Abstract
Acute pharmacological blockade of α1 adrenoreceptors (ARs) attenuates the locomotor response to amphetamine (LRA). We took a genetic approach to study how norepinephrine (NE) signaling modulates psychostimulant responses by testing LRA in dopamine β-hydroxylase knockout (Dbh−/−) mice that lack NE. Surprisingly, Dbh−/− animals were hypersensitive to the behavioral effects of amphetamine. Amphetamine (2 mg/kg) elicited greater locomotor activity in Dbh−/− mice compared to controls, whereas 5 mg/kg caused stereotypy in Dbh−/− mice, which is only observed in control mice at higher doses. Prazosin, an α1AR antagonist, attenuated LRA in Dbh+/− mice but had no effect in Dbh−/− mice. Changes in the sensitivity of dopamine (DA)-signaling pathways may contribute to the altered amphetamine responses of Dbh−/− mice because they were relatively insensitive to a D1 agonist and hypersensitive to a D2 agonist. Daily amphetamine administration resulted in behavioral sensitization in both Dbh+/− and Dbh−/− mice, demonstrating that NE is not required for the development or expression of behavioral sensitization. Daily prazosin administration blunted but did not completely block locomotor sensitization in Dbh+/− mice, suggesting that α1AR signaling contributes to, but is not required for sensitization in Dbh+/− control animals. We conclude that in contrast to acute α1AR blockade, chronic NE deficiency induces changes similar to sensitization, perhaps by altering DA-signaling pathways.
Amphetamine is a psychostimulant that is used both recreationally and therapeutically for diseases such as attention-deficit/hyperactivity disorder. The primary sites of amphetamine action are monoamine transporters, where it blocks reuptake and facilitates release of dopamine (DA), norepinephrine (NE), and serotonin. Although DA signaling is the focus of most amphetamine research, it is also clear that NE plays an important role in modulating cellular and behavioral responses to psychostimulants. Lesions of the locus coeruleus (the major central noradrenergic nucleus) or administration of prazosin, an α1-adrenoreceptor (α1AR) antagonist, attenuate amphetamine-induced locomotion (1–3). Prazosin decreases burst firing of dopaminergic ventral tegmental area neurons and blocks the excitatory effect of amphetamine on these cells (4–6). Finally, inactivation of the α1bAR gene in mice attenuates amphetamine responses (7). These results suggest that NE signaling through α1ARs is important for the locomotor and cellular responses to amphetamine.
Amphetamine produces behavioral sensitization, an enhancement of the locomotor response after repeated administration, which may model drug craving and psychosis. Sensitization is thought to involve long-term changes in both DA and other signaling pathways (8, 9). Because amphetamine sensitization is blunted in mice lacking α1bARs and rats treated with prazosin, α1AR signaling may influence the neural and behavioral changes that result from chronic psychostimulant use (7, 10).
Drug addiction develops over time and is a chronic disease, and genetic polymorphisms likely contribute to addiction susceptibility. Therefore, it is important to understand how long-term changes in neurotransmitter systems that are targets of psychostimulants affect the responses to these drugs. Dopamine β-hydroxylase knockout (Dbh−/−) mice lack NE from birth and develop postnatally in the absence of NE (11). Lesions and pharmacological blockade inhibit NE signaling acutely, whereas Dbh−/− mice represent a model of chronic NE deficiency. In this study, we used Dbh−/− mice to explore the consequences of chronic NE deficiency on the locomotor response to amphetamine (LRA).
Materials and Methods
Animals.
Dbh−/− mice, maintained on a mixed 129/SvEv and C57BL/6J background, were developed and generated as described (11, 12). Dbh+/− mice have normal catecholamine levels and are indistinguishable from WT littermates for all previously tested phenotypes (11–13). Therefore, heterozygous (+/−) littermates were used as controls for all experiments in this study. Mice between 3 and 6 mo of age were used for all experiments.
Experimental protocols were approved by the animal care committee at the University of Washington and meet the guidelines of the American Association for Accreditation of Laboratory Animal Care.
Measurement of Locomotor Activity.
