Abstract
Activation of ionotropic glycine receptors potentiates glutamate release in mature calyceal nerve terminals of the rat medial nucleus of the trapezoid body, an auditory brainstem nucleus. In young rats, glycine and its receptors are poorly expressed. We therefore asked whether GABA (γ-aminobutyric acid) might play a larger role than glycine in the regulation of glutamate release in the absence of glycine receptors. Indeed, in rats younger than postnatal day 11 (P11), and before the onset of hearing, calyces expressed high levels of ionotropic GABAA receptors but few glycine receptors. Isoguvacine, a selective agonist at GABAA receptors, strongly enhanced excitatory postsynaptic currents in young rats but had little effect in rats older than P11. Down-regulation of presynaptic GABAA receptors did not reflect global changes in receptor expression, because the magnitude of GABA and glycine responses was similar at P13 in the parent-cell bodies of the calyces, the bushy cells of the cochlear nucleus. In outside-out patches excised from the nonsynaptic face of calyces, GABA and glycine evoked single-channel currents consistent with the properties of postsynaptic GABAA and glycine receptors. Inhibitory GABAB receptors were present on the calyx at all developmental stages examined. Thus, GABA initially acts on two receptor subtypes, both promoting and inhibiting glutamate release. With age, the former role is transferred to the glycine receptor during the period in which postsynaptic glycinergic transmission is acquired.
Ligand-gated ion channels (ionotropic receptors) aggregate in subsynaptic membrane shortly after pre- and postsynaptic cells come in close contact (1–4). During maturation of the synapse, changes often occur in the subtypes of postsynaptic receptor (5, 6). Recently, it has become clear that many synapses contain presynaptic ionotropic receptors, such as glutamate, GABAA (γ-aminobutyric acid type A), or acetylcholine receptors, whose activation regulates transmitter release in response to presynaptic action potentials (7). Unlike many postsynaptic receptors, it is not known whether presynaptic receptors are expressed in parallel with the development of their corresponding transmitter systems. This problem is accentuated by the fact that presynaptic receptors generally are not directly apposed to presynaptic terminals (axo-axonic synapses) but, rather, are activated by “spillover” of transmitter from distant terminals (8, 9). Moreover, it is not known whether these receptors are selectively targeted to axon terminals or, instead, appear in proportion to global changes in receptor expression over the entire neuron.
The calyx of Held is a large glutamatergic nerve terminal found on neurons of the medial nucleus of the trapezoid body (MNTB), a brainstem auditory relay nucleus. Postsynaptic neurons in MNTB are glycinergic and supply fast inhibition to diverse targets in the superior olivary complex. We previously have shown that presynaptic glycine receptors are expressed on the calyx terminal and that activation of these receptors gates Cl− channels that weakly depolarize the terminals, causing an enhancement of glutamate release (10). Presynaptic glycine receptors apparently are activated by diffusion from boutons on the postsynaptic cells; thus, glycinergic terminals both generate an IPSC (inhibitory postsynaptic current) in the postsynaptic cell and enhance the ability of calyceal action potentials to drive glutamate release. Other studies have described several major changes in inhibitory transmission in nuclei of the superior olivary complex. In the lateral superior olive, inhibitory transmission shifts from depolarizing to hyperpolarizing in the first week after birth (11, 12). Moreover, IPSCs generated by MNTB neurons in the lateral superior olive and the medial superior olive initially are mediated in part by GABA and, later, become exclusively glycinergic in the 2 weeks after birth (11, 13). Such changes may reflect changes in postsynaptic receptor expression or transmitter synthesis by the MNTB neurons themselves.
We have examined presynaptic glycine and GABA receptors during postnatal development of the MNTB to determine which receptor subtypes are present, how they couple to regulation of glutamate release, and how such changes parallel the remarkable alterations in inhibitory transmission described above. We find that a few days after birth, glycine receptors are expressed only weakly by calyces. Instead, at these ages, GABAA receptors are present and their activation results in an increase in the excitatory postsynaptic currents (EPSCs). After 11 days, GABAA receptors are largely removed from the calyx, apparently replaced by glycine receptors, even though both receptors are expressed by the parent-cell body of the calyceal nerve terminals. Thus, mechanisms that target presynaptic receptors to the calyx membrane show preference for glycine receptors as glycine becomes a dominant transmitter in the superior olivary complex.
