Abstract
Synaptobrevins or VAMPs are vesicle-associated membrane proteins, often called v-SNARES, that are important for vesicle transport and fusion at the plasma membrane. Drosophila has two characterized members of this gene family: synaptobrevin (syb) and neuronal synaptobrevin (n-syb). Mutant phenotypes and gene-expression patterns indicate that n-Syb is exclusively neuronal and required only for synaptic vesicle secretion, whereas Syb is ubiquitous and, as shown here, essential for cell viability. When the eye precursor cells were made homozygous for syb−, the eye failed to develop. In contrast, n-syb− eye clones developed appropriately but failed to activate downstream neurons. To determine whether the two proteins are structurally specialized to accomplish these distinct in vivo functions, we have driven the expression of each gene in the absence of the other to look for phenotypic rescue. We find that expression of n-syb during eye development can rescue the cell lethality of the syb mutations, as can rat VAMP2 and cellubrevin. Expression of syb can restore synaptic transmission to n-syb mutants as assayed both by electroretinogram and recordings of excitatory junctional currents at the neuromuscular junction. Therefore, we find that Syb, which usually is not involved in synaptic function, can mediate Ca2+-triggered synaptic activity and that no particular specialization of the v-SNARE is required to differentiate synaptic exocytosis from other forms.
Synaptobrevins, also called vesicle-associated membrane protein (VAMPs), reside on exocytotic vesicles and mediate their fusion by interacting with the plasma membrane proteins syntaxin and SNAP-25. This complex, often called the SNAP-receptor (SNARE) complex, may closely appose the two membranes to create an activated docked state and drive vesicle fusion. Abundant evidence implicates these proteins in a late stage of membrane fusion (1). Moreover, this mechanism seems to be shared with many forms of intracellular trafficking in which homologous proteins function to fuse a vesicle with its target membrane (2). The SNARE proteins on the vesicles are referred to as v-SNAREs, and those on the target membrane are called t-SNAREs.
At present, it is unclear to what extent the SNAREs that are involved in a particular form of trafficking can substitute for one another and to what extent they are specialized for their individual tasks. Different intracellular membranes contain different SNAREs, and even within the plasma membrane, different domains such as the apical and basolateral domains of epithelial cells may be marked with different forms of these proteins (1, 3). v- and t-SNAREs have been hypothesized to have precise cognate partners and thereby provide the specificity by which fusion of each vesicle class with its appropriate target membrane is accomplished (1, 4–7). However, there also is evidence that the formation of tight SNARE complexes is not always highly selective among v- and t-SNARE family members. Moreover, the removal of a SNARE protein, either with toxins or genetically, does not prevent synaptic vesicles from docking at their appropriate sites on the presynaptic membrane (1, 8–12). Finally, the localization of some SNAREs is insufficiently precise to provide the sole determinant of specificity in trafficking. For example, the plasma membrane t-SNAREs, syntaxin and SNAP-25, which have been shown to mediate synaptic transmission, are not found exclusively at the nerve terminal but rather occur along the entire axon (13). Other individual SNAREs are reported on multiple intracellular compartments and are implicated in multiple trafficking steps (14). It seems likely, therefore, that alternative tethering and docking proteins provide a primary layer of specificity to membrane targeting and that specificity may be enhanced further by preferences for certain v-SNARE/t-SNARE pairs (1, 15).
Beyond the recognition of appropriate target membranes, SNAREs may be specialized in other ways for their particular tasks. Such specializations could include interactions with regulatory proteins that are associated with a particular instance of membrane fusion. Particularly in the case of synaptic transmission, properties of the SNARE proteins may confer some of the unique features of the nerve terminal: a stable pool of docked, fusion-competent, readily releasable vesicles and the efficient ability to fuse vesicles in that pool within hundreds of microseconds in response to a transient rise in cytosolic Ca2+. Thus, for example, the t-SNARE SNAP-25 has been shown to contain a set of three aspartate residues that are necessary for efficient Ca2+-evoked release of transmitter, and loss of particular synaptic SNAREs in Drosophila and mammalian cells causes a selective loss of rapid Ca2+-evoked release while permitting slower modes of release to remain (16–19).
Drosophila genetics offers an opportunity to test the specificity of two v-SNAREs and inquire whether the presence of these two genes encodes either selectivity for target membranes or susceptibility to Ca2+-dependent regulation at the synapse. Drosophila has two characterized synaptobrevin genes. Mutations of these genes, synaptobrevin (syb) and neuronal-synaptobrevin (n-syb), produce very different phenotypes, suggesting a functional specialization. The n-syb gene is specific to neurons and synapses, and n-syb mutants have a strictly synaptic phenotype. n-syb null embryos develop normally, but their nerve terminals do not release transmitter in response to an action potential (16, 17). Despite this, the n-syb−/− synapse evinces spontaneous transmitter release. Thus the triggered release of transmitter is mechanistically distinct from spontaneous fusions as well as from those fusions that brought membrane to the cell surface and allowed the development of the cell and the elaboration of its axon.