Amphetamine, prazosin, SKF81297, and quinpirole were obtained from Sigma, dissolved in 0.9% NaCl (with the exception of prazosin, which was dissolved in 1.5% DMSO, 1.5% Chremophor EL), and administered in a volume of 10 ml/kg body weight. Ambulations (consecutive beam breaks) were measured in activity cages with IR photobeams (San Diego Instruments). Vehicle (1.5% DMSO, 1.5% Cremaphor EL), 0.9% NaCl, prazosin (0.5 or 1 mg/kg), or SCH23390 (0.2 mg/kg) was injected i.p. 90 min after mice were placed into the chambers. Amphetamine (1, 2, 3, 5, or 10 mg/kg), SKF81297 (5 mg/kg; ± racemic mixture), quinpirole (2.5 mg/kg), or 0.9% NaCl was injected i.p. 30 min later, and ambulations were recorded for 2–4 h. For the l-3,4-dihydroxyphenylserine (DOPS) experiment, Dbh−/− mice received 1 mg/g DOPS (Sumitomo Pharmaceutical, Osaka), 0.125 mg/g S-(-)-carbidopa (ICN) and 0.3 mg/g ascorbic acid s.c. 3 h before placement in the activity chambers. Mice were then injected with vehicle 90 min after placement in the chambers and amphetamine (5 mg/kg) 30 min later. Amphetamine was administered 5 h after DOPS + carbidopa injection because central NE levels in Dbh−/− mice are highest at this time (12). Amphetamine-naive mice were used for all experiments (except those in the sensitization experiments, which received daily amphetamine injections; see below).
Stereotypy.
A subset of animals in the locomotor activity study were videotaped, and stereotypy was scored for 30 min between 30 and 60 min after saline or amphetamine administration. Behavior was broken down into 10-sec bins, and predominant behavior was recorded for each bin. Behaviors scored were quiet wake/sleeping, ambulating, rearing, grooming, head-bobbing, sniffing, circling, and nail biting. Ambulating and rearing were considered locomotory/exploratory behaviors and the last four were considered stereotypy.
Sensitization.
Mice were subjected to the above amphetamine (2 mg/kg) paradigm for 6 days, given 1 day of rest, then retested on day 8. Mice were returned to their home cage each day. Locomotor activity was recorded days 1–5 and 8. Some mice that received vehicle + amphetamine for the duration of sensitization received prazosin (0.5 or 1 mg/kg) + amphetamine on day 9, vehicle + amphetamine on d 10, vehicle + quinpirole (2.5 mg/kg) on day 11, vehicle + SKF81297 (5 mg/kg) on day 12, and vehicle + amphetamine on days 15 and 43. Some mice also were scored for stereotypy on day 8 (percentage time spent locomoting vs. time spent in stereotypy for 5 min between 30 and 60 min after amphetamine administration). The effects of acute α1AR blockade on sensitization were determined by pretreating Dbh+/− mice with prazosin (0.5 or 1 mg/kg) during the sensitization paradigm (see Results for details).
Statistics.
Data were analyzed by Student's t test or Wilcoxon-Mann–Whitney U test when comparing two groups, and ANOVA, followed by Student Newman-Keuls or Bonferroni post hoc tests if significant differences among groups were found, when comparing more than two groups.
Results
Dbh−/− Mice Are Hypersensitive to Amphetamine.
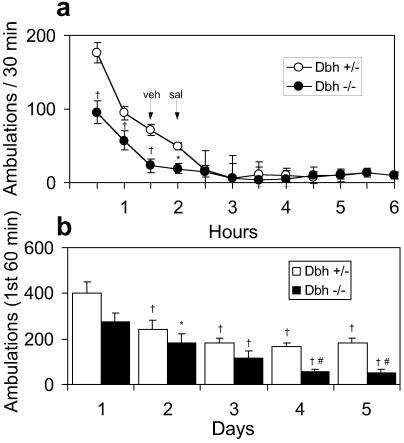
The LRA was measured in activity cages equipped with IR light beams. When naive rodents are placed in new cages they manifest exploratory activity that gradually subsides. The Dbh−/− mice had approximately half the exploratory activity as Dbh+/− mice (Fig. 1a). Injections of either vehicle or saline did not stimulate locomotion (Fig. 1a). Placing mice in the same cage on successive days also led to a gradual reduction in initial exploratory activity. Dbh−/− mice manifested less exploratory behavior than Dbh+/− mice over the five daily trials (Fig. 1b).
