Abstract
The bystander effect, originating from cells irradiated in vitro, describes the biologic response(s) of surrounding cells not directly targeted by a radiation insult. To overcome the limitations of in vitro tissue culture models and determine whether a bystander effect that is initiated by the in vivo decay of a radionuclide can be demonstrated in an animal, the ability of 5-[125I]iodo-2′-deoxyuridine (125IUdR)-labeled tumor cells to exert a damaging effect on neighboring unlabeled tumor cells growing s.c. in nude mice has been investigated. When mice are injected with a mixture of human colon LS174T adenocarcinoma cells and LS174T cells prelabeled with lethal doses of DNA-incorporated 125I, a distinct inhibitory effect on the growth of s.c. tumor (derived from unlabeled cells) is observed. Because (i) the 125I present within the cells is DNA-bound, (ii) ≈99% of the electrons emitted by the decaying 125I atoms have a subcellular range (<0.5 μm), and (iii) the overall radiation dose deposited by radiolabeled cells in the unlabeled cells within the growing tumor is <10 cGy, we conclude that the results obtained are a consequence of a bystander effect that is generated in vivo by factor(s) present within and/or released from the 125IUdR-labeled cells. These in vivo findings significantly impact the current dogma for assessing the therapeutic potential of internally administered radionuclides. They also call for reevaluation of the approaches currently used for estimating the risks to individuals and populations inadvertently exposed internally to radioactivity as well as to patients undergoing routine diagnostic nuclear medical procedures.
Studies in recent years have demonstrated that a radiobiologic phenomenon termed the “bystander effect” can be observed in mammalian cells grown in vitro. Bystander damage describes biologic effects, originating from irradiated cells, that occur in unirradiated neighboring cells. Several investigators have reported that when α-particles traverse a small fraction of a cell population in vitro, lower rates of survival and higher rates of genetic change are observed than those predicted from direct-ionization-only models (1–6). These changes include increased levels of sister chromatid exchanges, mutations, and micronuclei formation, changes in gene expression, and oncogenic transformation. Cell survival is likewise compromised when cells are cocultured with tritiated thymidine-labeled cells (7, 8) and iodine-125 (9). Similarly, the bystander effect has been reported for microcolonies that have been γ irradiated (10) and for cells exposed to media from γ-irradiated cells (10, 11). Evidence from these reports challenges the past half-century's tenet that radiation produces effects only in cells whose DNA has been damaged either through direct ionization or indirectly (for example, through hydroxyl radicals produced in water molecules in the immediate vicinity of the DNA).
Whether radiation-induced bystander effects represent a phenomenon that occurs only ex vivo, i.e., are a byproduct of in vitro conditions and manipulations, or whether they are factual in vivo events has not been fully examined. Consequently, the extension of conclusions derived from in vitro studies to the in vivo situation is uncertain. The demonstration of a bystander effect with an in vivo system and the elucidation of the underlying mechanisms of an in vivo bystander effect would go a long way in translating its implications for humans.
Recently, Watson et al. (12) demonstrated chromosomal instability in the progeny of unirradiated bone marrow cells mixed with cells exposed ex vivo to neutrons and transplanted into recipient mice. In this novel system, a sex-mismatch transplantation protocol provides a three-way marker system and allows the investigators to distinguish not only host-derived cells from donor-derived cells, but also irradiated donor stem-cell-derived cells from nonirradiated donor stem-cell-derived cells. These studies thus provide the first demonstration of an in vivo bystander mechanism, albeit with cells that were irradiated ex vivo before their administration.
To determine whether a bystander effect can be demonstrated with mammalian cells irradiated in vivo, we have investigated the ability of tumor cells, prelabeled with lethal radioactive concentrations of 5-[125I]iodo-2′-deoxyuridine (125IUdR) and, thus, destined to die, to exert a radiobiologic effect on surrounding unlabeled tumor cells growing s.c. in nude mice. To this end, human colon adenocarcinoma LS174T cells, preincubated with the thymidine analog 125IUdR, were coinjected, s.c. with unlabeled cells into nude mice, and tumor growth was measured at various times to determine the tumor responsiveness to the coinjected “dying” 125IUdR-labeled cells. Under these experimental conditions (i) the vast majority of the radiation dose is accumulated in the radiolabeled cells after s.c. injection, (ii) ≈99% of the electrons emitted by the decaying atoms of the Auger electron emitter 125I have a subcellular range (<0.5 μm) (13–15) and are, therefore, confined principally to the radiolabeled cells (15, 16), and (iii) the overall radiation dose deposited by radiolabeled cells in the unlabeled cells within the growing tumors is <10 cGy. Consequently, any retardation/inhibition of tumor growth would be a consequence of an in vivo induced bystander effect, presumably initiated by the irradiation of the labeled cell DNA by Auger electrons.
Materials and Methods
Mice.