Syb is the primary candidate for mediating the fusion of vesicles with the plasma membrane for cell growth and maintenance. The homology of syb with vertebrate synaptobrevins strongly suggests a role in fusion at the plasma membrane. Syb is also widespread within the organism and is not concentrated at the synapse (20, 21). As described here, loss of syb in a somatic clone of cells is lethal.
This paper describes the syb phenotype and investigates whether n-Syb and Syb can substitute for one another despite their normally disparate roles. We thereby tested the hypotheses that v-SNAREs confer specificity on the interaction of vesicles and target membranes and that Ca2+-triggered fusion would place unique requirements on the relevant v-SNARE. We find that the two genes are capable of rescuing each other's cellular defects and therefore, in at least some cases, SNARE proteins may be interchangeable in vivo.
Materials and Methods
DNA Constructs.
pUAST-syb, pUAST-n-syb, pUAST-VAMP2, and pUAST-cellubrevin were made by cloning the syb-a, n-syb, rat VAMP2, and cellubrevin ORFs into pUAST with added 5′-XhoI and 3′-XbaI sites. The n-syb and syb-a ORFs were subsequently transferred to pCaSpeR-hs by linearizing the pUAST constructs with XhoI and blunting the ends with Klenow and dNTPs. The DNA then was cut with XbaI, and this fragment was ligated to HpaI- and XbaI-cut pCaSpeR-hs. Clones were sequenced before microinjection for germ-line transformation. Numerous transformant lines were obtained with each construct including pHs-sybA-1-2, pHs-sybB-2-1, pHs-n-sybII-7-1, pHs-n-sybII-10-1, and pHs-n-sybII-10-2, which were used here. Heat-shock induction of the transgenes was either for 30 min twice a day or 1 h once daily.
Stocks.
Df(2R)X1 was from P. Taghert (Washington University, St. Louis). Df(2R)12 was generated by X-irradiation of a stock (B-1-2nd-5; courtesy of L. Jan, University of California, San Francisco) that bore a w+ P element at 47A and by selection for w chromosomes that were both homozygous-lethal and lethal in combination with Df(2R)X1. syb21-15 and syb25-77 were identified among ethylmethane sulfonate-induced lethal mutations that mapped to the overlap of Df(2R)X1 and Df(2R)12 and were provided by E. Goldstein (Arizona State University, Tempe). syb144 is a deletion in the syb gene generated by the imprecise excision of a P element insertion in the second intron, l(2)k07705 (B.D.M., Y. Guo, K. Kaiser, and C.J.O., unpublished data). n-sybΔF33B is a characterized null allele (16).
Construction of Lines for Phenotypic Rescue.
Rescue of syb eye morphology.
y,w;FRT42D,GMR-hid/CyO;ey-GAL4,UAS-FLP stock (22) was crossed to y,w;pRESCUE,FRT42D,syb144 (or syb21-15)/CyO,y+. pRESCUE refers to one of the following constructs: pHs-sybB-2-1, pHs-n-sybII-10-2, pUAST-VAMP2, or pUAST-cellubrevin.
Rescue of n-syb electroretinogram (ERG) defects.
y,w;ey-GAL4, UAS-FLP;FRT80B,GMR-hid/TM2 was crossed to y,w;pHs-sybB-2-1/CyOy+;FRT80B,n-sybΔF33Bb/TM6,y+. In controls, the above cross was carried out without pHs-sybB-2-1.
Rescue of n-syb neuromuscular physiology.
n-syb homozygotes were obtained by selecting for y− larvae in stocks of y,w;pHs-n-sybII-7-1;NsybΔF33B/TM6,y+ or y,w;pHs-sybB-2-1;NsybΔF33B/TM6,y+.
Production of Recombinant Proteins and Gel Electrophoresis of SNARE Complexes.
Construction, expression, purification, and cleavage of GST moiety of GST-tagged Drosophila SNAP-25, syntaxin 1A (amino acid 4–269), n-Syb (amino acid 1–104), and Syb (amino acid 1–110) were performed as described (23). Complex formation was obtained by incubating 2 μM of each recombinant protein in binding buffer (50 mM Tris, pH 8.0/150 mM NaCl/1 mM EDTA or 2 mM CaCl2, final volume 30 μl) overnight at 4°C. After the addition of 2× SDS loading buffer (final SDS concentration, 0.67%), samples were incubated for 3 min at the given temperatures. Complexes were resolved on 12% SDS/PAGE gels, transferred to nitrocellulose membranes, and visualized by anti-syntaxin antibody 8C3 (a gift of S. Benzer, California Institute of Technology, Pasadena, CA).