Figure 1.
Locomotor response of Dbh+/− and Dbh−/− mice to a novel environment. (a) Naive mice (Dbh+/−, n = 13; Dbh−/−, n = 13) were placed in activity chambers, injected with vehicle (1.5% DMSO/1.5% Cremophor EL in 0.9% NaCl) at 90 min, 0.9% NaCl at 120 min, and activity was recorded for an additional 4 h. *, P < 0.05; †, P < 0.001 compared to Dbh+/−. (b) In the course of the amphetamine sensitization experiments, mice (Dbh+/−, n = 16; Dbh−/−, n = 8) were placed in the activity chambers once per day for 5 days. Shown are total ambulations over the first 60 min (before any drug treatment). *, P < 0.05 compared to Dbh−/− day 1; †, P < 0.001 compared day 1 for that genotype; #, P < 0.01 compared to Dbh−/− day 2.
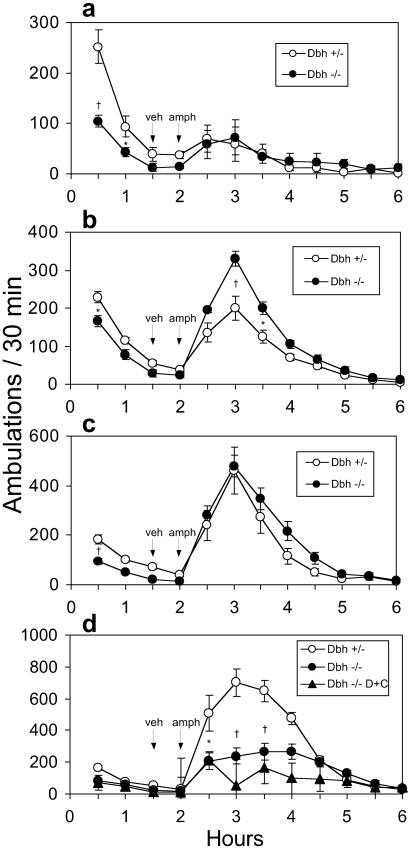
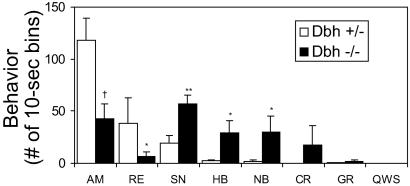
Amphetamine was administered 2 h after mice were placed in the activity cages, when exploratory activity had subsided. The locomotor responses to amphetamine administered at 1, 2, 3, or 5 mg/kg (i.p.) are shown in Fig. 2 a–d. The Dbh+/− mice show a dose-dependent increase in locomotor responses up to 5 mg/kg, whereas Dbh−/− mice have greater activity than Dbh+/− mice at 2 mg/kg, peak activity at 3 mg/kg, and much less activity than Dbh+/− mice at 5 mg/kg. We hypothesized that the Dbh−/− mice were hypersensitive to amphetamine and that the 5 mg/kg dose produced stereotypy at the expense of locomotion. Videotaping the mice and scoring various behaviors confirmed this notion; the Dbh−/− mice displayed significantly more sniffing, head bobbing, nail biting, and circling than the Dbh+/− mice (Fig. 3). This degree of stereotypy is observed in Dbh+/− mice at 10 mg/kg of amphetamine (data not shown). These results indicate that Dbh−/− mice are hypersensitive to the behavioral effects of amphetamine at 2 or 5 mg/kg. Because psychostimulant-induced locomotion is a combination of a drug effect and an environmentally influenced exploratory response, we believe that the reduced exploratory response of Dbh−/− mice (Fig. 1) likely masks the increased sensitivity to 1 mg/kg amphetamine, although this hypothesis is difficult to test directly.
Figure 2.
