Abstract
Epithelial carcinoma and leukemia cells express sialyl Lewis x oligosaccharides as tumor-associated carbohydrate antigens. To determine the role of sialyl Lewis x oligosaccharides in tumor dissemination, human melanoma MeWo cells, which do not express sialyl Lewis x, were transfected with α1,3-fucosyltransferase III (FTIII), and cell lines expressing different amounts of sialyl Lewis x were isolated. When these cells were injected into the tail vein of nude mice, cells expressing moderate amounts of sialyl Lewis x (MeWo-FTIII⋅M) produced a significantly greater number of lung tumor foci than did parental MeWo cells. In contrast, cells expressing large amounts of sialyl Lewis x (MeWo-FTIII⋅H) produced few lung tumor foci in nude mice but were highly tumorigenic in beige mice, which have defective natural killer (NK) cells. In vitro assays demonstrated that MeWo-FTIII⋅H cells are much more sensitive to NK cell-mediated cytotoxicity than are MeWo-FTIII⋅M cells or parental MeWo cells and the susceptibility of MeWo-FTIII⋅H cells to NK cell-mediated cytolysis can be inhibited by preincubating MeWo-FTIII⋅H cells with anti-sialyl Lewis x antibody. Moreover, we discovered that NK cell-mediated cytolysis of MeWo-FTIII⋅H cells can be inhibited by the addition of an antibody against the NK cell receptor CD94 or sialyl Lewis x oligosaccharides. These results, combined with structural analysis of MeWo-FTIII⋅H cell carbohydrates, indicate that moderate amounts of sialyl Lewis x lead to tumor metastasis, whereas expression of high levels of sialyl Lewis x leads to an NK cell attack on tumor cells, demonstrating that expression of different amounts of sialyl Lewis x results in entirely different biological consequences.
Distinct cell-surface carbohydrates are expressed in tissue- and cell-specific manners during development and in adulthood. Aberrations in cell-surface carbohydrates often are associated with malignant transformation and other pathological conditions (1–3). Among various cell-type-specific carbohydrates, sialyl Lewis x, NeuNAcα2→3Galβ1→4(Fucα1→3)GlcNAcβ→R, is expressed on neutrophils, monocytes, and certain T lymphocytes (4, 5) and plays a key role in the recruitment of leukocytes. E- and P-selectin expressed on activated endothelial cells capture these leukocytes through binding to sialyl Lewis x, allowing them to roll, which leads to extravasation of leukocytes at inflammatory sites (6, 7). Lymphocyte circulation is directed by interaction between L-selectin on lymphocytes and the sulfated form of sialyl Lewis x present on L-selectin receptors that are restricted to high endothelial venules (8–10). Such an initial interaction leads to extravasation of lymphocytes from the intravascular compartment to the lymphatic compartment.
The amounts of sialyl Lewis x and sialyl Lewis a, NeuNAcα2→3Galβ1→3(Fucα1→3)GlcNAc→R, which also has been shown to bind to E-selectin (11, 12), are increased significantly in tumor cells such as carcinoma and leukemia (13–16). In breast and colonic carcinoma patients, the presence of sialyl Lewis x and sialyl Lewis a is correlated with poor prognosis (17–19). These results strongly suggest that blood-borne tumor cells may use a carbohydrate–selectin (or a selectin-related molecule) interaction when tumor cells adhere to the endothelia at metastatic sites. In fact, we have shown recently that B16 melanoma cells become highly metastatic when they express moderate amounts of sialyl Lewis x as a result of transfection of α1,3-fucosyltransferase III (FTIII) (20). Similarly, a lung carcinoma cell line becomes highly metastatic after transfection of fucosyltransferase VII, which directs expression of increased amounts of sialyl Lewis x (21). These results indicate that sialyl Lewis x (or sialyl Lewis a) plays a direct role in metastasis. During our studies of B16 melanoma cells, we also discovered that B16 melanoma cells overexpressing sialyl Lewis x, B16-FTIII⋅H cells, were not tumorigenic in wild-type mice but were highly tumorigenic in beige mice, which have defective natural killer (NK) cells (20). The studies, however, did not determine whether B16 melanoma cells expressing high levels of sialyl Lewis x can be directly targeted by NK cells.
The above studies also suggest that tumor dissemination may be inhibited by use of sialyl Lewis x oligosaccharides as antagonists. To test this hypothesis, a peptide-displaying phage library was screened for peptides binding to E selectin (22, 23). Specifically, we screened a peptide-displaying library by using an anti-Lea antibody as a target and isolated peptides that compete with sialyl Lewis x for binding to E-, L-, and P-selectin in a calcium-dependent manner (23). One of these isolated peptides, IELLQAR, inhibited lung tumor formation by B16 melanoma cells transfected with FTIII or human lung carcinoma cells (23, 24).