Scanning Electron Microscopy.
Prepared flies (22) were analyzed with a Philips Electron Optics model 505 SEM (Eindhoven, The Netherlands).
Electrophysiology.
ERGs were performed as described (22). For neuromuscular junction recordings, transgenes were induced at 37°C for 1 h, twice, ≈24 h apart. At least 2 h elapsed between the final heat pulse and the recordings. Embryos were glued to Sylgard (Dow-Corning)-coated slides with Nexaband (Veterinary Laboratory Products, Phoenix), applied although a glass micropipette, and then covered with physiological saline. The embryos were dissected open along the dorsal midline by using glass needles, and the dorsal edges were glued to the slide. The preparations then were treated with 1 mg/ml Sigma collagenase type IV for 1 min and washed with saline repeatedly. The physiological saline was HL3 (24) with the exception that Ca2+ was 4 mM, and Mg2+ was 12 mM. Synaptic currents were recorded in the whole-cell mode with 8–10 MΩ borosilicate glass pipettes filled with an intracellular solution (17), an Axopatch 1D amplifier, and pClamp. Suction pipettes were applied lightly to the segmental nerves near their exit from the ventral ganglion, and 1-ms stimuli were delivered to the nerve at 1 Hz.
Results
syb Mutations Are Cell-Lethal.
The syb gene, which resides at 46F on the second chromosome, was found to be removed by two deficiencies, Df(2R)X1 and Df(2R)12. Two complementation groups of lethal ethylmethane sulfonate-induced mutations that fail to complement both of these deficiencies were identified (see Materials and Methods), and one of these complementation groups was determined to be the syb gene by sequencing of the ORF. Two alleles were identified: syb25-77 has a C-to-T change that replaces Q95 with a stop codon, and syb21-15 has a G-to-A change that replaces W105 with a stop codon. Both alleles thus truncate the protein before the transmembrane domain that anchors Syb to the vesicle membrane. Alternative splicing generates two forms, Syb-a and Syb-b, that differ in their C termini, and both forms are truncated in syb25-77 and syb21-15. An additional allele, syb144, is a deletion derived by imprecise excision of a P element insertion. This deletion removes all of the syb ORF apart from 16 N-terminal amino acids encoded by exon 2 and 25 C-terminal amino acids of Syb-a encoded by exon 5 and therefore is adjudged to be a null allele. syb25-77 and syb21-15 behaved identically to the deletion allele with regard to lethal period and other phenotypes and appear to be equivalent to null mutations when placed over the larger deficiencies. No dominant effect of the truncated proteins was detected. Thus, although some functional SNARES are known to lack transmembrane domains, the removal of the transmembrane domain from Syb prevented its function.
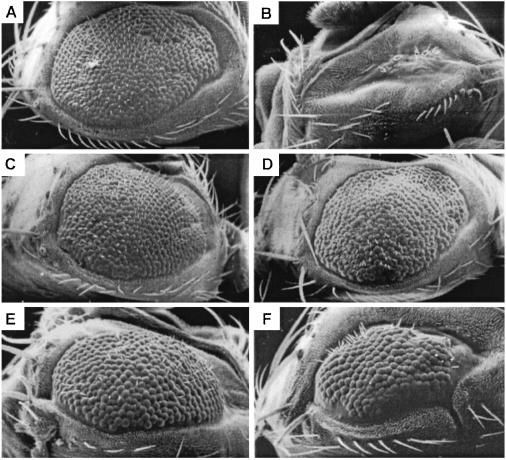
The distinct functions of Syb and n-Syb are apparent from an analysis of the eye. The eyeless-GAL4 UAS-FLP (EGUF)/hid technique (22) was used to generate flies in which all the cells of the eye, and only the cells of the eye, were made homozygous for mutations of either syb or n-syb. In control experiments, a wild-type chromosome arm is made homozygous in the eye by the same method (Fig. 1A) so as to factor out the modest reduction in size and roughening of the eye that arises as a consequence of the EGUF/hid method. When any of the three alleles of syb were made homozygous in the eye, the eye failed to develop, and all or nearly all the ommatidia were absent (Fig. 1B). Cell lethality also was observed in eye mosaics made with heat-shock-driven FLP recombinase and without the EGUF/hid method (data not shown). These findings demonstrate the cell lethality of syb mutations.
Figure 1.