Locomotor response of Dbh+/− and Dbh−/− mice to amphetamine. Naive mice were placed in activity chambers and injected with vehicle at 90 min and amphetamine at 120 min, and activity was recorded for an additional 4 h. Amphetamine doses were: 1 mg/kg (Dbh+/−, n = 13; Dbh−/−, n = 13) (a); 2 mg/kg (Dbh+/−, n = 32; Dbh−/−, n = 24) (b); 3 mg/kg (Dbh+/−, n = 24; Dbh−/− n = 24) (c); and 5 mg/kg [Dbh+/−, n = 20; Dbh−/−, n = 17, Dbh−/− DOPS + carbidopa (D + C), n = 4] (d). *, P < 0.05 compared to Dbh+/−; †, P < 0.001 compared to Dbh+/−.
Figure 3.
Stereotypy in Dbh+/− and Dbh−/− mice in response to 5 mg/kg amphetamine. Naive mice (Dbh+/−, n = 6; Dbh−/−, n = 6) were placed in activity chambers and injected with vehicle at 90 min and amphetamine at 120 min, and mice were videotaped for 2 additional h. Behavior was scored in 10-s bins. AM, ambulating; RE, rearing; SN, sniffing; HB, head bobbing; NB, nail biting; CR, circling; GR, grooming; QWS, quiet wake/sleeping. *, P < 0.05; **, P < 0.01; †, P < 0.001 compared to Dbh+/−.
To determine whether acutely restoring NE centrally to Dbh−/− mice could restore normal amphetamine sensitivity, DOPS and carbidopa were administered 5 h before amphetamine (5 mg/kg) administration. DOPS can be converted to NE by l-aromatic amino acid decarboxylase (AADC), thus bypassing the requirement for DBH, and carbidopa is an inhibitor of AADC that cannot cross the blood–brain barrier. DOPS + carbidopa treatment did not restore normal amphetamine sensitivity to Dbh−/− mice; they had reduced LRA and increased stereotypy compared to Dbh+/− mice (Fig. 2d, data not shown). This result suggests that the amphetamine hypersensitivity of Dbh−/− mice is not caused by an “acute” lack of NE, but rather is a chronic condition that was established after birth.
Prazosin Has No Effect on LRA in Dbh−/− Mice.
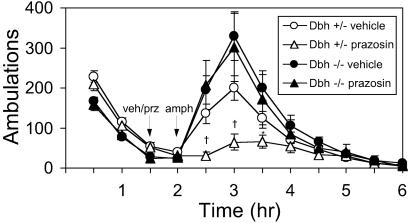
To confirm that α1AR signaling is required for LRA, Dbh+/− and Dbh−/− mice were pretreated with prazosin (0.5 or 1 mg/kg) 30 min before amphetamine (2 mg/kg). As reported (1, 2, 10), amphetamine-induced locomotion was blocked in control mice (Fig. 4 and data not shown). Genetic disruption of the α1bAR gene results in a similar attenuation but not abolishment of LRA (7). However, the LRA of Dbh−/− mice persisted in the presence of prazosin (Fig. 4 and data not shown), demonstrating that α1AR signaling is critical in control but not Dbh−/− mice. This result is consistent with the fact that Dbh−/− mice lack NE that could activate α1ARs and suggests that acute and chronic block of NE signaling have different consequences on amphetamine response. The D1 antagonist SCH23390 completely blocked LRA in both Dbh+/− and Dbh−/− mice (data not shown), demonstrating that D1 signaling is still required in both groups.
Figure 4.
Effects of prazosin on amphetamine-induced locomotion in Dbh+/− and Dbh−/− mice. Naive mice were placed in activity chambers and injected with vehicle (Dbh+/−, n = 32; Dbh−/−, n = 24; replicated from Fig. 1b) or prazosin (0.5 mg/kg; Dbh+/−, n = 18; Dbh−/−, n = 9) at 90 min and amphetamine (2 mg/kg) at 120 min, and activity was recorded for an additional 4 h. *, P < 0.05; †, P < 0.001 compared to vehicle control for that genotype.
Dbh−/− Mice Are Insensitive to a D1 Agonist and Hypersensitive to a D2 Agonist.