In the present study, we first examined lung tumor formation of human MeWo melanoma cells after transfection with FTIII to allow expression of sialyl Lewis x. We found that MeWo cells expressing moderate amounts of sialyl Lewis x (MeWo-FTIII⋅M) form numerous lung tumor foci. MeWo cells expressing large amounts of sialyl Lewis x (MeWo-FTIII⋅H) were not tumorigenic in nude mice but were highly tumorigenic in beige mice. In vitro assays demonstrated that MeWo-FTIII⋅H cells as well as B16-FTIII⋅H cells are highly susceptible to NK cell-mediated cytotoxicity, whereas MeWo-FTIII⋅M and B16-FTIII⋅M cells are less so. We also showed that the susceptibility of MeWo-FTIII⋅H cells to NK cell-mediated cytolysis can be inhibited by preincubating them with anti-sialyl Lewis x antibody or by adding IELLQAR peptide during the cytotoxicity assay. Moreover, we found that the CD94 receptor on NK cells recognizes sialyl Lewis x expressed on tumor cells.
Materials and Methods
Transfection of MeWo Cells with pcDNA-FTIII.
Human MeWo melanoma cells, which are negative for sialyl Lewis x expression as assessed by immunostaining, were transfected with pcDNA3(neo)-FTIII (25) as described (20). Transfected cells were cultured in RPMI medium 1640 containing 10% (vol/vol) FCS and G418 (800 μg/ml; GIBCO/BRL), and single colonies were selected as described (20). The cells were dissociated into monodispersed cells by using an enzyme-free cell dissociation solution (Hanks' based) purchased from Cell and Molecular Technologies (Phillipsburg, NJ). Monodispersed cells were incubated with anti-sialyl Lewis x antibody (CSLEX-1; Becton Dickinson) followed by FITC-conjugated goat affinity-purified F(ab′)2 fragments specific to mouse IgM (Cappel). The cells were separated by cell sorting into cells negative for sialyl Lewis x expression (MeWo-FTIII⋅N), cells expressing moderate amounts of sialyl Lewis x (MeWo-FTIII⋅M), and cells strongly positive for sialyl Lewis x (MeWo-FTIII⋅H). B16-FTIII⋅H, B16-FTIII⋅M, and B16-FTIII⋅N were established as described (20).
Tumor Formation in Mice.
The above sorted cell populations were cultured approximately 1 week to obtain a sufficient number of cells. A total of 5 × 106 monodispersed cells (>90% viability) were suspended in 100 μl of serum-free RPMI medium 1640 and injected into the tail vein of BALB/c nude (nu/nu) or beige mice (C.B-17 scid-beige; 6–8 weeks, female). After 2 (beige mice) or 3 (nude mice) weeks, mice were killed, organs were fixed with Bouin's solution, and tumor foci were counted under a dissecting microscope. Because the majority of tumors occurred in the lung, this organ was selected for evaluation of tumor formation.
Isolation of IELLQAR Peptide That Mimics Sialyl Lewis x.
Peptides mimicking sialyl Lewis x were isolated by screening a peptide-harboring phage library by using anti-Lewis a antibody (clone 7LE; ref. 11) as a target molecule as described (23). IELLQAR, the most potent, calcium-dependent E selectin-binding peptide, and an unrelated VTSIAQA control peptide were synthesized as octameric peptides containing the (Lys)4→(Lys)2→Lys backbone. To test the inhibitory activity of the IELLQAR peptide on tumor formation, IELLQAR or control peptide (500 μg/100 μl per mouse) was injected through a tail vein of a nude mouse. After 20 min, MeWo-FTIII⋅M cells were injected into a second tail vein and tumor formation was assessed as described above.
Preparation of NK Cells.
NK cells were purified from human peripheral blood essentially as described (26). Briefly, human peripheral mononuclear cells were obtained by using a Ficoll/Paque cushion and then passed through a nylon wool column (Wako Pure Chemical, Osaka) to remove adherent cells. To obtain IL-2-activated NK cells (26), 1 × 106 per ml of the cells prepared above were cultured in RPMI medium 1640 containing 10% (vol/vol) FCS, 2 mM l-glutamine, and 1 mM sodium pyruvate in the presence of 1,000 units/ml of IL-2 (Cetus) for 3 days. Mouse NK cells were isolated from spleens of C57BL/6 and nude mice and activated by IL-2 as described (26).
Cytotoxicity Assay.
Cytotoxicity was measured by using the CytoTox96 Nonradioactive Cytotoxicity Assay kit (Promega). This assay is based on the release of lactate dehydrogenase from lysed cells. All assays were performed in triplicate. Percent cytotoxicity = (experimental lactate dehydrogenase release − effector spontaneous release − target spontaneous release) ÷ (target maximum release − target spontaneous release) × 100.
mAb and Peptide Treatment.
To assay the inhibition of tumor formation and cytotoxicity by anticarbohydrate antibodies, MeWo-FTIII cells were incubated with anti-sialyl Lewis x antibody (CSLEX-1) or anti-Lewis x antibody (CD15; Immunotech, Westbrook, ME) at a concentration of 10 μg/ml for 30 min at 4°C and then subjected to assays for tumor formation or cytotoxicity as described above. To analyze inhibition of cytotoxicity by peptides, the IELLQAR peptide or the control VTSIAQA peptide was added at 10 μM or 50 μM to the cytotoxicity assay mixture. Similarly, sialyl Lewis x oligosaccharides attached to polyacrylamide gel containing 30% or 10% mole of sialyl Lewis x (Glyco- Tech, Williamsport, PA; ref. 27) was added at 50 μM to the cytotoxic assay mixture. To determine which NK cell receptors bind to sialyl Lewis x, anti-L-selectin antibody (PharMingen; ref. 28), anti-CD69 antibody (Calbiochem; ref. 29), or anti-CD94 antibody (Immunotech; ref. 30) was added at 10 μg/μl to the cytotoxicity assay mixture.