Eye phenotypes of syb and n-syb. To analyze eye phenotypes by scanning electron microscopy, somatic recombination with the EGUF/hid method was used to make the entire eye and only the eye homozygous for either a wild-type (A), syb144 (B), or n-sybΔF33B (C) chromosome. (A) The control eye is slightly roughened and reduced in consequence of the recombination and cell death entailed in the EGUF/hid method. (B) syb144 ablates all or nearly all the ommatidia, and the eye is reduced to a scar. (C) In contrast, n-sybΔF33B appears similar to control. (D Left) ERG of a control eye similar to that in A illustrating the response to a 1-s flash of light (bar). On and off transients corresponding to synaptically evoked responses are marked by arrows. (D Right) Similar ERG recording from an eye homozygous for n-sybΔF33B, as in C. A robust response to light is obtained. On and off transients, however, were always completely absent (arrows). Specific genotypes were y,w;FRT42D, GMR-hid/FRT42D;ey-GAL4,UAS-FLP/+ (A), y,w;FRT42D,GMR-hid/FRT42D, syb144;ey-GAL4,UAS-FLP/+ (B) y,w;ey-GAL4,UAS-FLP/+;FRT80B,GMR-hid/FRT80B,n-sybΔF33B (C and D Right), and y,w;ey-GAL4,UAS-FLP/+;FRT80B, GMR-hid/FRT80B (D Left).
n-syb Mutations Alter the Synapse-Dependent Component of the ERG.
Eyes made homozygous for a null allele of n-syb (n-sybΔF33B) were externally indistinguishable from eyes in which a wild-type chromosome had been made homozygous (Fig. 1C). Thus, n-Syb is not essential for cell division, viability, or differentiation of the photoreceptors (25). A field-potential recording from the surface of the eye, the ERG, was used to assay synaptic transmission, because the ERG response has been shown to contain components, transient signals observed at light-on and light-off, that are manifestations of the activation of second-order cells and therefore indicate successful synaptic transmission (22, 26). In an eye in which a wild-type chromosome has been made homozygous, the ERG is normal (Fig. 1D Left). In contrast, in n-syb−/− eyes, the on and off transients are absent (Fig. 1D Right). Thus transmission at the photoreceptor synapse requires n-Syb, as expected from observations with tetanus toxin and studies of the neuromuscular junction (16, 17, 25).
Both Syb and n-Syb Form SDS-Resistant Complexes with Syntaxin and SNAP-25.
Biochemical analyses were performed to determine whether the distinct phenotypes of syb and n-syb mutants arose from the relative ability of these proteins to complex with the known synaptic t-SNAREs, Drosophila syntaxin 1A and SNAP-25. To this end, recombinant proteins were purified and allowed to form complexes (see Materials and Methods). Cognate SNARE complexes are highly stable even in the presence of SDS, and to probe the relative stability of Syb- and n-Syb-containing complexes further, the stability of the complexes was tested at 37, 65, 75, 85, and 100°C. Syb and n-Syb were capable of coassembling into higher molecular weight SDS-resistant complexes with SNAP-25 and syntaxin (Fig. 2). Moreover, these complexes had similar thermal stabilities in SDS, dissociating partially at 65°C and completely at 75°C and above (higher temperature data not shown). Thus there was no indication of a significant selectivity of n-Syb over Syb in its ability to interact with its synaptic partners. Similarly, no selectivity was detected previously in the binding of these v-SNARES to complexes containing the nonsynaptic t-SNARE, SNAP-24 (23). Although in these in vitro assays, the in vivo functional differences of Syb and n-Syb do not appear to be reflected, subtle but functionally important differences between the complexes could still exist. Therefore, we tested the interchangeability of synaptobrevins in vivo.
Figure 2.
Both n-Syb and Syb form SDS-resistant complexes. Recombinant SNAP-25, syntaxin 1A, and either Syb or n-Syb were allowed to form complexes, and the temperature sensitivity of the complex in SDS was assayed. Complexes were visualized with antibodies to syntaxin. The position of monomeric syntaxin is marked, as are the positions of the SNARE complexes. A small amount of immunoreactivity at ≈55 kDa is due to uncleaved GST-syntaxin that comigrates with SNARE complex 1 but does not depend on the presence of other SNARE partners.
Other Synaptobrevins Can Rescue the Cell Lethality of syb.