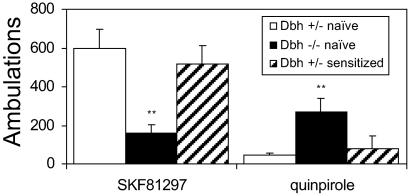
To determine whether changes in DA receptor sensitivity were responsible for the hypersensitivity to amphetamine-induced behaviors in Dbh−/− mice, locomotion was assessed after administration of a D1 agonist (SKF81297; 5 mg/kg) or a D2 agonist (quinpirole, 2.5 mg/kg). Compared to Dbh+/− controls, Dbh−/− mice had a reduced locomotory response to SKF81297 but an increased response to quinpirole (Fig. 5). These results suggest that changes in DA receptor signaling may contribute to amphetamine hypersensitivity in Dbh−/− mice. Radioligand-binding experiments suggest that the D1 insensitivity and D2 hypersensitivity of Dbh−/− mice are not caused by changes in DA receptor number (D. Kim and S. Thomas, personal communication).
Figure 5.
Locomotor response of Dbh+/− and Dbh−/− mice to a D1 and D2 agonist. Naive or sensitized mice were placed in activity chambers and injected with vehicle at 90 min and the D1 agonist SKF81297 (5 mg/kg; Dbh+/−, n = 10; Dbh−/−, n = 10) or the D2 agonist quinpirole (2.5 mg/kg; Dbh+/−, n = 19; Dbh−/−, n = 15) at 120 min, and activity was recorded for an additional 2 h. Shown are the total ambulations for 2 h after drug administration. **, P < 0.01 compared to Dbh+/−.
Effects of Prazosin and Chronic NE Deficiency on Amphetamine Sensitization.
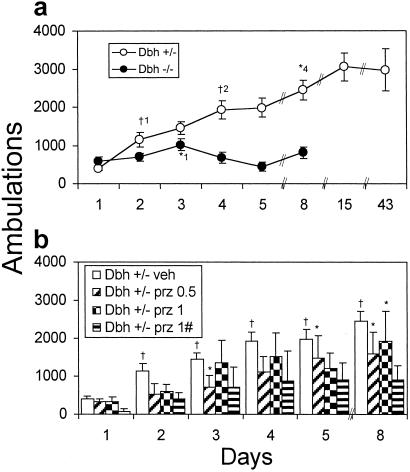
Daily administration of amphetamine (2 mg/kg) produced robust sensitization in Dbh+/− mice. A greater LRA was observed after only 1 day; it continued to increase with repeat exposures, and it persisted for at least a month (Fig. 6a).
Figure 6.
Amphetamine sensitization in Dbh+/− and Dbh−/− mice. (a) Mice (Dbh+/−, n = 23; Dbh−/−, n = 8) were placed in activity chambers and injected with vehicle at 90 min and amphetamine (2 mg/kg) at 120 min, and activity was measured for an additional 2 h. This paradigm was repeated every day for 6 days, mice were rested on day 7, then retested on days 8, 15, and 43. Shown are the total ambulations for 2 h after amphetamine administration. *, P < 0.05 compared to Dbh−/− day 1; †1, P < 0.001 compared to Obh+/− day 1; †2, P < 0.001 compared to Dbh+/− day 2; *4, P < 0.05 compared to Obh+/− day 4. (b) For “Dbh+/− prazonsin (prz) 0.5” and “Dbh+/− prz 1”, mice were given prazosin (0.5 mg/kg or 1 mg/kg) at 90 min on days 2–6. For “Dbh+/− prz 1#”, mice were given prazosin (1 mg/kg) at 90 min on days 1–6. All mice were given amphetamine (2 mg/kg) at 120 min. Dbh+/− vehicle (veh) (n = 23, replicated from a), Dbh+/− prz 0.5 (n = 8), Dbh+/− prz 1 (n = 4), and Dbh+/− prz 1# (n = 3). Shown are the total ambulations for 2 h after amphetamine administration. *, P < 0.05; †, P < 0.001 compared to day 1 for that treatment.
There was apparently only modest sensitization in Dbh−/− mice (Fig. 6a). LRA increased slightly over the first 3 days and then decreased. Observation of Dbh−/− mice revealed an increase of stereotypical behaviors in response to amphetamine over time at the expense of horizontal locomotion (percentage time spent in stereotypy after day 8 amphetamine injection: Dbh+/− 48 ± 9%, Dbh−/− 82 ± 4%, P < 0.05). This result suggests that Dbh−/− mice are capable of sensitization but sensitize into stereotypy instead of increasing locomotion.