Characterization of Carbohydrates Attached to MeWo-FTIII Cells.
MeWo-FTIII⋅N, MeWo-FTIII⋅M, and MeWo-FTIII⋅H cells were metabolically labeled with [3H]galactose, and glycopeptides were prepared as described (31). The glycopeptides were then applied to a ConA-Sepharose column. Glycopeptides unbound to ConA-Sepharose, representing O-glycans and tri- and tetra-antennary N-glycans, were applied to a column of LEA (tomato lectin)-agarose and eluted as described (31, 32). Tomato lectin binds glycans having three or more N-acetyllactosamine repeats (31–33). The glycopeptides unbound to LEA-agarose were applied to a column of Datura stramonium agglutinin (DSA)-agarose as described (31, 32).
Results
Isolation of MeWo Cells Expressing Different Amounts of Sialyl Lewis x.
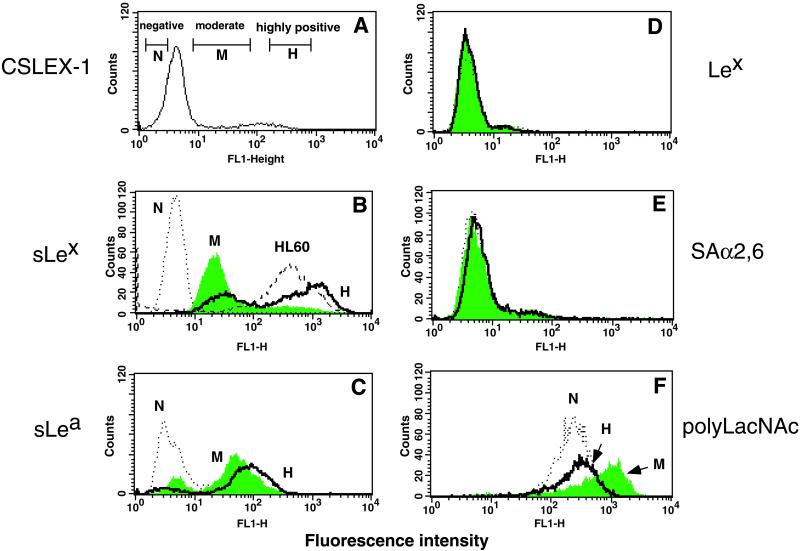
To create MeWo cell lines expressing sialyl Lewis x, cells were transfected with FTIII. FTIII is widely distributed in various tissues and directs the expression of both sialyl Lewis x and sialyl Lewis a (25). Stably transfected MeWo-FTIII cells were subjected to cell sorting with an anti-sialyl Lewis x antibody and fractionated into three groups: those highly positive (MeWo-FTIII⋅H), moderately positive (MeWo-FTIII⋅M), and negative (MeWo-FTIII⋅N) for sialyl Lewis x (Fig. 1A). After several passages, the cells maintained the phenotypes seen in Fig. 1B. Among these lines, the difference in sialyl Lewis x expression levels is similar to that of sialyl Lewis a (Fig. 1C). By contrast, there was almost no difference between MeWo-FTIII⋅M and MeWo-FTIII⋅H cells in staining with an anti-Lewis x antibody (Fig. 1D), α2,3-sialic acid-specific (Macchia amurensis) agglutinin, or α2,6-sialic acid-specific (S. nigra) agglutinin (Fig. 1E). MeWo-FTIII⋅M cells, however, showed slightly stronger staining by tomato lectin than did MeWo-FTIII⋅H cells (Fig. 1F), suggesting that MeWo-FTIII⋅M cells express more poly-N-acetyllactosamines than MeWo-FTIII⋅H cells.
Figure 1.
Cell sorting and flow cytometric analysis of MeWo-FTIII cells. (A) MeWo-FTIII cells were stained with anti-sialyl Lewis x antibody (CSLEX-1) then by FITC-conjugated secondary antibody, and sorted by FACStar. Cells indicated by open bars were pooled and designated as MeWo-FTIII⋅N (negative), MeWo-FTIII⋅M (moderate), and MeWo-FTIII⋅H (highly positive). (B–F) Cultured MeWo-FTIII⋅N (N), MeWo-FTIII⋅M (M), and MeWo-FTIII⋅H (H) cells were subjected to flow cytometry analysis after staining with anti-sialyl Lewis x (B), anti-sialyl Lewis a (C), or anti-Lewis x antibody (D), followed by FITC-conjugated secondary antibody or FITC-conjugated Sambucus nigra agglutinin (E) or FITC-conjugated tomato lectin (F). HL-60 cells also were stained in B.
Metastatic Properties of Different MeWo-FTIII Cells.