To test whether synaptobrevins could substitute for one another in vivo, separate transgenic lines were generated that expressed Syb, n-Syb, rat VAMP2, and rat cellubrevin. Syb-a and n-Syb each were expressed under the control of a heat-shock-inducible promoter (hsp70), which was activated by placing the flies at 37°C at regular intervals (see Materials and Methods). Rat VAMP2 and rat cellubrevin were expressed under the control of a UAS promoter that was activated by the eyeless-GAL4 construct used in the EGUF/hid system. Multiple transgenic lines were established with these constructs. These transgenic constructs were crossed into the appropriate genetic background for using the EGUF/hid system to create eyes homozygous for either syb144 or syb21-15. Two Syb and three n-Syb transgenic lines were tested, and each of these rescued the syb cell-lethal phenotype and restored the ommatidia (Fig. 3). The extent of the rescue varied within the progeny of a single cross, which is likely to arise from variation in the time of gene activation by heat shock relative to critical moments in the development of the eye. Nonetheless, whether the transgene was syb or n-syb, the majority of the eyes were rescued to greater than 80% of the size of the control [Syb, 95 of 137 (69%); n-Syb, 76 of 112 (68%)], and no eyes were encountered that did not show some degree of rescue. The transgenes rescued syb144 and syb21-15 equally. Thus, n-Syb is capable of substituting for Syb in vivo. The VAMP2 and cellubrevin transgenes (one transformant of each) also were able to rescue the syb defect (Fig. 3 E and F).
Figure 3.
The cell lethality of a syb mutation in the eye was rescued by other synaptobrevins. (A) Scanning electron micrograph of a control eye with no syb mutation. (B) An eye that is homozygous for syb144 lacks most ommatidia. syb mutant eyes, were rescued with transgenes bearing syb (C), n-syb (D), rat VAMP2 (E), and rat cellubrevin (F). Specific genotypes were y,w;FRT42D,GMR-hid/FRT42D;ey-GAL4,UAS-FLP/+ (A), y,w;FRT42D,GMR-hid/FRT42D,syb144;ey-GAL4,UAS-FLP/+ (B), y,w;FRT42D,GMR-hid/pHs-sybB-2-1,FRT42D,syb21-15; ey-GAL4,UAS-FLP/+ (C), y,w;FRT42D,GMR-hid/pHs-n-sybII-10-2,FRT42D,syb21-15;ey-GAL4,UAS-FLP/+ (D), y,w;FRT42D, GMR-hid/pUAST-VAMP2,FRT42D,syb21-15;ey-GAL4,UAS- FLP/+ (E), and y,w;FRT42D,GMR-hid/pUAST-cellubrevin FRT42D,syb144;ey-GAL4,UAS-FLP/+ (F).
Syb Expression Can Rescue Synaptic Transmission in n-syb.
Although n-Syb could substitute for Syb in restoring cell viability, the reciprocal ability of Syb to substitute for n-Syb was potentially more doubtful, because action potential-driven neurosecretion involves regulatory controls and potential modifications for speed that might not be compatible with SNAREs of a slow, general trafficking pathway. The Syb transgene therefore was crossed into the appropriate genetic background to create eyes homozygous for n-sybΔF33B, and Syb expression was induced by periodic heat shocks. In the ERG, Syb expression restored the on and off transients (Fig. 4). Thus the Syb protein, which normally does not mediate synaptic transmission, is able to substitute for n-Syb in the functioning of this synapse.
Figure 4.
syb can rescue the ERG defect of n-syb. ERG recordings of n-syb+ (A) and n-syb homozygous null (B) eyes formed by somatic recombination by the EGUF/hid system. The n-syb mutation removes the on and off transients. However, the expression of a syb transgene restores the transient deflections as shown by the arrows in C. (A) y,w;ey-GAL4,UAS-FLP/+;FRT80B,GMR-hid/FRT80B; (B) y,w;ey-GAL4,UAS-FLP/+;FRT80B,GMR-hid/FRT80Bn-sybΔF33Bb; (C) y,w;ey-GAL4,UAS-FLP/pHs-sybB-2-1;FRT80B,GMR-hid/FRT80Bn-sybΔF33Bb.
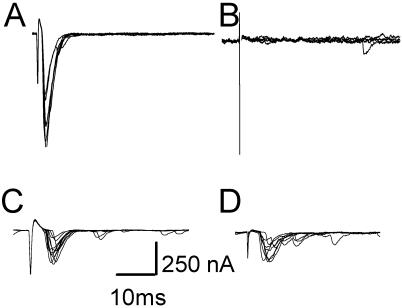
We recorded from the embryonic neuromuscular junction for a more direct assay of transmission (Fig. 5). The neuromuscular junctions of n-sybΔF33B are incapable of synchronous, evoked synaptic transmission at the neuromuscular junction even in the presence of elevated Ca2+ or K+-channel blockers to enhance release (16). The expression of either Syb or n-Syb in embryos homozygous for n-sybΔF33B was accomplished with the same transgenes used above. When n-Syb was expressed in the embryo, a partial rescue of the phenotype was observed (390.8 ± 115.9 nA, n = 4; Fig. 5C). The smaller amplitude of the synaptic current likely results from lower levels of n-Syb at the neuromuscular junction, as compared with wild-type flies in which n-Syb expression is driven by its own promoter; synaptic vesicle proteins may need to be expressed in a very narrow developmental window to be packaged successfully into synaptic vesicles. The ability of a Syb transgene to rescue synaptic transmission, however, was similar in magnitude to that of n-Syb (398.7 ± 78.2 nA, n = 3; Fig. 5D), and thus Syb was capable of functionally substituting for n-Syb in synaptic transmission.