To determine the effect of acute α1AR blockade on amphetamine sensitization in normal animals, Dbh+/− mice were given vehicle plus amphetamine (2 mg/kg) on day 1. The mice were then split into two groups with similar average responses. One group continued to receive vehicle plus amphetamine on days 2–6, and the other group received prazosin (0.5 or 1 mg/kg) plus amphetamine on days 2–6. Both groups received vehicle plus amphetamine on day 8. Prazosin administration retarded but did not abolish amphetamine sensitization (Fig. 6b).
Interestingly, only 1 day of amphetamine treatment attenuated the effect of prazosin. Whereas prazosin decreased LRA in naive Dbh+/− mice (Fig. 4), animals treated with prazosin plus amphetamine on day 2 had slightly increased LRA compared to day 1 (no prazosin), although still less LRA than mice receiving vehicle plus amphetamine on both days (Fig. 6b). This finding suggests that a single dose of amphetamine can partially bypass the α1AR requirement for LRA. We tested whether this phenomenon was responsible for the persistence of sensitization in the prazosin-treated groups by treating a group of mice with prazosin (1 mg/kg) plus amphetamine (2 mg/kg) starting on day 1. This paradigm was the most effective at attenuating sensitization, but the mice still sensitized (Fig. 6b). This residual increase in LRA after repeated doses is reminiscent of the ability of α1bAR knockout mice to undergo a small amount of amphetamine sensitization (7). Taken together, these results indicate that α1AR signaling contributes to but is not absolutely required for the development of sensitization.
Sensitized Dbh+/− Mice Are Indifferent to Prazosin.
A single dose of amphetamine reduced the ability of prazosin to attenuate LRA (see above). To determine the contribution of α1AR signaling to the expression of preestablished sensitization, Dbh+/− mice that had been given daily vehicle + amphetamine (2 mg/kg) injections for 1 wk were tested for LRA after pretreatment with prazosin (0.5 mg/kg). In contrast to the attenuation of LRA in naive Dbh+/− mice, prazosin (0.5 mg/kg) had no effect on LRA in sensitized Dbh+/− mice (combined ambulations for 2 h after 2 mg/kg amphetamine: naive vehicle 531 ± 72 n = 32, naive prazosin 214 ± 49 n = 18, P < 0.01; sensitized vehicle 1,700 ± 304, n = 12, sensitized prazosin 1,606 ± 233, n = 12, P = 0.75). Similar results were obtained by using 1 mg/kg prazosin. These results suggest that neuroadaptive changes during the sensitization process bypass the requirement for α1AR signaling.
DA Agonist Sensitivity Is Normal in Sensitized Dbh+/− Mice.
D1 receptor insensitivity and D2 receptor hypersensitivity have been observed in rodents that have undergone psychostimulant sensitization or have sensitized-like phenotypes (14, 15). To determine whether our sensitization paradigm induces these changes, locomotor activity of sensitized Dbh+/− mice was tested in response to the D1 agonist SKF81297 (5 mg/kg) or the D2 agonist quinpirole (2.5 mg/kg). Neither D2 agonist hypersensitivity nor D1 agonist insensitivity was observed in sensitized Dbh+/− mice (Fig. 5), demonstrating that this particular sensitization paradigm did not alter DA receptor signaling.
Discussion
Why Are Dbh−/− Mice Hypersensitive to Amphetamine?
Although acute blockade of α1ARs reduces LRA, Dbh−/− mice that lack all NE signaling are hypersensitive to amphetamine. We propose that in naive normal animals, NE signaling through α1ARs is critical for LRA. However, compensation occurs in animals that develop in the absence of NE, and this compensation confers a “sensitized-like” phenotype that bypasses the α1AR-dependent pathway. Because changes in DA receptor signaling can alter responses to amphetamine, we predict that D1 receptor insensitivity and D2 receptor hypersensitivity are part of the compensatory mechanism that functions in Dbh−/− mice and directly contribute to amphetamine hypersensitivity. α1bAR knockout mice have reduced sensitivity to amphetamine and therefore resemble prazosin-treated Dbh+/− mice and not Dbh−/− mice (7). Therefore, the adaptations in Dbh−/− mice resulting in amphetamine hypersensitivity are likely caused by the chronic absence of signaling by other ARs.