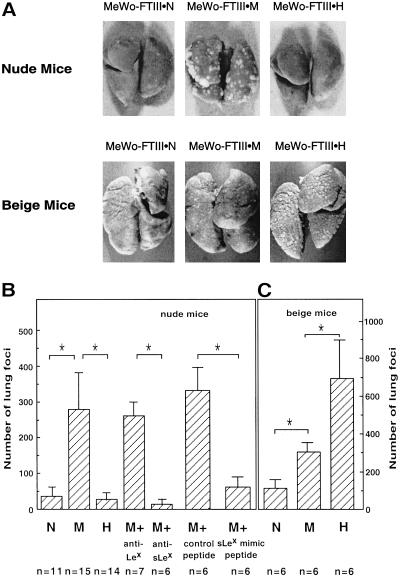
Tail vein injection of the above sorted cell lines showed that MeWo-FTIII⋅M cells produced a much higher number of tumor foci in nude mice compared with the parental MeWo or MeWo-FTIII⋅N cells (Fig. 2A). Lung tumor formation by MeWo-FTIII⋅M cells was inhibited by preincubation of the cells with anti-sialyl Lewis x but not by anti-Lewis x antibody, indicating that tumor metastasis by MeWo-FTIII⋅M cells depended on sialyl Lewis x expressed on the cells (Fig. 2B). Similarly, preinjection of the IELLQAR peptide, which mimics sialyl Lewis x, inhibited tumor formation by MeWo-FTIII⋅M cells (Fig. 2B). The most striking result, however, was that MeWo-FTIII⋅H cells produced as few tumor foci as did MeWo-FTIII⋅N cells in nude mice (Fig. 2 A and B).
Figure 2.
Lung tumor foci formed in nude and beige mice. (A–C) Lungs were examined 3 (nude mice) or 2 (beige mice) weeks after injection of MeWo-FTIII cells. In B, MeWo-FTIII⋅M (M) cells were preincubated with anti-Lewis x antibody (anti-Lex) or anti-sialyl Lewis x antibody (anti-sLex) before i.v. injection. Similarly, IELLQAR or control VTSIAQ peptide was injected i.v. before injection of MeWo-FTIII⋅M cells. The differences in the number of tumor nodules formed, denoted by asterisks, are statistically significant by the Mann–Whitney U test (P < 0.01).
Formation of Lung Tumor Foci in Beige Mice.
Our results indicate that the growth rate, measured as described (20), is almost the same among three different MeWo-FTIII lines. These results thus suggest that MeWo-FTIII⋅H cells may be more susceptible to immune cell attack than MeWo-FTIII⋅M cells. Because tumor formation described above was assayed in nude mice, which are defective in T cell-dependent immune responses, we chose next to examine tumor formation in beige mice, which are deficient in NK cell activity. The results shown in Fig. 2 A and C clearly demonstrate that the number of tumor foci in beige mice is increased after injection of all of the different MeWo-FTIII cell lines, and, yet, the number of tumor foci produced by different MeWo-FTIII cells is directly proportional to the amount of sialyl Lewis x expressed on the cell surface (Fig. 2C). These results are consistent with the results obtained on B16-FTIII cells (20). These results strongly suggest that high expression of sialyl Lewis x on the cell surface results in attack by NK cells, and MeWo-FTIII⋅H and B16-FTIII⋅H cells are eliminated during or after the attachment to endothelial cells.
NK Cells Can Directly Lyse MeWo-FTIII Cells.
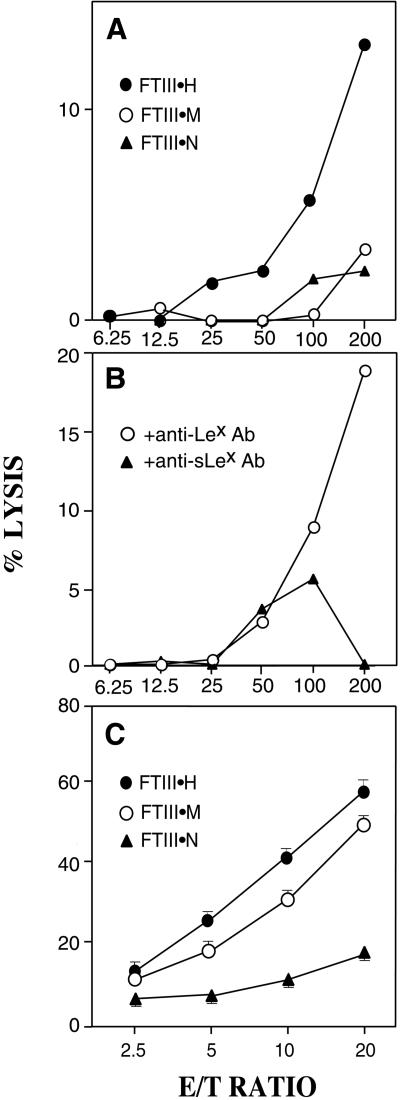
To determine whether different B16-FTIII and MeWo-FTIII cell lines differ in sensitivity to direct lysis by NK cells, we assayed the cytotoxicity of NK cells against B16 and MeWo melanoma cell lines expressing different amounts of sialyl Lewis x. As shown in Fig. 3A, mouse NK cells most efficiently cytolysed B16-FTIII⋅H cells but only marginally cytolysed B16-FTIII⋅M and B16-FTIII⋅N cells. This cytotoxicity is dependent on sialyl Lewis x on mouse B16-FTIII⋅H cells, because preincubation with anti-sialyl Lewis x antibody but not with anti-Lewis x antibody reduced the cytotoxicity (Fig. 3B). Similarly, mouse NK cells cytolysed MeWo-FTIII cells corresponding to the amount of sialyl Lewis x expressed on the cell surface (Fig. 3C).