Figure 5.
syb and n-syb transgenes can restore synaptic transmission in n-syb null mutants. (A) Representative whole-cell recordings of nerve-evoked excitatory junctional currents in a wild-type embryo. (B) No evoked responses are observed in an n-syb mutant, but responses are restored partially by the presence of either n-Syb (C) or Syb (D) transgenes. In each panel, responses to 10 consecutive stimuli are shown. Specific genotypes are OrR (A), y,w;n- sybΔF33B (B), y,w;pHs-n-sybII-7-1;n-sybΔF33B (C), and y,w;pHs-sybB-2-1; n-sybΔF33B (D).
Discussion
Membrane trafficking to the cell surface is a complex process in which different classes of transport vesicle bear different cargoes to different membrane domains. In neurons, distinct classes of vesicles have been observed for synaptic vesicle proteins, for proteins required to build presynaptic active zones, and for postsynaptic proteins such as transmitter receptors. Neurite outgrowth in cultured mammalian neurons has been shown to involve distinct SNAREs from those found in the terminal (27–29). Does the cognate recognition of v- and t-SNARE partners, which is hypothesized to play an important role in the selective fusion of transport vesicles at intracellular compartments, also selectively direct cargoes to appropriate domains of the plasma membrane? Differential distribution of SNAREs in epithelial cells and neurons has suggested such a mechanism, but differential distribution does not mean that the SNAREs are actually restricting the site of fusion by virtue of their selective ability to form complexes.
The isolation of mutations in both syb and n-syb has provided an opportunity to examine the phenotypes of two v-SNAREs and the degree to which they are interchangeable. The phenotype that we report for syb null mutations is extremely different from that of n-syb null mutants and is consistent with a disparate in vivo role for Syb. The phenotypic differences are most apparent when the phenotypes are compared in the same organ, the well described retina of Drosophila. The ubiquitous distribution of Syb (20, 21) had predicted a role in general cellular traffic to the membrane surface, and consistent with this hypothesis, the absence of Syb proved lethal to the homozygous cells.
In contrast, cells homozygous for n-syb do not die and instead develop a normal retina that, however, is defective in synaptic transmission. This finding is consistent with studies in which tetanus toxin, a highly selective protease that cleaves n-Syb, was expressed in the photoreceptors (25). Thus one SNARE has an essential role in cell viability, whereas the other functions exclusively at the synapse. In vivo, therefore, these proteins are likely to mediate different classes of membrane fusion and to be associated with vesicles bearing distinct cargoes. In particular, n-Syb is uniquely associated with fast Ca2+-triggered fusion at synaptic active zones.
A similar distinction has been proposed for one of the Drosophila t-SNAREs at the plasma membrane. SNAP-25 is concentrated at synapses and is primarily neuronal, whereas a closely related homolog, SNAP-24, has a ubiquitous distribution and is implicated in nonneuronal exocytosis (23, 30). In contrast, the other plasma membrane t-SNARE, syntaxin 1, is shared by both general cellular traffic and transmitter secretion. Mutations of syntaxin are cell-lethal in clones in the eye, the female germ line, and wing discs (22, 31) and disrupt cellularization of the early embryo (32). The same syntaxin 1 gene is essential for synaptic transmission (31, 33). These studies led to a hypothesis that synaptic transmission is mediated by vesicular n-Syb binding selectively to syntaxin 1 and SNAP-25, whereas general cellular traffic is mediated by Syb binding to syntaxin 1 and SNAP-24.
To test this hypothesis, we examined the ability of the two v-SNAREs to interact with the synaptic t-SNARES and observed no differences in their ability to form tight, SDS-resistant complexes (Fig. 2). It has been suggested, however, that the ability to form a complex in vitro need not correlate with an in vivo ability to undergo the necessary conformational changes that may underlie membrane fusion (7). We therefore examined the functional competence of these v-SNAREs to substitute for one another and found that they behaved interchangeably in the eye in assays of cell viability (Fig. 3) and synaptic function (Fig. 4). The ability of syb to function at the synapse was confirmed with recordings from neuromuscular junctions; rapid synaptic responses could be evoked from n-syb null synapses that were rescued with either syb or n-syb transgenes (Fig. 5). Although this does not exclude the possibility that subtle or quantitative differences exist in regulatory aspects of synaptic transmission, such as the synchrony of release or ability of the synapse to be modulated by second messengers such as cAMP (17, 34), it indicates that Syb-containing complexes are competent for mediating the regulated fusion of synaptic vesicles.