Methods
Coronal slices of brainstem were prepared from postnatal day (P)5–15 Wistar rats as described previously (10), following approved procedures. Animals were decapitated, and transverse, 250- to 300-μm-thick slices were cut with a vibratome (VT1000S; Leica, Deerfield, IL). Slices were stored in an extracellular solution composed of 125 mM NaCl, 25 mM glucose, 2.5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 1.25 mM NaH2PO4, 25 mM NaHCO3, 0.4 mM ascorbic acid, 3 mM myo-inositol, and 2 mM sodium pyruvate, bubbled with 5% CO2/95% O2 and warmed to 37°C. During recordings (21–23°C), slices were perfused at 4 ml/min with either the above HCO3-based extracellular solution (but without ascorbic acid, myo-inositol, and sodium pyruvate) or a Hepes-based solution containing 145 mM NaCl, 25 mM glucose, 2.5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, and 10 mM Hepes (pH 7.3), bubbled with O2.
Neurons were viewed by using a Zeiss Axioskop FS with differential interference contrast optics and a ×60 water-immersion objective. Postsynaptic principal cells in the MNTB were identified by their typical morphology (spherical cells, diameter ≈15–20 μm), their ability to generate an EPSC and, upon steady depolarization in current clamp, a single action potential. For recordings from globular bushy cells, slices included the anteroventral cochlear nucleus, and bushy cells were identified as oval cells with eccentric nuclei (14) and a single bushy dendrite observed when labeled with Lucifer yellow. Presynaptic terminals were filled with Lucifer yellow in each case and identified visually as a calyx surrounding the MNTB principal cell. Calyces had no spontaneous EPSCs and could generate repetitive action potentials upon depolarization (15).
Borosilicate glass electrodes for whole-cell postsynaptic recording had resistances of 2–3 MΩ when filled with pipette solution; series resistances during recordings were <5 MΩ and were compensated electronically by 90%. Pre- and postsynaptic cells were voltage-clamped to −60 mV or 0 mV, unless otherwise indicated. For recordings from presynaptic terminals, the electrodes were 5–6 MΩ, with series resistances of 10–15 MΩ, compensated by 80–90%. Pipettes for whole-cell recording of EPSCs contained 135 mM CsF, 5 mM CsCl, 5 mM EGTA, 10 mM Hepes, and 2 mM QX314 (286 mOsM) at pH 7.25 with CsOH. Pipettes for pre- and postsynaptic whole-cell recording of glycine and GABA responses contained 125 mM cesium methanesulfonate, 15 mM CsCl, 5 mM EGTA, 1 mM MgCl2, 10 mM Hepes, 2 mM ATP, 0.3 mM GTP, 10 mM phosphocreatine, and 0.2 mM Lucifer yellow (290 mOsM) at pH 7.2 with CsOH. Membrane voltages were corrected for junction potentials.
EPSCs were elicited at 0.1 Hz by voltage pulses (100 μsec, 10- to 20-V stimuli) delivered through a glass pipette. EPSCs were recorded with an Axopatch 200B (Axon Instruments, Foster City, CA); signals were filtered at 5 kHz, digitized at 20 kHz, and analyzed by using pclamp software (Axon Instruments). Ten consecutive ESPCs were averaged for each treatment in each cell. Glycine and GABA responses were filtered at 1 kHz and sampled at 2 kHz. Pre- or postsynaptic conductances were obtained by the slope of a line fitted to the GABA- or glycine-evoked current–voltage relation between −60 mV and +40 mV.
For single-channel recording, outside-out patches were excised after allowing the terminal to load with Lucifer yellow. Recording pipettes contained 140 mM CsCl, 5 mM EGTA, 1 mM MgCl2, 10 mM Hepes, 2 mM ATP, 0.3 mM GTP, 10 mM phosphocreatine, and 0.2 mM Lucifer yellow (290 mOsM) at pH 7.2 with CsOH. The patch first was held away from the slice in a flowing control solution and checked for spontaneous channel openings. Silent patches then were moved into one of two streams of agonist solution, and channel activity was recorded. Recordings were filtered at 2 kHz and analyzed with fetchan 6.0.6. Opening and closing transitions were detected by using a 50% threshold criterion. Openings briefer than 330 μs (twice the filter rise time) were excluded from the analysis. Amplitude histograms were fitted by the sum of 4 or 5 Gaussian curves, as needed (pstat 6.0.4).
Drugs were applied by pressure ejection (Picospritzer II; General Valve, Fairfield, NJ) or by bath perfusion. Pressure pipette conditions were identical for application of both GABA and glycine to cell bodies and to terminals [3-μm pipette tips, placed about 20 μm from the cells, using 1- to 2-psi pressure (1 psi = 6.89 kPa)]. Responses to agonists in the two locations had similar rise times, ≈100 ms, and were stable over time. Reagents were obtained from Sigma and Tocris Neuramin (Bristol, U.K.). Statistical significance was established by using paired and unpaired t tests, as indicated, and errors were reported as ±1 SD.