Figure 3.
Mouse NK cells directly lyse B16-FTIII and MeWo-FTIII cells in proportion to sialyl Lewis x content. (A and B) B16-FTIII⋅N, B16-FTIII⋅M, and B16-FTIII⋅H cells were subjected to cytolysis before and after preincubation of tumor cells with anti-sialyl Lewis x or anti-Lex antibody. (C) MeWo-FTIII⋅ N, MeWo-FTIII⋅M, and MeWo-FTIII⋅H cells were subjected to cytolysis by NK cells derived from nude mice.
The CD94 Receptor on Human NK Cells Is Responsible for Cytolysis of MeWo-FTIII⋅H Cells.
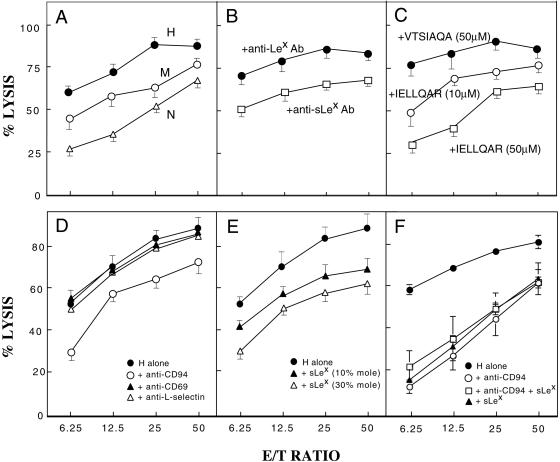
We then investigated how human NK cells exhibit cytotoxicity for human MeWo-FTIII cells to gain insight into human tumor progression. Fig. 4A illustrates that MeWo-FTIII⋅H cells are most sensitive to NK cell cytotoxicity and the susceptibility of different MeWo-FTIII cells to such cytotoxicity is proportional to the amount of sialyl Lewis x expressed on the cell surface. This cell lysis was inhibited by preincubation of MeWo-FTIII⋅H cells with anti-sialyl Lewis x antibody but not with anti-Lewis x antibody (Fig. 4B) and by the presence of IELLQAR peptide, which mimics sialyl Lewis x (23), in a dose-dependent manner but not by the control peptide (Fig. 4C).
Figure 4.
Human NK cells directly lyse MeWo-FTIII⋅H through CD94 recognition of sialyl Lewis x on tumor cells. (A) MeWo-FTIII⋅H (H), MeWo-FTIII⋅M (M), and MeWo-FTIII⋅N (N) cells were subjected to cytotoxic lysis by NK cells in different ratios of effector (E) and target (T) cells. (B and C) Inhibition of NK cell-mediated cytotoxic lysis by preincubation of MeWo-FTIII⋅H cells with anti-Lewis x or anti-sialyl Lewis x antibody (B) or by 10 μM or 50 μM IELLQAR peptide or by 50 μM control VTSIAQA peptide (C). (D–F) Inhibition of NK cell-mediated cytotoxic lysis of MeWo-FTIII⋅H cells by incubation of NK cells with anti-CD94, anti-CD69, or anti-L selectin antibody (D), by addition of sialyl Lewis x oligosaccharides attached to polyacrylamide gel (sLex) (E), or by anti-CD94 antibody or sLex (30% mole) oligosaccharide alone or anti-CD94 plus sLex (30% mole) oligosaccharides (F).
We then determined which cell surface receptors on NK cells, containing a lectin-like domain, are involved in recognition of sialyl Lewis x overexpressed on tumor cells. First, we found that anti-CD94 but not anti-CD69 or anti-L-selectin can inhibit NK cell-mediated cytolysis of MeWo-FTIII⋅H cells (Fig. 4D). Second, we showed that sialyl Lewis x oligosaccharides attached to polyacrylamide gel can inhibit the same NK cell-mediated cytolysis. The extent of the inhibition was more significant when highly dense sialyl Lewis x polymer was used than when less dense sialyl Lewis x polymer was used (Fig. 4E). Finally, we found that simultaneous addition of anti-CD94 antibody plus sialyl Lewis x oligosaccharide polymer does not result in an additive effect over the addition of anti-CD94 antibody or sialyl Lewis x oligosaccharide polymer alone (Fig. 4F). These results establish that the CD94 receptor on NK cells recognizes sialyl Lewis x expressed on tumor cells.
MeWo-FTIII⋅M Cells Express More Sialyl Lewis x on Poly-N-Acetyllactosamines than Do MeWo-FTIII⋅H Cells.