The ability of alternative SNAREs to substitute for Syb extended to rat VAMP2 and rat cellubrevin. VAMP2, similar to n-Syb, is associated primarily with synaptic vesicles (35). Cellubrevin (also called VAMP3) may be comparable to Syb and has been implicated in recycling of receptors from endosomes to the plasma membrane (36, 37) and neurite outgrowth (28). The rescue of cell viability in the eye by these additional SNAREs further confirmed the lack of highly restricted cognate pairing.
In Drosophila nerve terminals lacking n-Syb, earlier work from this and other labs has demonstrated that spontaneous fusion of vesicles occurred (although at reduced rates) despite the complete blockade of evoked, synchronous transmitter release (9, 16, 17, 34). A similar phenotype has been observed in murine syb mutants (18). It was tempting to speculate at the time that spontaneous release (often called “minis”) was mediated by the general trafficking machinery, including Syb, but that this machinery was incapable of supporting evoked release. This hypothesis is less likely, however, in light of the present finding that Syb can support evoked release. If Syb were present on synaptic vesicles in the n-Syb mutants at levels adequate to mediate the spontaneous fusion of vesicles, why wasn't at least a small amount of evoked release retained? These spontaneous fusions may have a much lower requirement for the presence of a v-SNARE, may involve no v-SNARE at all, may involve an as-yet-uncharacterized third v-SNARE, or may be mediated by syntaxin on vesicles substituting for a v-SNARE.
One question that arises is: Why doesn't the substitution of Syb for n-Syb happen in these mutants in the absence of expression from transgenes? In the case of syb mutants, n-Syb expression is not activated until synaptogenesis occurs, and thus n-Syb is not available to rescue the viability of the eye precursor cells. In the case of n-syb mutants, the situation is more complicated in that some Syb must be present to support the survival of these cells (Fig. 1). The likely explanation is that, unless expressed by the heterologous promoter of the transgene, Syb is not incorporated adequately into synaptic vesicles.
The interchangeability of the particular SNAREs used in this study does not imply that cognate SNARE pairing does not promote specificity for other membrane-trafficking events such as polarized transport in epithelial cells or selective delivery to an intracellular compartment. SNAREs from the endoplasmic reticulum or Golgi, for example, may not be competent to substitute for either Syb or n-Syb. Additional genetic tests of SNARE functioning may clarify this matter but will be complicated by the need to overcome the selective localization of the SNAREs to their normal compartments. Our findings, however, are consistent with the observation that mutations and toxins that remove SNAREs do not prevent synaptic vesicles from being transported to terminals, clustering in the vicinity of the synapse, and docking at appropriate sites of the active zone. The results presented here indicate that the structural requirements of SNARE complexes that form subsequent to these steps are not so strict as to preclude the substitution of related SNAREs for one another in vivo.
Acknowledgments
We are grateful to Cara Empey and Irene Inman for outstanding technical assistance and to Drs. Elliot Goldstein, Yuqian Guo, and Kim Kaiser for providing Drosophila strains. This work was supported by National Institutes of Health Grant NS40053 (to T.L.S.) and a Marie Curie Fellowship from the European Commission (to B.D.M.).
Abbreviations
- VAMP
vesicle-associated membrane protein
- SNARE
SNAP receptor
- syb
synaptobrevin
- n-syb
neuronal syb
- ERG
electroretinogram
- EGUF
eyeless-GAL4 UAS-FLP
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 13359.