Results
Regulation of Glutamatergic EPSCs.
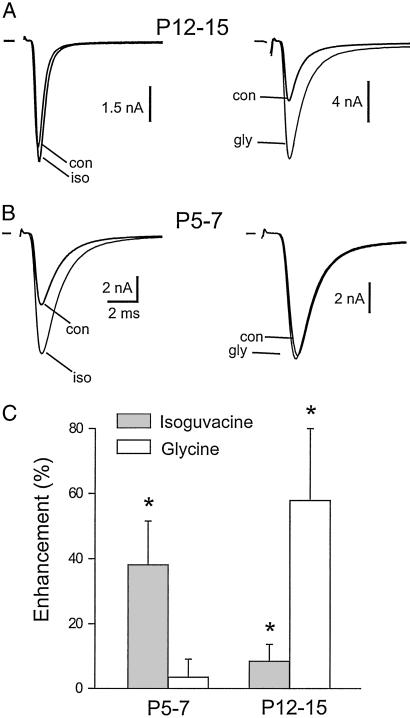
Whole-cell recordings were made in MNTB principal cells, and EPSCs were evoked by stimulation of presynaptic axons. EPSCs were all-or-nothing events, consistent with the presence of a single excitatory terminal, the calyx of Held, on each postsynaptic cell body. Postsynaptic responses to glycine or GABA were suppressed by loading the cell bodies with CsF (10) and holding at −60 mV. As described previously, pressure application of 100 μM glycine in neurons of P12–15 animals significantly (P < 0.01, n = 7) enhanced EPSCs, as shown in Fig. 1 A and C. This effect is consistent with our previous report of a presynaptic glycine receptor (10). For P12–15 rats, application of 100 μM isoguvacine, a selective agonist of GABAA receptors, produced an increase in EPSC amplitude of only 8 ± 5% (P < 0.02, n = 9). However, as shown in Fig. 1 B and C, when recordings were made from animals of age range P5–7, glycine had relatively little effect, whereas isoguvacine strongly enhanced EPSC amplitude (increase by 38 ± 13%, P < 0.001, n = 9). In the presence of 0.5–1 μM strychnine, a glycine receptor antagonist, glycine increased the EPSC by only 6 ± 6% (n = 4) in P12–15 rats, whereas for P5–7 rats with 20–30 μM SR95531, a GABAA receptor antagonist, isoguvacine, increased EPSCs by only 5 ± 7% (n = 4). These data suggest that there is a developmental switch from expression of GABAA receptors to glycine receptors on the calyx membrane.
Figure 1.
Potentiation of EPSCs by isoguvacine and glycine. (A and B) Pressure ejection of 100 μM isoguvacine (iso) increased EPSC amplitude in cells from P5–7 rat MNTB but not from P12–15 rats. By contrast, 100 μM glycine (gly) had the opposite developmental profile. Vhold = −60 mV. (C) Average data. Enhancement is calculated as 100 × (drug − control)/control (n = 5–9 cells per age group; error is given as SD). *, P < 0.01 except for isoguvacine at P12–15 (P < 0.02).
Application of GABA, instead of isoguvacine, in P12–15 rats caused an inhibition of the EPSC because of activation of the metabotropic GABAB receptors (not shown; see refs. 10, 16, and 17). Application of 100 μM dl-baclofen, an agonist at the GABAB receptor, inhibited EPSCs of P6 cells by 88 ± 2% (n = 3) and of P12–15 cells by 85 ± 4% (n = 4). These values were not significantly different (P = 0.25); it is clear that GABAB receptors are present even in the youngest calyces we examined and have large effects in both age groups. Thus, developmental control of GABA receptors in the MNTB over this age range is selective for the ionotropic receptor.
GABA- and Glycine-Gated Ionic Currents in the Calyx.