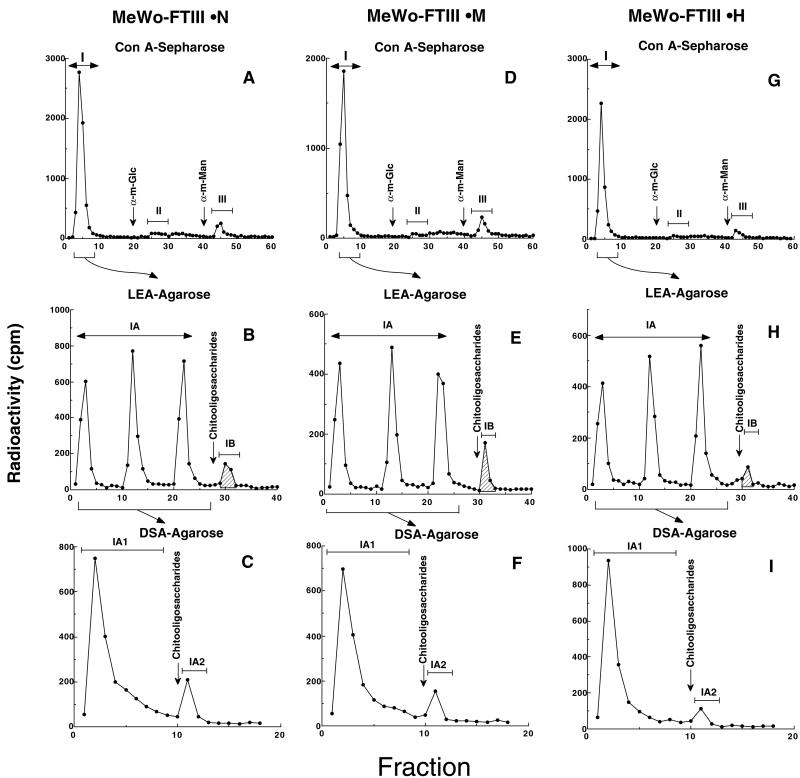
To understand the structural basis for differential susceptibility of MeWo-FTIII cell lines to NK cell attack, cells were metabolically labeled with [3H]galactose and the prepared glycopeptides were fractionated by ConA-Sepharose and LEA-agarose followed by DSA-agarose. MeWo-FTIII⋅M cells produced high levels of glycopeptides that bound to and eluted from LEA-agarose (IB in Fig. 5E). By contrast, fewer glycopeptides from MeWo-FTIII⋅H cells were bound to LEA-agarose (IB in Fig. 5H). When the glycopeptides unbound to LEA-agarose were applied to DSA-agarose, no significant difference was observed among three MeWo-FTIII cell lines in the amount of glycopeptides bound to DSA-agarose (IA2 in Fig. 5). It has been shown that the glycopeptides bound to LEA-agarose contain three or more N-acetyllactosamine repeats, whereas those bound to DSA-agarose include N-glycans containing one or two N-acetyllactosamines (31–33). These glycopeptides (IB and IA2 in Fig. 5) were then digested with endo-β-galactosidase before and after defucosylation (34). These results combined with the results shown in Fig. 1 indicate that MeWo-FTIII⋅M cells contain more sialyl Lewis x in poly-N-acetyllactosamine than do MeWo-FTIII⋅H cells. By contrast, MeWo-FTIII⋅H cells express the highest amount of sialyl Lewis x in short N-glycans among the different MeWo-FTIII cells.
Figure 5.
Serial lectin affinity chromatography of [3H]galactose-labeled glycopeptides. MeWo-FTIII⋅N (A–C), MeWo-FTIII⋅M (D–F), and MeWo-FTIII⋅H (G–I) were metabolically labeled with [3H]galactose, and glycopeptides prepared were subjected to affinity chromatography on ConA-Sepharose, LEA-agarose, and DSA-agarose. Horizontal bars indicate the glycopeptides subjected to the next step. Vertical arrows indicate the initiation of elution by a mixture of chitobiose and chitotriose. ConA-unbound glycopeptides were applied in portions to LEA-agarose, because poly-N-acetyllactosaminyl glycopeptides tend to become insoluble after lyophilization at this stage.
Discussion
The present study provides direct evidence that sialyl Lewis x plays a critical role in tumor metastasis and NK cell-mediated cytolysis. MeWo-FTIII⋅M cells expressing moderate amounts of sialyl Lewis x produced many more lung tumor foci than did the parental MeWo cells or MeWo-FTIII⋅N cells, which express negligible amounts of sialyl Lewis x. Furthermore, preincubation with anti-sialyl Lewis x antibody or preinjection of a sialyl Lewis x-mimicking peptide prevented tumor formation by MeWo-FTIII⋅M cells. The most striking discovery in the present study, however, is that overexpression of sialyl Lewis x on tumor cells, as seen in MeWo-FTIII⋅H cells, does not result in tumor formation in nude mice. By contrast, the same MeWo-FTIII⋅H cells produce a large number of tumor foci in beige mice, which have defective NK cells. These results are consistent with our previous findings showing that B16 melanoma cells overexpressing sialyl Lewis x can produce lung tumor foci only in beige mice or in C57BL/6 mice that have been depleted of NK cells by treatment with anti-asialo-GM1 antibody (20). Most importantly, the present study extends these findings by demonstrating that NK cells directly lyse B16-FTIII and MeWo-FTIII cells in proportion to the amount of sialyl Lewis x expressed on the cell surface. We demonstrate that NK cell-mediated lysis of MeWo-FTIII⋅H cells can be inhibited by preincubation of MeWo-FTIII⋅H cells with anti-sialyl Lewis x antibody or addition of sialyl Lewis x-mimicking IELLQAR peptide or sialyl Lewis x oligosaccharide polymer during the cytotoxicity assay. These combined results indicate that moderate expression of sialyl Lewis x on tumor cells results in tumor metastasis, whereas expression of high levels of sialyl Lewis x on tumor cells results in cytolysis by NK cells.
