies now can be directed toward further elucidation of the physiological role of IL-2-mediated regulation of IL-7Rα expression.
Acknowledgments
We thank Dr. Jian-Xin Lin for useful suggestions, Ms. Julie Bollenbacher for technical support, and John Kelly for sharing unpublished observations with us. We thank Drs. Larry Samelson, Scott Durum, and Silvio Gutkind for critical comments.
Abbreviations
- γc
common cytokine receptor γ chain
- IL-2R
IL-2 receptor
- Jak
Janus tyrosine kinase
- STAT
signal transducer and activator of transcription
- MAP
mitogen-activated protein
- PI3-kinase
phosphatidylinositol 3-kinase
- MAPK
MAP kinase
- myr-Akt
myristylated Akt
- GSK
glycogen synthase kinase
- TCR
T cell antigen receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Leonard W J. Nat Rev Immunol. 2001;73:200–208. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 2.Leonard W J. In: Fundamental Immunology. Paul W E, editor. Philadelphia: Lippincott–Raven; 1999. pp. 741–774. [Google Scholar]
- 3.Waldmann T, Tagaya Y, Bamford R. Int Rev Immunol. 1998;16:205–226. doi: 10.3109/08830189809042995. [DOI] [PubMed] [Google Scholar]
- 4.Sadlack B, Merz H, Schorle H, Schimpl A, Feller A C, Horak I. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 5.Willerford D M, Chen J, Ferry J A, Davidson L, Ma A, Alt F W. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki H, Kundig T M, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard J J, Ohashi P S, Griesser H, et al. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 7.Van Parijs L, Refaeli Y, Lord J D, Nelson B H, Abbas A K, Baltimore D. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 8.Lin J X, Leonard W J. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 9.Friedmann M C, Migone T S, Russell S M, Leonard W J. Proc Natl Acad Sci USA. 1996;93:2077–2082. doi: 10.1073/pnas.93.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J X, Migone T S, Tsang M, Friedmann M, Weatherbee J A, Zhou L, Yamauchi A, Bloom E T, Mietz J, John S, Leonard W J. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 11.Ravichandran K S, Igras V, Shoelson S E, Fesik S W, Burakoff S J. Proc Natl Acad Sci USA. 1996;93:5275–5280. doi: 10.1073/pnas.93.11.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taichman R, Merida I, Torigoe T, Gaulton G N, Reed J C. J Biol Chem. 1993;268:20031–20036. [PubMed] [Google Scholar]
- 13.Karnitz L M, Sutor S L, Abraham R T. J Exp Med. 1994;179:1799–1808. doi: 10.1084/jem.179.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriggl R, Gouilleux-Gruart V, Jahne R, Berchtold S, Gartmann C, Liu X, Hennighausen L, Sotiropoulos A, Groner B, Gouilleux F. Mol Cell Biol. 1996;16:5691–5700. doi: 10.1128/mcb.16.10.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriggl R, Topham D J, Teglund S, Sexl V, McKay C, Wang D, Hoffmeyer A, Van Deursen J, Sangster M Y, Bunting K D, et al. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 16.Arcaro A, Wymann M P. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlahos C J, Matter W F, Hui K Y, Brown R F. J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 18.Reif K, Burgering B M, Cantrell D A. J Biol Chem. 1997;1272:14426–14433. doi: 10.1074/jbc.272.22.14426. [DOI] [PubMed] [Google Scholar]
- 19.Gomez J, Pitton C, Garcia A, Martinez de Aragon A, Silva A, Rebollo A. Exp Cell Res. 1995;218:105–113. doi: 10.1006/excr.1995.1136. [DOI] [PubMed] [Google Scholar]
- 20.Chou M, Hou M, W, Johnson J, Graham L K, Lee M H, Chen C S, Newton A C, Schaffhausen B S, Toker A. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi T. Science. 1995;268:251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 22.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Kim J L, Newcomb J R, Rose P E, Stover D R, Toledo L M, Zhao H, Morgenstern K A. Structure Fold Des. 1999;7:651–661. doi: 10.1016/s0969-2126(99)80086-0. [DOI] [PubMed] [Google Scholar]
- 24.Schluns K S, Kieper W C S, Jameson C, Lefrancois L. Nat Immunol. 2000;5:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 25.Hofmeister R, Khaled A R, Benbernou N, Rajnavolgyi E, Muegge K, Durum S K. Cytokine Growth Factor Rev. 1999;10:41–60. doi: 10.1016/s1359-6101(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 26.de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, Banchereau J, Liu Y J, Lebecque S, Caux C. J Immunol. 1998;160:1666–1676. [PubMed] [Google Scholar]
- 27.von-Freeden-Jeffry U, Vieira P, Lucian L A, McNeil T, Burdach S E, Murray R. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peschon J J, Morrssey P J, Grabstein K H, Ramsdell F J, Maraskovsky E, Gliniak B C, Park L S, Ziegler S F, Williams D E, Ware C B. J Exp Med. 1994;181:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puel A, Ziegler S F, Buckley R H, Leonard W J. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 30.Tan J T, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg K I, Surh C D. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrack P. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 32.Pandey A, Ozaki K, Baumann H, Levin S D, Puel A, Farr A G, Ziegler S F, Leonard W J, Lodish H F. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 33.Park L S, Martin U, Garka K, Gliniak B, Di Santo J P, Muller W, Largaespada D A, Copeland N G, Jenkins N A, Farr A G, et al. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin S D, Koelling R M, Friend S L, Isaksen D E, Ziegler S F, Perlmutter R M, Farr A G. J Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 35.Friend S L, Hosier S, Nelson A, Foxworthe D, Williams D E, Farr A. Exp Hematol (Charlottesville, Va) 1994;22:321–328. [PubMed] [Google Scholar]
- 36.Grayson J M, Zajac A J, Altman J D, Ahmed R. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 37.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman I L. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 38.Maraskovsky E, O'Reilly L A, Teepe M, Corcoran L M, Peschon J J, Strasser A. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 39.Nakajima H, Leonard W J. J Immunol. 1999;162:782–790. [PubMed] [Google Scholar]
- 40.Hassan J, Reen D J. Eur J Immunol. 1998;28:3057–3065. doi: 10.1002/(SICI)1521-4141(199810)28:10<3057::AID-IMMU3057>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 41.Migone T S, Rodig S, Cacalano N A, Berg M, Schreiber R D, Leonard W J. Mol Cell Biol. 1998;18:6416–6422. doi: 10.1128/mcb.18.11.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H-P, Kelly J, Leonard W J. Immunity. 2001;15:159–172. doi: 10.1016/s1074-7613(01)00167-4. [DOI] [PubMed] [Google Scholar]