Male NCr nude mice, the standard athymic model for the National Cancer Institute, were obtained at 4–5 weeks of age from Taconic Farms. The mice were housed in a Harvard University animal facility and fed ad libitum. We used the mice for experiments when they were 6–8 weeks old.
Tissue Culture.
LS174T cells, an adenocarcinoma developed from human colon cancer, were procured from American Type Culture Collection. The cells were grown at 37°C with 5% CO2 in Dulbecco's Modified Essential Medium, supplemented with sodium pyruvate, nonessential amino acids (Invitrogen), and 10% FBS (HyClone).
For all experiments, we trypsinized logarithmically growing LS174T cells, determined their number per milliliter with a hemocytometer, and assessed their viability with trypan blue. The cells were suspended in complete medium at a concentration of 2 × 107 cells/ml before being mixed with other cell preparations. All injectates contained a total of 2 × 106 tumor cells (dead, 125IUdR-labeled, unlabeled, and/or externally irradiated).
Dead Cell Preparations.
Dead LS174T tumor cells, used as “spacers,” ensured a consistent spatial arrangement of viable cells in all groups. These cells, harvested from growing healthy monolayers and suspended in complete medium (2 × 107 cells/ml), underwent three successive freeze–thaw cycles, from −135°C (Queue Cryostar −135°C freezer) to 37°C (water bath). Trypan-blue staining confirmed that 100% of the cells were no longer viable.
125IUdR-Labeled Cell Preparations.
Logarithmically growing LS174T tumor cells were incubated with 3–3.5 μCi/ml 125IUdR (ICN; 1 Ci = 37 GBq) for 48 h at 37°C with 5% CO2. They were then washed three times with PBS to remove non-DNA-associated 125IUdR. The labeled cells were trypsinized and passed repeatedly through a 20-gauge needle to produce a single-cell suspension, thus ensuring that each injection bolus contained the same cell count. The trypan-blue dye-exclusion test indicated that >90% of the labeled cell population were viable in all experiments. Trichloroacetic acid precipitation confirmed that all remaining cell-associated radioactivity was covalently bound to DNA. The radioactivity per cell was assessed with a Wallac 1480 γ counter and was found to range from 0.15 pCi per cell to 0.25 pCi per cell (with a targeted value of 0.20 pCi per cell). Autoradiography illustrated that the labeling index in all experiments was, on average, ≈80%. The radioiodinated cells were resuspended in complete medium at a concentration of 2 × 107 cells/ml before use. When required, dead 125IUdR-labeled cells were prepared by freezing and thawing (three cycles) a portion of the labeled cells.
Externally Irradiated Cell Preparations.
Logarithmically growing LS174T cells were trypsinized and suspended in complete medium (2 × 107 cells/ml). The cells were irradiated with a Gammacell 220 cobalt irradiator (15 cGy/sec). The radiation doses varied from 50 cGy to 2,000 cGy, depending on the experimental requirements. The irradiated cells were immediately mixed with an equal number of live cells and injected into mice.
Tumor Cell Administration.
Cells in 0.1 ml of medium were administered by s.c. injection in the anterior right flank of nude mice. Any visible leakage from the injection site resulted in elimination of the animal from the study. Each study group consisted of 10 animals. For 125I-cell retention studies, cells were introduced s.c. into both hind legs of each mouse.
Autoradiography.
A small fraction (1 × 106 to 2 × 106) of the prepared 125IUdR-labeled cells were seeded into Lab-Tek II chamber slides (Nalge Nunc) and returned to the incubator for 2–3 h. This time frame allowed for cell adhesion, but was not sufficiently long for cell division. After the cells attached, the medium was decanted and the cells were fixed with 70% ethyl alcohol for 3 min. Distilled water provided a final rinse.
In a darkroom, the slides were dipped once or twice in a mixture of NTB2 emulsion (Eastman Kodak) and distilled water (1:1) at 42°C, the emulsion was allowed to dry for 10 min, and the slides were stored in a dark box with Drierite (anhydrous CaSO4) at 4°C for 3–5 days. Finally, the slides were dipped for 5 min in Kodak Developer D-19, washed in distilled water, immersed in Rapid Fixer for 5 min, and washed again in distilled water. The cells were assessed under a light microscope for the presence of nuclear grains.
125I-Cell Retention Studies.
To obtain 125IUdR-labeled-cell radioactivity-retention data, mice previously injected with a mixture of 5 × 105 125IUdR-labeled cells and an equal number of unlabeled tumor cells were killed at various time points (3 h to 2 weeks). Their tumor-bearing hind legs were removed and counted in a γ counter.
Tumor Measurement.
One week after tumor-cell implantation in mice, tumor growth was examined. Volume assessment commenced once the tumors reached a palpable size. Tumors were measured in three dimensions, length (L), width (W), and height (H), by using calipers. After subtracting skin thickness from each, tumor volumes were calculated with the formula V = (L × W × H)/2.