References
- 1.Chen Y A, Scheller R H. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- 2.Wickner W, Haas A. Annu Rev Biochem. 2000;69:247–275. doi: 10.1146/annurev.biochem.69.1.247. [DOI] [PubMed] [Google Scholar]
- 3.Gaisano H Y, Ghai M, Malkus P N, Sheu L, Bouquillon A, Bennett M K, Trimble W S. Mol Biol Cell. 1996;7:2019–2027. doi: 10.1091/mbc.7.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothman J E, Warren G. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 5.Parlati F, Varlamov O, Paz K, McNew J A, Hurtado D, Sollner T H, Rothman J E. Proc Natl Acad Sci USA. 2002;99:5424–5429. doi: 10.1073/pnas.082100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNew J A, Parlati F, Fukuda R, Johnston R J, Paz K, Paumet F, Sollner T H, Rothman J E. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- 7.Scales S J, Chen Y A, Yoo B Y, Patel S M, Doung Y C, Scheller R H. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 8.Yang B, Gonzalez L, Jr, Prekeris R, Steegmaier M, Advani R J, Scheller R H. J Biol Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]
- 9.Broadie K, Prokop A, Bellen H J, O'Kane C J, Schulze K L, Sweeney S T. Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 10.Hunt J M, Bommert K, Charlton M P, Kistner A, Habermann E, Augustine G J, Betz H. Neuron. 1994;12:1269–1279. doi: 10.1016/0896-6273(94)90443-x. [DOI] [PubMed] [Google Scholar]
- 11.Fasshauer D, Antonin W, Margittai M, Pabst S, Jahn R. J Biol Chem. 1999;274:15440–15446. doi: 10.1074/jbc.274.22.15440. [DOI] [PubMed] [Google Scholar]
- 12.Fasshauer D, Sutton R B, Brunger A T, Jahn R. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia E P, McPherson P S, Chilcote T J, Takei K, De Camilli P. J Cell Biol. 1995;129:105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Mollard G F, Nothwehr S F, Stevens T H. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sollner T H. Dev Cell. 2002;2:377–378. doi: 10.1016/s1534-5807(02)00161-2. [DOI] [PubMed] [Google Scholar]
- 16.Deitcher D L, Ueda A, Stewart B A, Burgess R W, Kidokoro Y, Schwarz T L. J Neurosci. 1998;18:2028–2039. doi: 10.1523/JNEUROSCI.18-06-02028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshihara M, Ueda A, Zhang D, Deitcher D L, Schwarz T L, Kidokoro Y. J Neurosci. 1999;19:2432–2441. doi: 10.1523/JNEUROSCI.19-07-02432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof T C, Kavalali E T. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 19.Washbourne P, Thompson P M, Carta M, Costa E T, Mathews J R, Lopez-Bendito G, Molnar Z, Becher M W, Valenzuela C F, Partridge L D, Wilson M C. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- 20.DiAntonio A, Burgess R W, Chin A C, Deitcher D L, Scheller R H, Schwarz T L. J Neurosci. 1993;13:4924–4935. doi: 10.1523/JNEUROSCI.13-11-04924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin A C, Burgess R W, Wong B R, Schwarz T L, Scheller R H. Gene. 1993;131:175–181. doi: 10.1016/0378-1119(93)90291-a. [DOI] [PubMed] [Google Scholar]
- 22.Stowers R S, Schwarz T L. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niemeyer B A, Schwarz T L. J Cell Sci. 2000;113:4055–4064. doi: 10.1242/jcs.113.22.4055. [DOI] [PubMed] [Google Scholar]
- 24.Stewart B A, Atwood H L, Renger J J, Wang J, Wu C F. J Comp Physiol A. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- 25.Hiesinger P R, Reiter C, Schau H, Fischbach K F. J Neurosci. 1999;19:7548–7556. doi: 10.1523/JNEUROSCI.19-17-07548.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pak W L, Grossfield J, White N V. Nature. 1969;222:351–354. doi: 10.1038/222351a0. [DOI] [PubMed] [Google Scholar]
- 27.Zhai R G, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger E D, Ziv N E, Garner C C. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 28.Coco S, Raposo G, Martinez S, Fontaine J J, Takamori S, Zahraoui A, Jahn R, Matteoli M, Louvard D, Galli T. J Neurosci. 1999;19:9803–9812. doi: 10.1523/JNEUROSCI.19-22-09803.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Arca S, Coco S, Mainguy G, Schenk U, Alberts P, Bouille P, Mezzina M, Prochiantz A, Matteoli M, Louvard D, Galli T. J Neurosci. 2001;21:3830–3838. doi: 10.1523/JNEUROSCI.21-11-03830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao S S, Stewart B A, Rivlin P K, Vilinsky I, Watson B O, Lang C, Boulianne G, Salpeter M M, Deitcher D L. EMBO J. 2001;20:6761–6771. doi: 10.1093/emboj/20.23.6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulze K L, Broadie K, Perin M S, Bellen H J. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- 32.Burgess R W, Deitcher D L, Schwarz T L. J Cell Biol. 1997;138:861–875. doi: 10.1083/jcb.138.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saitoe M, Schwarz T L, Umbach J A, Gundersen C B, Kidokoro Y. Science. 2001;293:514–517. doi: 10.1126/science.1061270. [DOI] [PubMed] [Google Scholar]
- 34.Yoshihara M, Suzuki K, Kidokoro Y. J Neurosci. 2000;20:8315–8322. doi: 10.1523/JNEUROSCI.20-22-08315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elferink L A, Trimble W S, Scheller R H. J Biol Chem. 1989;264:11061–11064. [PubMed] [Google Scholar]
- 36.McMahon H T, Ushkaryov Y A, Edelmann L, Link E, Binz T, Niemann H, Jahn R, Sudhof T C. Nature. 1993;364:346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- 37.Galli T, Chilcote T, Mundigl O, Binz T, Niemann H, De Camilli P. J Cell Biol. 1994;125:1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]