The above conclusion is corroborated by experiments in which MeWo-FTIII⋅H cells were analyzed after many passages. After more than 10 passages, MeWo-FTIII⋅H cells tend to lose sialyl Lewis x and are comparable in levels of sialyl Lewis expression to MeWo-FTIII⋅M cells. The resultant cells produce large numbers of tumor foci in nude mice, as do MeWo-FTIII⋅M cells, but are no longer highly susceptible to NK cell-mediated cytolysis (data not shown). These results, taken together, strongly support our conclusion that differences in the level of sialyl Lewis x expression lead to entirely different biological consequences.
Although it has been shown that NK cells can mediate cytolysis of tumor cells (for review, see ref. 35), ours is a clear-cut demonstration that a particular carbohydrate structure is recognized by NK cells as a cytolytic signal. We found that the CD94 receptor complex on NK cells recognizes sialyl Lewis x overexpressed on MeWo-FTIII. Moreover, we showed that simultaneous addition of anti-CD94 antibody and sialyl Lewis x oligosaccharide polymer did not have an additive inhibitory effect over the addition of either reagent alone. These results indicate that the CD94 receptor is solely responsible for the recognition of sialyl Lewis x overexpressed on tumor cells. The CD94 receptor complex can be classified into inhibitory and activating receptors, depending on subunits associated with CD94 (36, 37). Because anti-CD94 antibody inhibited NK-cell-mediated cytotoxicity toward MeWo-FTIII⋅H cells, it can be concluded that the activating CD94 receptor complex functions in this cytotoxicity; otherwise, anti-CD94 antibody treatment should enhance the cytotoxicity to the tumor cells.
Our structural analysis and cell-surface staining by different lectins indicate that MeWo-FTIII⋅M and MeWo-FTIII⋅H exhibit few differences in the level and composition of cell-surface carbohydrates except that MeWo-FTIII⋅H cells contain more heavily fucosylated short N-glycans than do MeWo-FTIII⋅M cells, whereas MeWo-FTIII⋅M cells contain more poly-N-acetyllactosamine than do MeWo-FTIII⋅H cells. We also have shown that B16-FTIII⋅H cells express more sialyl Lewis x on short N-glycans than do B16-FTIII⋅M cells (20). These results taken together indicate that dense expression of sialyl Lewis x leads to cytolysis by NK cells.
In conclusion, we have shown that sialyl Lewis x is recognized by CD94 on NK cells. Apparently, CD94 binds more efficiently to sialyl Lewis x densely attached to carrier glycans than to sialyl Lewis x sparsely attached to carrier glycans. By contrast, sparsely or moderately attached sialyl Lewis x facilitates tumor metastasis. These results clearly indicate that the difference in sialyl Lewis x expression leads to entirely different biological consequences, providing a potential carbohydrate-based tumor therapy.
Acknowledgments
We thank Dr. John Lowe for the gift of FTIII cDNA, Dr. Wayne Yokoyama for useful discussions, Dr. Elise Lamar for critical reading of the manuscript, and Joseph P. Henig and Tracy Keeton for organizing the manuscript. This work was supported by National Cancer Institute, National Institutes of Health Grants R37 CA33000 and PO1 CA71932. S.C. was supported by a Cancer Research Institute fellowship.