Cell Sizing.
Tumor, from an injectate containing 1 × 106 125IUdR-labeled cells and 1 × 106 live LS174T cells, was removed from nude mice 14 days after inoculation. The tissue was fixed with formalin, sectioned (5-μm thick), and stained with hematoxylin/eosin. Cell diameters were measured under a microscope.
Results
Distinct Patterns of in Vivo Bystander Effect.
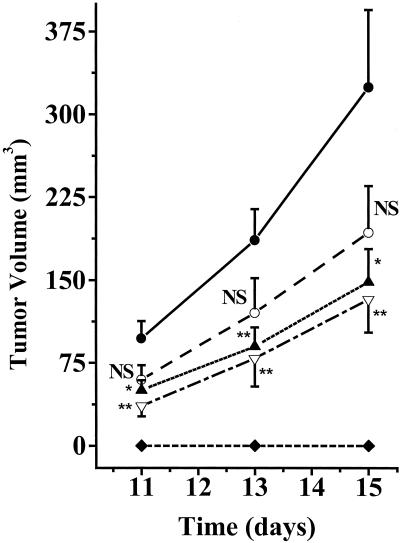
To characterize the in vivo bystander effect of the dying 125IUdR-labeled cells on neighboring or distant cells, the effect of varying numbers of 125IUdR-labeled cells on tumor growth in nude mice was assessed (Fig. 1). There was no detectable tumor growth when 1 × 106 125IUdR-labeled cells (0.19 pCi per cell) were exclusively inoculated into nude mice. However, when 125IUdR-labeled cells were coadministered with live LS174T cells, distinct patterns of in vivo tumor growth inhibition/retardation were observed. For example, when 1 × 106 125IUdR-labeled cells and 1 × 106 unlabeled cells were injected, the rate of tumor growth was substantially and significantly (P < 0.01) lower than that in the control animals (1 × 106 unlabeled tumor cells + 1 × 106 dead unlabeled cells). Dosimetric estimates indicate that under these experimental conditions, the total dose deposited in these s.c. tumors was 6.9 cGy (see Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org, for dose calculations). Tumor growth retardation was also significant when 2 × 105 radiolabeled cells and 1 × 106 unlabeled cells were injected; in this case the ratio of labeled-to-unlabeled cells is lower (1:5) and the cumulative dose in the unlabeled tumor cells is 2.3 cGy only. Because such a reduction in the ratio (i) increases the distances between the radiolabeled cells, (ii) decreases the number of radiolabeled cells in contact with unlabeled cells, and (iii) leads to a corresponding decrease in the total radiation dose deposited in these s.c. tumors (from ≈7 to ≈2 cGy), we conclude that the presence of the dying 125IUdR-labeled cells among the growing tumor cells resulted in a distinct bystander effect. A somewhat lesser decrease in the growth of tumors was observed when the ratio of 125IUdR-labeled cells to the surrounding unlabeled cells was only 1:10 (Fig. 1); under these experimental conditions, the differences in the growth of these tumors were not significantly different from the controls (P > 0.05), though the trend is clear.
Figure 1.
Patterns of in vivo, 125IUdR-labeled, LS174T-cell-induced bystander effect. Five groups of nude mice were injected s.c. with 1 × 106 live unlabeled cells and 1 × 106 dead unlabeled cells (●); 1 × 105 125IUdR-labeled cells, 1 × 106 live unlabeled cells, and 9 × 105 dead unlabeled cells (○); 2 × 105 125IUdR-labeled cells, 1 × 106 live unlabeled cells, and 8 × 105 dead unlabeled cells (▴);1 × 106 125IUdR-labeled cells and 1 × 106 live unlabeled cells (▿); and 1 × 106 125IUdR-labeled cells and 1 × 106 dead unlabeled cells (⧫). Tumor volumes were assessed, and the data were plotted as mean ± SEM (n = 10 per group). At each time point, the differences between the mean tumor volumes for each experimental group and those for the controls were determined by using the Student's t test. NS, not significant; *, P < 0.05; **, P < 0.01.