The DBH enzyme functions in the NE biosynthetic pathway and converts DA to NE. Thus, Dbh−/− mice not only lack NE, but synthesize and probably release DA from their “noradrenergic” neurons. It is possible that this ectopic DA and not the absence of NE causes the phenotypes observed in the Dbh−/− mice, especially because DA can apparently increase ventral tegmental area excitability via the α1AR (6). However, we favor the absence of NE as the primary mechanism for a number of reasons. First, Dbh−/− and Dbh+/− mice have comparable amounts of DA in relevant brain areas such as the midbrain, striatum, and cortex (12). Second, Dbh−/− mice do not display other phenotypes such as basal hyperactivity associated with chronically elevated extracellular DA levels (16). Third, LRA in Dbh−/− mice is not blocked by prazosin, which would prevent receptor activation by either NE or DA. Fourth, a few studies demonstrate similar changes in DA receptor and amphetamine sensitivity in animals with lesions of the locus coeruleus that do not result in ectopic DA (17, 18). However, because ectopic DA remains a possible mechanism, it will be important to examine LRA in mice that lack NE without producing ectopic DA.
NE in the Prefrontal Cortex (PFC).
The PFC has emerged as an important site in controlling psychostimulant responses. Regulation of PFC excitability by NE appears to be critical for both the initial locomotor response to amphetamine as well as the development of sensitization. The locus coeruleus projects to the PFC, and α1AR stimulation activates pyramidal cells within the PFC and is required for LRA in naive animals (1, 19). One model is that amphetamine causes release of NE in the PFC, pyramidal cells are activated, glutamate is released and acts on relevant target neurons in the ventral tegmental area and NAc, and locomotion ensues.
α1AR Signaling and Sensitization.
Because α1AR activation appears to be the major excitatory influence on PFC neurons in response to amphetamine and the PFC is required for the development of amphetamine sensitization (20), it follows that α1AR signaling would be required for sensitization. In support of this model, it was recently demonstrated (10) that prazosin administration prevented amphetamine sensitization in rats. Sensitization was significantly attenuated in Dbh+/− mice treated with prazosin starting on day 1 of the sensitization paradigm, and sensitization also was blunted in α1bAR knockout mice (7). However, we found that even a single dose of amphetamine without prazosin pretreatment reduced the ability of prazosin to block subsequent sensitization. Consistent with previous reports (21), this finding suggests that a single dose of amphetamine results in some degree of sensitization. Sensitized animals are hypersensitive to amphetamine and therefore “experience” increasingly higher doses as sensitization proceeds. The locomotor response to the 2 mg/kg dose of amphetamine that is blocked by prazosin in naive mice may in essence be experienced as a much higher dose in sensitized mice. This phenomenon, in combination with changes in DA receptor-signaling pathway sensitivity, may bypass noradrenergic regulation. In support of this model, Dbh−/− mice, which have phenotypes similar to sensitized animals, are still capable of further sensitization, although they tend to sensitize into stereotypy instead of horizontal locomotion at this dose.
DBH Activity and Psychostimulant Addiction.
What can the amphetamine phenotypes of Dbh−/− mice tell us about psychostimulant addiction in humans? Dbh-deficient humans do exist, although it is an exceedingly rare condition (22). More importantly, relatively common polymorphisms in the DBH gene confer very low DBH activity (23, 24). Strikingly, cocaine abusers with low-activity DBH haplotypes have increased sensitivity to cocaine-induced paranoia (23) and euphoria (R. Malison, personal communication), suggesting that DBH enzyme levels modulate both dysphoric and rewarding effects of psychostimulants in humans. Furthermore, the DBH inhibitor disulfiram has recently been used successfully to treat cocaine dependence, leading to speculation that DBH may play an important role in cocaine reward and/or relapse (25, 26). Dbh−/− mice provide a preclinical animal model to test the role of NE in psychostimulant addiction.