Abbreviations
- FTIII
α1,3-fucosyltransferase III
- NK
natural killer
- LEA
tomato lectin
- DSA
Datura stramonium agglutinin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hakomori S. Cancer Res. 1985;45:2405–2414. [PubMed] [Google Scholar]
- 2.Feizi T. Nature. 1985;314:53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda M. Cancer Res. 1996;56:2237–2244. [PubMed] [Google Scholar]
- 4.Fukuda M, Spooncer E, Oates J E, Dell A, Klock J C. J Biol Chem. 1984;259:10925–10935. [PubMed] [Google Scholar]
- 5.Mizoguchi A, Takasaki S, Maeda S, Kobata A. J Biol Chem. 1984;259:11949–11957. [PubMed] [Google Scholar]
- 6.Springer T A. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 7.Homeister J W, Lowe J B. In: Molecular and Cellular Glycobiology. Fukuda M, Hindsgaul O, editors. Oxford: Oxford Univ. Press; 2000. pp. 62–115. [Google Scholar]
- 8.Hiraoka N, Petryniak B, Nakayama J, Tsuboi S, Suzuki M, Yeh J C, Izawa D, Tanaka T, Miyasaka M, Lowe J B, et al. Immunity. 1999;11:79–89. doi: 10.1016/s1074-7613(00)80083-7. [DOI] [PubMed] [Google Scholar]
- 9.Hemmerich S, Bistrup A, Singer M, van Zante A, Lee J K, Tsay D, Peters M, Carminati J L, Brennan T J, Carver-Moore K, et al. Immunity. 2001;15:237–247. doi: 10.1016/s1074-7613(01)00188-1. [DOI] [PubMed] [Google Scholar]
- 10.Yeh J-C, Hiraoka N, Petryniak B, Nakayama J, Ellies L G, Rabuka D, Hindsgaul O, Marth J D, Lowe J B, Fukuda M. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 11.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Cancer Res. 1993;53:354–361. [PubMed] [Google Scholar]
- 12.Berg E L, Robinson M K, Mansson O, Butcher E C, Magnani J L. J Biol Chem. 1991;266:14869–14872. [PubMed] [Google Scholar]
- 13.Magnani J L, Nilsson B, Brockhausen M, Zopf D, Steplewski Z, Koprowski H, Ginsburg V. J Biol Chem. 1982;257:14365–14369. [PubMed] [Google Scholar]
- 14.Itzkowitz S H, Yuan M, Fukushi Y, Lee H, Shi Z R, Zurawski V, Jr, Hakomori S, Kim Y S. Cancer Res. 1988;48:3834–3842. [PubMed] [Google Scholar]
- 15.Fukushima K, Hirota M, Terasaki P I, Wakisaka A, Togashi H, Chia D, Suyama N, Fukushi Y, Nudelman E, Hakomori S. Cancer Res. 1984;44:5279–5285. [PubMed] [Google Scholar]
- 16.Fukuda M, Bothner B, Ramsamooj P, Dell A, Tiller P R, Varki A, Klock J C. J Biol Chem. 1985;260:12957–12967. [PubMed] [Google Scholar]
- 17.Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y, Irimura T. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- 18.Shimodaira K, Nakayama J, Nakamura N, Hasebe O, Katsuyama T, Fukuda M. Cancer Res. 1997;57:5201–5206. [PubMed] [Google Scholar]
- 19.Renkonen J, Paavonen T, Renkonen R. Int J Cancer. 1997;74:296–300. doi: 10.1002/(sici)1097-0215(19970620)74:3<296::aid-ijc11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Ohyama C, Tsuboi S, Fukuda M. EMBO J. 1999;18:1516–1525. doi: 10.1093/emboj/18.6.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Satué M, de Castellarnau C, Blanco J. Br J Cancer. 1999;80:1169–1174. doi: 10.1038/sj.bjc.6690482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martens C L, Cwirla S E, Lee R Y, Whitehorn E, Chen E Y, Bakker A, Martin E L, Wagstrom C, Gopalan P, Smith C W, et al. J Biol Chem. 1995;270:21129–21136. doi: 10.1074/jbc.270.36.21129. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda M N, Ohyama C, Lowitz K, Matsuo O, Pasqualini R, Ruoslahti E, Fukuda M. Cancer Res. 2000;60:450–456. [PubMed] [Google Scholar]
- 24.Zhang J, Nakayama J, Ohyama C, Suzuki M, Suzuki A, Fukuda M, Fukuda M N. Cancer Res. 2002;62:4194–4198. [PubMed] [Google Scholar]
- 25.Kukowska-Latallo J F, Larsen R D, Nair R P, Lowe J B. Genes Dev. 1990;4:1288–1303. doi: 10.1101/gad.4.8.1288. [DOI] [PubMed] [Google Scholar]
- 26.Takeda K, Oshima H, Hayakawa Y, Akiba H, Atsuta M, Kobata T, Kobayashi K, Ito M, Yagita H, Okumura K. J Immunol. 2000;164:1741–1745. doi: 10.4049/jimmunol.164.4.1741. [DOI] [PubMed] [Google Scholar]
- 27.Weitz-Schmidt G, Stokmaier D, Scheel G, Nifant'ev N E, Tuzikov A B, Bovin N V. Anal Biochem. 1996;238:184–190. doi: 10.1006/abio.1996.0273. [DOI] [PubMed] [Google Scholar]
- 28.Gallatin W M, Weisman I L, Butcher E C. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 29.De Maria R, Cifone M G, Trotta R, Rippo M R, Festuccia C, Santoni A, Testi R. J Exp Med. 1999;180:1999–2004. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, López-Botet M, Geraghty D E. Proc Natl Acad Sci USA. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee N, Wang W C, Fukuda M. J Biol Chem. 1990;265:20476–20487. [PubMed] [Google Scholar]
- 32.Saitoh O, Wang W C, Lotan R, Fukuda M. J Biol Chem. 1992;267:5700–5711. [PubMed] [Google Scholar]
- 33.Merkle R K, Cummings R D. J Biol Chem. 1987;262:8179–8189. [PubMed] [Google Scholar]
- 34.Maemura K, Fukuda M. J Biol Chem. 1992;267:24379–24386. [PubMed] [Google Scholar]
- 35.Trinchieri G. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama W M. Curr Opin Immunol. 1998;10:298–305. doi: 10.1016/s0952-7915(98)80168-4. [DOI] [PubMed] [Google Scholar]
- 37.Lanier L L. Nat Immunol. 2001;2:23–27. doi: 10.1038/83130. [DOI] [PubMed] [Google Scholar]