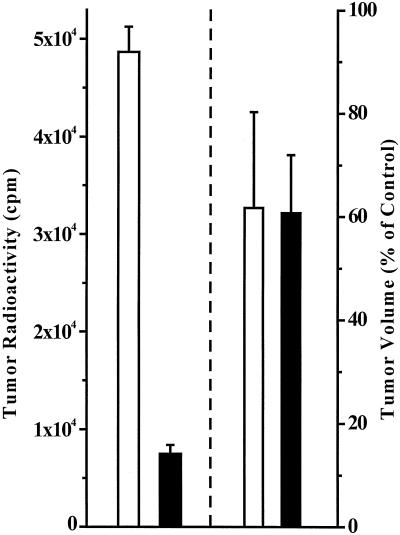
In another set of experiments, the radioactivity present within the growing tumors was determined after the coinjection of 1 × 106 125IUdR-labeled cells and 1 × 106 unlabeled cells or the coadministration of 2 × 105 125IUdR-labeled cells, 1 × 106 unlabeled cells, and 8 × 105 dead unlabeled cells. No differences were observed between the tumor volumes for the two groups of animals 15 days after tumor inoculation (respectively 61% and 62% of the volumes found in the control animals) (Fig. 2). However, there was a substantial difference at that time in the amount of radioactivity still present within the tumor masses of the two groups, further demonstrating the absence of any correlation between the radiation dose and the bystander effect.
Figure 2.
Relationship of LS174T tumor-associated radioactivity and tumor size in bystander study. Two groups of nude mice were injected s.c. with 1 × 106 125IUdR-labeled cells + 1 × 106 live cells (□) or 2 × 105 125IUdR-labeled cells + 1 × 106 live cells + 8 × 105 dead cells (■). Tumor radioactivity and tumor volume were determined 15 days after the injection, and the data were plotted as mean ± SEM (n = 5 per group). Left bars represent radioactivity; right bars are tumor volumes (as percentage of control mice injected with 1 × 106 live cells + 1 × 106 dead cells).
Reutilization of Released 125IUdR by Tumor Cells.
Mammalian cells are efficiently killed after the decay of DNA-incorporated 125I (15, 17–19). In such studies, very low doses of 125I (≈0.05 pCi per cell) are cytocidal. Because the DNA-incorporated 125IUdR within such cells may be released after their death and degeneration and could, therefore, be taken up by adjoining DNA-synthesizing tumor cells, the retention of radioactivity was measured in the legs of mice injected s.c. with 0.25 × 106 dead (frozen and thawed three times) 125IUdR-labeled tumor cells premixed with an equal number of LS174T cells. The data indicate that 98% of the radioactivity released by the killed 125IUdR-labeled cells was eliminated from the s.c. tumors within 24 hours of their injection. After this initial rapid washout of radioactivity, the decrease in radioactivity was slowed and reached background levels by day 14. These results show minimal reutilization by the coinjected tumor cells of the radioactivity released by killed 125IUdR-labeled cells. The findings agree with those reported for a different tumor-cell line (20).
Radiation Dose Calculations.
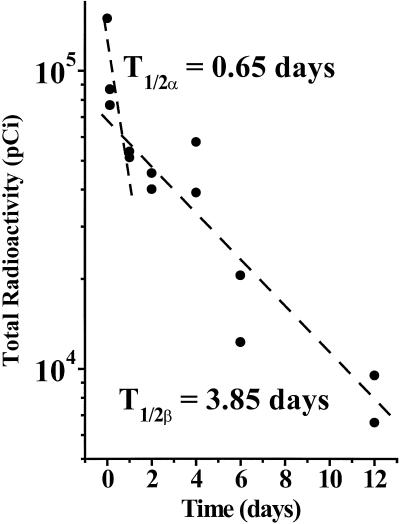
The decay of 125I produces both low-energy electrons and low-energy gamma radiation. With ≈90% and 99% of the emitted electrons having, respectively, a range of <50 nanometers and <0.5 micrometers (13, 14), most of the electrons emitted from 125IUdR-labeled cells cannot cause direct damage to neighboring or distant cells (15, 16). However, because (i) some of the electrons emitted have ranges that are greater than a mammalian cell diameter, and (ii) the 35.5 keV photons emitted during the decay of 125I have a mean free path for energy absorption in soft tissue of 5–10 cm (21), the 125IUdR-labeled cells will deposit a small dose that might damage unlabeled neighboring or distant cells by cross fire. To determine the absorbed dose from these radiations and to estimate possible radiobiologic effects, we adopted the approaches recommended for calculating radiation dose from homogeneously distributed radioactivity (22). The retention of radioactivity was measured in the legs of mice injected s.c. with 5 × 105 125IUdR-labeled cells premixed with an equal number of LS174T cells. An apparent two-phase washout of radioactivity was observed: an initial rapid component that reached ≈35% by day 1 and a slower component that reached 10% by day 6 (Fig. 3). We used these data, tumor growth kinetics, cell size, and the medical internal radiation dose (MIRD) S values for iodine-125 (23) to calculate the absorbed dose (see supporting information for details). The calculations indicate that under the experimental conditions that lead to statistically significant retardation/inhibition of tumor growth (Fig. 1), ≈1–7 cGy were deposited in the growing tumors (day 0 to day 15) when varying numbers of 125IUdR-labeled cells were premixed with unlabeled LS174T cells.
Figure 3.