Acknowledgments
We thank Sumitomo Pharmaceutical (Osaka) for their generous donation of DOPS, J. P. Tassin, M. Sczcypka, and D. Kim for critical comments on the manuscript, and S. A. Thomas and D. Kim for sharing unpublished data. D.W., N.S.M., K.B., and M.L.L. were supported by the Howard Hughes Medical Institute.
Abbreviations
- Dbh
dopamine β-hydroxylase
- AR
adrenoreceptor
- LRA
locomotor response to amphetamine
- NE
norepinephrine
- DA
dopamine
- PFC
prefrontal cortex
- DOPS
l-3,4-dihydroxyphenylserine
References
- 1.Darracq L, Blanc G, Glowinski J, Tassin J P. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snoddy A M, Tessel R E. Eur J Pharmacol. 1985;116:221–228. doi: 10.1016/0014-2999(85)90156-6. [DOI] [PubMed] [Google Scholar]
- 3.Mohammed A K, Danysz W, Ogren S O, Archer T. Neurosci Lett. 1986;64:139–144. doi: 10.1016/0304-3940(86)90089-3. [DOI] [PubMed] [Google Scholar]
- 4.Grenhoff J, Svensson T H. Eur J Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- 5.Shi W X, Pun C L, Zhang X X, Jones M D, Bunney B S. J Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paladini C A, Fiorillo C D, Morikawa H, Williams J T. Nat Neurosci. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- 7.Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin J P. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce R C, Kalivas P W. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 9.Wolf M E. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 10.Drouin C, Blanc G, Villegier A S, Glowinski J, Tassin J P. Synapse. 2002;43:51–61. doi: 10.1002/syn.10023. [DOI] [PubMed] [Google Scholar]
- 11.Thomas S A, Matsumoto A M, Palmiter R D. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 12.Thomas S A, Marck B T, Palmiter R D, Matsumoto A M. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- 13.Thomas S A, Palmiter R D. Cell. 1997;91:583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- 14.Vanderschuren L J, Schoffelmeer A N, Mulder A H, De Vries T J. Psychopharmacology. 1999;143:244–253. doi: 10.1007/s002130050943. [DOI] [PubMed] [Google Scholar]
- 15.Xu F, Gainetdinov R R, Wetsel W C, Jones S R, Bohn L M, Miller G W, Wang Y M, Caron M G. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- 16.Giros B, Jaber M, Jones S R, Wightman R M, Caron M G. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson I, Dolphin A, Jenner P, Marsden C D, Pycock C. Eur J Pharmacol. 1976;39:179–191. doi: 10.1016/0014-2999(76)90126-6. [DOI] [PubMed] [Google Scholar]
- 18.Harro J, Merikula A, Lepiku M, Modiri A R, Rinken A, Oreland L. Pharmacol Toxicol. 2000;86:197–202. doi: 10.1034/j.1600-0773.2000.d01-35.x. [DOI] [PubMed] [Google Scholar]
- 19.Marek G J, Aghajanian G K. Eur J Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- 20.Wolf M E, Dahlin S L, Hu X T, Xue C J, White K. Neuroscience. 1995;69:417–439. doi: 10.1016/0306-4522(95)00248-h. [DOI] [PubMed] [Google Scholar]
- 21.Vanderschuren L J, Schmidt E D, De Vries T J, Van Moorsel C A, Tilders F J, Schoffelmeer A N. J Neurosci. 1999;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson D, Haile V, Perry S E, Robertson R M, Phillips J A, III, Biaggioni I. Hypertension. 1991;18:1–8. doi: 10.1161/01.hyp.18.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Cubells J F, Kranzler H R, McCance-Katz E, Anderson G M, Malison R T, Price L H, Gelernter J. Mol Psychiatry. 2000;5:56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- 24.Zabetian C P, Anderson G M, Buxbaum S G, Elston R C, Ichinose H, Nagatsu T, Kim K S, Kim C H, Malison R T, Gelernter J, Cubells J F. Am J Hum Genet. 2001;68:515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll K M, Nich C, Ball S A, McCance E, Rounsavile B J. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- 26.George T P, Chawarski M C, Pakes J, Carroll K M, Kosten T R, Schottenfeld R S. Biol Psychiatry. 2000;47:1080–1086. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]