Retention by s.c. LS174T tumor of radioactivity from the inoculation of 125IUdR-labeled tumor cells. Nude mice were injected s.c. with 5 × 105 125IUdR-labeled cells mixed with an equal number of live tumor cells. The total radioactivity in the tumors was measured at various time points after injection. Dashed line represents linear fit of data points (●).
In Vivo Growth of γ-Irradiated Tumor Cells and Substantiation of the 125I-Based Bystander Effect.
To evaluate the effects of radiation dose on the growth of LS174T tumor cells in vivo, groups of nude mice were injected s.c. with tumor-cell suspensions composed of 1 × 106 cells that had just been exposed to external γ-radiation doses of 0, 50, 100, or 200 cGy and an equal number of dead tumor cells. The growth of these tumor cells was monitored up to 14 days. With the exception of the cells irradiated with 200 cGy, there were no differences in the rate of tumor growth among the other study groups.
Based on this evidence and our dosimetric estimates indicating that <7 cGy was deposited by the 125IUdR-labeled cells in the unlabeled cells within the growing s.c. tumors (Fig. 1), the contribution of direct γ-radiation dose to the in vivo inhibition of tumor growth can be ruled out. We conclude, therefore, that the inhibitory effect of 125IUdR-labeled cells on in vivo growth of unlabeled tumor cells is clearly a consequence of a bystander effect.
Effect of γ-Irradiated “Dying” Tumor Cells on Growth of Unirradiated Cells.
The demonstration of an inhibitory bystander effect on tumor growth with 125IUdR-labeled cells led us to ask whether a similar effect could be shown with dying γ-irradiated tumor cells. Tumor cells exposed to either 5 or 20 Gy of γ radiation (1 × 106 cells) were mixed immediately with live LS174T tumor cells (1 × 106 cells). The cells were then injected s.c. into groups of nude mice. The in vivo tumor sizes were compared, and no bystander effect from the γ-irradiated tumor cells on the in vivo tumor growth of unirradiated tumor cells was observed.
Discussion
It has long been accepted that radiation-induced biologic effects, such as decreased cell survival, increased levels of sister chromatid exchanges, mutations, and micronuclei formation, changes in gene expression, and oncogenic transformation, occur only as a consequence of direct/indirect ionization and damage to the intranuclear DNA of mammalian cells. However, recent in vitro studies have provided strong evidence that unirradiated cells react to signals produced by irradiated cells (1–11), and there is now data indicating that this phenomenon also occurs when ex vivo irradiated cells are administered to animals (12). If a bystander effect were to occur after the irradiation of cells within an animal, it would greatly impact current (i) interpretations of the mechanisms underlying cell death after external beam and radionuclide therapy, (ii) assessment of therapeutic response to radionuclide therapy, and (iii) risk estimations after the administration of diagnostic radiopharmaceuticals to patients or the inadvertent exposure of individuals or populations to radioactivity.
To determine whether the bystander effect could be a consequence of in vivo irradiation, we selected a radionuclide (125I) with unique decay characteristics and a well established animal tumor model (human LS174T adenocarcinoma cells that grow as s.c. tumors in nude mice as approximately spherical tumors). The experimental approach we have used is simple. LS174T cells are labeled in vitro with 125I at a DNA-incorporated radioactive concentration that is lethal to the labeled cells but that deposits minimal energy in neighboring cells, i.e., there is insignificant cross fire. The radiolabeled cells are then mixed with unlabeled tumor cells, injected s.c. into mice, and the size of the consequent tumors is determined over time. Under such conditions, any alteration in the growth of the tumor will be a consequence of an in vivo, radiation-initiated bystander effect.
The Auger electron emitter iodine-125 is an isotope with physical decay characteristics that are ideal for the intended studies. Auger electron emission from certain radioactive atoms occurs whenever an inner shell vacancy is created. These primary vacancies can be induced by electron capture or internal conversion. The highly excited residual atom de-excites with the emission of several to many low-energy (<1 keV) electrons (Auger, Coster–Kronig, and super Coster–Kronig transitions) or atomic shell X-rays. Monte Carlo calculations, performed to determine the electron spectra of such radionuclides (13, 14), have shown them to be extremely complex and to consist primarily of electrons having subcellular ranges (nanometers). For 125I, the average Auger and Coster–Kronig electron spectra give a total of about 20 electrons per decay with energies ranging from ≈15 to 24 keV. The ejection of these electrons leaves the decaying atoms transiently with a high positive charge and results in the deposition of a concentrated amount of energy (1 × 106 to 1 × 109 cGy per decay) in an extremely small volume around the decay site, typically on the order of a few cubic nanometers (15). The highly localized nature of energy absorption leads to severe damage of molecular structures only in the immediate vicinity of the decaying atom (19, 24). In vitro studies at the molecular and cellular levels have repeatedly demonstrated (i) the very high toxicity and efficient double-strand break formation when such radionuclides decay in close proximity to nuclear DNA (15, 17–19, 25, 26), and (ii) the relative ineffectiveness of these radionuclides when the decay occurs in the cytoplasm or outside the cell (16, 27).
The LS174T cells were radiolabeled with 125I by incubating logarithmically growing cells with the thymidine analog 125IUdR. These cells were then mixed with unlabeled cells and injected s.c. into mice. The unique properties of 125IUdR offer significant advantages for the exploration of the bystander effect in vivo. First, after its phosphorylation and DNA incorporation, the radioactive atom has been found to be indefinitely bound to DNA for the life of the cell and/or its progeny (15, 28, 29). When we followed the time course of radioactivity in s.c. tumors (Fig. 3), we also did not detect evidence of reutilization. As a control, we coinjected labeled cells that had been killed (three freeze–thaw cycles) before injection and observed that 98% of the radioactivity disappears within 24 h. Consequently, we conclude that reutilization of 125I does not account for/contribute to the decreased tumor growth observed. Second, as the 125IUdR-labeled cells are destined to die, the factor(s) responsible for the observed bystander effect should be present only during the first few days after tumor-cell injection. Third, even though 125I also emits low-energy γ photons that travel multicellular distances, potentially damaging cells by cross fire, our dose estimates (see supporting information for details of dose calculation) indicate that <7 cGy are deposited in the s.c. tumors during their growth (days 0–15), whereas γ irradiation of these tumor cells with up to 100 cGy, i.e., more than 10 times the calculated γ-radiation dose from 125IUdR-labeled cells, has no detectable effect on their growth in vivo. The distinct differences between the lethal doses to the labeled cells and the insignificant nonlethal doses to the unlabeled cells lead us to conclude that the growth inhibitory or toxic effect of 125IUdR-labeled cells on the neighboring or distant unlabeled tumor cells is a consequence of an in vivo induced bystander effect, presumably initiated by the irradiation of the labeled cell DNA by Auger electrons.
The tumor in our experiments is assumed to be made of a closely packed collection of cells. In such a configuration, each tumor cell will be in contact with a number of neighboring cells. Therefore, if physical contact between each of the 125IUdR-labeled and unlabeled cells is necessary for the observed bystander effect to manifest itself, as has been reported in several in vitro studies (4, 7), one would expect a substantial decrease in tumor growth retardation/inhibition when the ratio of the radiolabeled-to-unlabeled cells approaches a value that no longer assures each unlabeled cell to be in contact with a labeled cell. We observed similar growth retardation/inhibition whether the 125IUdR-labeled-to-unlabeled cell ratio is 1:1 or 1:5 (P < 0.05 when these tumor volumes are compared with those of the controls). When this ratio drops to 1:10 (Fig. 1), the effectiveness of the radiolabeled cells declines but the differences among the three experimental groups are statistically insignificant (P > 0.05). It thus appears that once the ratio of the radiolabeled cells to unlabeled cells rises above the minimum needed to cause the bystander effect, the same retardation/inhibition in tumor growth occurs, suggesting that in vivo the bystander effect is a binary “all or none” phenomenon. This conclusion is in agreement with the hypothesis recently expressed by Brenner and coworkers (6).
The mechanisms underlying the radiation-induced bystander effect are poorly understood. Many investigators have reported an increased expression of p53 in irradiated cells (30, 31), as well as of the Cdk inhibitor p21CIP1/WAF1 (32), CD95 (APO-1/Fas), death receptors and ligands (33, 34), cytokines (35, 36), reactive oxygen species (37), caspase-8 (38), and nitric oxide (39). Increased expression of p53 and p21WAF1 in unirradiated bystander cells cocultured with α-particle-irradiated cells has also been reported (4). Other workers have implicated the release of a factor into the culture medium from γ-irradiated cells as playing a role in the induction of an in vitro bystander effect (11). Additionally, investigators have presented evidence for the involvement of gap-junction-mediated intercellular communication in in vitro bystander effect studies (4, 8). These findings suggest that a damage signal or signals from irradiated cells is probably transferred to the unirradiated bystander cells through a range of signaling pathways. If similar pathways underlie the in vivo bystander effects of 125I decay described in this paper, new therapeutic regimens for cancer may be devised by combining both radionuclide-based therapy and biologic modification of, or intervention in, various steps of signaling pathways to achieve greater effectiveness. There is a good likelihood that the bystander effect observed (Fig. 1) is a direct consequence of signal(s) initiated by 125I decays occurring in vivo because, when the radiolabeled cells were killed by three freeze–thaw cycles before mixing with an equal number of unlabeled cells, only a very small and statistically insignificant decrease in tumor growth was observed. Finally, whether the bystander effect is restricted in vivo to the highly specific damage to DNA by ionization secondary to Auger electron cascades is yet to be determined.
The bystander effect induced in vivo by radioactive decay also introduces a concept that dramatically impacts our views on risk assessment after the administration of radiopharmaceuticals to patients or the inadvertent exposure of individuals/populations to radioactivity. Traditionally (22), dose estimations have been carried out by averaging the radiation dose to cells within a tissue/organ/tumor mass from radioactive atoms present on/within the cells (self dose) and that from radionuclides present in or on other cells or in the extracellular fluids (cross dose). Such absorbed dose estimates, based on the decay characteristics of the radionuclide in question, have played an important role in determining the amount of radioactivity to be administered to patients in diagnostic/therapeutic procedures as well as in assessing environmental radiation risks, for example, radon inhalation. When a bystander effect is factored in, the actual radiobiologic response should be greater than that predicted by dosimetric estimates alone.
The decay of an Auger-electron-emitting radionuclide in close proximity to nuclear DNA is highly toxic to mammalian cells in vitro (15, 17–19, 40, 41). Consequently, investigators have assessed the therapeutic potential of such radionuclides and have shown them to be efficacious in animals bearing ovarian tumors (42, 43), brain tumors (44), s.c. tumors (45), and, recently, spinal cord tumors (46). Despite these positive findings, some investigators have refrained from pursuing the therapeutic potential of Auger-electron-emitting radionuclides, because the short ranges of the emitted electrons (≈99% have a range <0.5 μm) would seem to require the presence of the radionuclide within each and every targeted tumor cell. They have, rather, focused their efforts on using medium- to high-energy, electron-emitting isotopes that permit cross fire. Our current findings, in which the presence of 125I-containing cells within a growing tumor mass leads to substantial and statistically significant retardation in the growth of a tumor (Fig. 1), demonstrate that the biologic effects are not limited to radiolabeled cells only but also extend to neighboring cells. The presence of this bystander effect should encourage investigators to use Auger-electron-emitting radionuclides for therapy.
Supplementary Material
Acknowledgments
We thank Alice D. Carmel and Nan-hui Ho for technical assistance, and Dr. H. Green for use of the cobalt γ irradiator. This work was supported by National Cancer Institute Grant CA 15523 (to A.I.K.).
Abbreviation
- 125IUdR
5-[125I]iodo-2′-deoxyuridine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Nagasawa H, Little J B. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 2.Hickman A W, Jaramillo R J, Lechner J F, Johnson N F. Cancer Res. 1994;54:5797–5800. [PubMed] [Google Scholar]
- 3.Nagasawa H, Little J B. Radiat Res. 1999;152:552–557. [PubMed] [Google Scholar]
- 4.Azzam E I, de Toledo S M, Little J B. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawant S G, Randers-Pehrson G, Geard C R, Brenner D J, Hall E J. Radiat Res. 2001;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Brenner D J, Little J B, Sachs R K. Radiat Res. 2001;155:402–408. doi: 10.1667/0033-7587(2001)155[0402:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Bishayee A, Rao D V, Howell R W. Radiat Res. 1999;152:88–97. [PMC free article] [PubMed] [Google Scholar]
- 8.Bishayee A, Hall H Z, Stein D, Rao D V, Howell R W. Radiat Res. 2001;155:335–344. doi: 10.1667/0033-7587(2001)155[0335:friagj]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howell R W, Bishayee A. Micron. 2002;33:127–132. doi: 10.1016/s0968-4328(01)00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mothersill C, Seymour C. Int J Radiat Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 11.Mothersill C, Seymour C B. Radiat Res. 1998;149:256–262. [PubMed] [Google Scholar]
- 12.Watson G E, Lorimore S A, Macdonald D A, Wright E G. Cancer Res. 2000;60:5608–5611. [PubMed] [Google Scholar]
- 13.Charlton D E, Booz J. Radiat Res. 1981;87:10–23. [PubMed] [Google Scholar]
- 14.Sastry K S R, Rao D V. In: Physics of Nuclear Medicine: Recent Advances. Rao D V, Chandra R, Graham M C, editors. Woodbury, NY: Am. Inst. Phys.; 1984. pp. 169–208. [Google Scholar]
- 15.Kassis A I, Sastry K S R, Adelstein S J. Radiat Res. 1987;109:78–89. [PubMed] [Google Scholar]
- 16.Kassis A I, Fayad F, Kinsey B M, Sastry K S R, Taube R A, Adelstein S J. Radiat Res. 1987;111:305–318. [PubMed] [Google Scholar]
- 17.Hofer K G, Hughes W L. Radiat Res. 1971;47:94–109. [PubMed] [Google Scholar]
- 18.Porteous D D. Br J Cancer. 1971;25:594–597. doi: 10.1038/bjc.1971.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan P C, Lisco E, Lisco H, Adelstein S J. Radiat Res. 1976;67:332–343. [PubMed] [Google Scholar]
- 20.Dethlefsen L A. J Natl Cancer Inst. 1970;44:827–840. [PubMed] [Google Scholar]
- 21.Storm E, Israel H I. Nucl Data Tables. 1970;A8:1–198. [Google Scholar]
- 22.Loevinger R, Budinger T F, Watson E E. MIRD Primer for Absorbed Dose Calculations, Revised. New York: Soc. Nucl. Med.; 1991. [Google Scholar]
- 23.Goddu S M, Howell R W, Rao D V. J Nucl Med. 1994;35:303–316. [PubMed] [Google Scholar]
- 24.Deutzmann R, Stöcklin G. Radiat Res. 1981;87:24–36. [PubMed] [Google Scholar]
- 25.Walicka M A, Adelstein S J, Kassis A I. Radiat Res. 1998;149:134–141. [PubMed] [Google Scholar]
- 26.Kassis A I, Walicka M A, Adelstein S J. Acta Oncol. 2000;39:721–726. doi: 10.1080/028418600750063785. [DOI] [PubMed] [Google Scholar]
- 27.Hofer K G, Harris C R, Smith J M. Int J Radiat Biol. 1975;28:225–241. doi: 10.1080/09553007514550991. [DOI] [PubMed] [Google Scholar]
- 28.Hofer K G, Prensky W, Hughes W L. J Natl Cancer Inst. 1969;43:763–773. [PubMed] [Google Scholar]
- 29.Dethlefsen L A. Cancer Res. 1969;29:1717–1720. [PubMed] [Google Scholar]
- 30.Lowe S W, Schmitt E M, Smith S W, Osborne B A, Jacks T. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 31.Lu X, Lane D P. Cell. 1993;75:765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- 32.Dulic V, Kaufmann W K, Wilson S J, Tisty T D, Lees E, Harper J W, Elledge S J, Reed S I. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 33.Albanese J, Dainiak N. Radiat Res. 2000;153:49–61. doi: 10.1667/0033-7587(2000)153[0049:irafal]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Gong B, Almasan A. Cancer Res. 2000;60:5754–5760. [PubMed] [Google Scholar]
- 35.Narayanan P K, LaRue K E A, Goodwin E H, Lehnert B E. Radiat Res. 1999;152:57–63. [PubMed] [Google Scholar]
- 36.Epperly M W, Gretton J A, DeFilippi S J, Sikora C A, Liggitt D, Koe G, Greenberger J S. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Hachiya M, Osawa Y, Akashi M. Biochim Biophys Acta. 2000;1495:237–249. doi: 10.1016/s0167-4889(99)00168-8. [DOI] [PubMed] [Google Scholar]
- 38.Belka C, Heinrich V, Marini P, Faltin H, Schulze-Osthoff K, Bamberg M, Budach W. Int J Radiat Biol. 1999;75:1257–1264. doi: 10.1080/095530099139412. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto H, Hayashi S, Hatashita M, Ohnishi K, Shioura H, Ohtsubo T, Kitai R, Ohnishi T, Kano E. Radiat Res. 2001;155:387–396. doi: 10.1667/0033-7587(2001)155[0387:iorban]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Kassis A I, Adelstein S J, Haydock C, Sastry K S R, McElvany K D, Welch M J. Radiat Res. 1982;90:362–373. [PubMed] [Google Scholar]
- 41.Makrigiorgos G M, Kassis A I, Baranowska-Kortylewicz J, McElvany K D, Welch M J, Sastry K S R, Adelstein S J. Radiat Res. 1989;118:532–544. [PubMed] [Google Scholar]
- 42.Bloomer W D, Adelstein S J. Nature. 1977;265:620–621. doi: 10.1038/265620a0. [DOI] [PubMed] [Google Scholar]
- 43.Baranowska-Kortylewicz J, Makrigiorgos G M, Van den Abbeele A D, Berman R M, Adelstein S J, Kassis A I. Int J Radiat Oncol Biol Phys. 1991;21:1541–1551. doi: 10.1016/0360-3016(91)90331-w. [DOI] [PubMed] [Google Scholar]
- 44.Kassis A I, Wen P Y, Van den Abbeele A D, Baranowska-Kortylewicz J, Makrigiorgos G M, Metz K R, Matalka K Z, Cook C U, Sahu S K, Black P M, et al. J Nucl Med. 1998;39:1148–1154. [PubMed] [Google Scholar]
- 45.Behr T M, Sgouros G, Vougioukas V, Memtsoudis S, Gratz S, Schmidberger H, Blumenthal R D, Goldenberg D M, Becker W. Int J Cancer. 1998;76:738–748. doi: 10.1002/(sici)1097-0215(19980529)76:5<738::aid-ijc20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 46.Kassis A I, Dahman B A, Adelstein S J. Acta Oncol. 2000;39:731–737. doi: 10.1080/028418600750063802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.