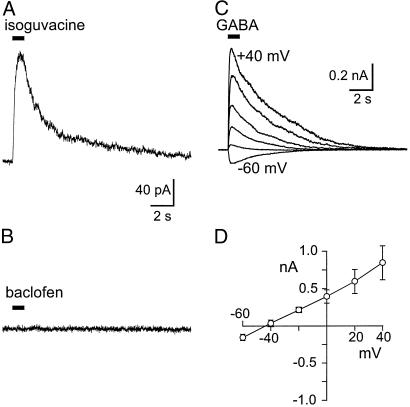
To document directly the changes in expression of functional presynaptic receptors, patch-clamp recordings were made directly from the terminals. As shown in Fig. 2 A and B, in recordings from P8 calyces, pressure application of 100 μM isoguvacine produced an outward current at a holding potential of 0 mV (mean 102 ± 5 pA, n = 3 terminals), whereas 100 μM baclofen had essentially no effect (mean 3.6 ± 0.6 pA, n = 3 terminals). GABA (1 mM) evoked a large ionic current that reversed close to the expected reversal potential for Cl− (Fig. 2 C and D). We then contrasted the amplitude of the GABA- and glycine-evoked currents in rats of different ages. In P12–13 rats, terminals loaded with 17 mM Cl− responded to glycine with a rapid and large outward current at a holding potential of 0 mV, whereas, in the same calyces, GABA evoked a much smaller current; both currents were blocked by agents appropriate for glycine and GABAA receptors (Fig. 3A). The opposite profile was observed in P5–7 calyces (Fig. 3A). In each case, current–voltage relations were obtained as in Fig. 2B, and, from the slope, the conductance of the response was determined and plotted in Fig. 3B. Reversal potentials for GABA and glycine currents were consistent with activation of Cl− channels (overall mean EGABA, −49 ± 4 mV, n = 44; Eglycine, −49 ± 3 mV, n = 46; Cl− Nernst potential, −56 mV) and were independent of age.
Figure 2.
Activation of GABAA receptors in the calyx of Held of neonatal rats. (A) Application of 100 μM isoguvacine evoked an outward current at 0 mV. (B) Baclofen (100 μM) had little effect at the same voltage. (C) Presynaptic recording (P5 rat) by using a cesium-methanesulfonate patch–electrode solution. One-second puff of 1 mM GABA evoked a transient current. Vhold varied in 20-mV increments from −60 mV to +40 mV. (D) Current–voltage relation for peak GABA-evoked current in 15 P5–7 terminals. Reversal is at −48 mV, close to the Nernst potential of −56 mV.
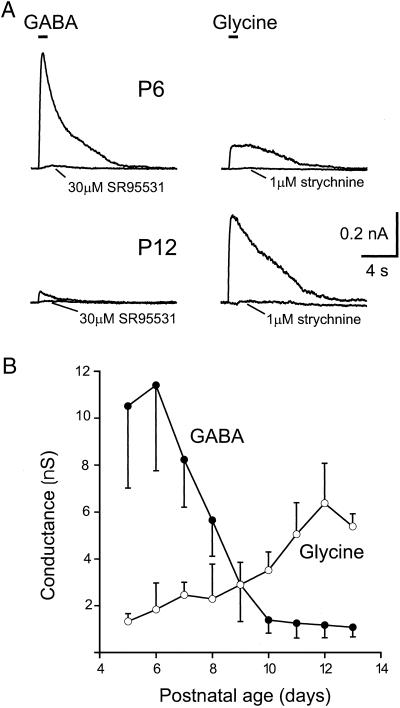
Figure 3.
Developmental regulation of presynaptic GABA- and glycine-evoked currents in the calyx. (A) In a P6 calyx, 1 mM GABA evoked larger currents than 1 mM glycine, whereas in a P12 calyx, glycine responses are larger than GABA responses. In each case, the indicated selective antagonist blocked the current. (B) Average data for calyces from P5-P13; mean of 3–7 calyces per point.
As detailed in Fig. 3B, in calyces from animals between P5 and P13, there was a progressive fall in the amplitude of the GABA response and increase in the glycine response, such that by P12–13, the glycine-induced conductance was 5.5 times larger than the GABA response. However, this switch in expression was not symmetrical: in the younger animals, the GABA-evoked currents were larger than the largest glycine responses of older calyces (mean peak GABA conductance at P5–7 terminals: 10.0 ± 3.3 nS, n = 15; mean peak glycine conductance at P12–13 terminals: 6.0 ± 1.4 nS, n = 8). In the younger age group, 10–30 μM SR95531 blocked the GABA response by 94 ± 2% (n = 4, P < 0.001), whereas the glycine currents on the older age group were blocked by 96 ± 2% by 1 μM strychnine (n = 4, P < 0.001). We were concerned that the developmental shift in response amplitude might occur if receptor affinities changed to render 1 mM either closer to saturation (for glycine) or nonsaturating (for GABA). We measured the ratio of the response to 10 mM and 1 mM GABA or glycine in calyces of the two age groups and found that 1 mM indeed was saturating at all ages for both agonists. For GABA, these ratios were 0.98 ± 0.04 (n = 3) at P5–7 and 0.97 ± 0.09 (n = 3) at P12–13, whereas for glycine, they were 1.01 ± 0.06 (n = 4) at P5–7 and 1.01 ± 0.10 (n = 3) at P12–13.
Single-Channel Recording.
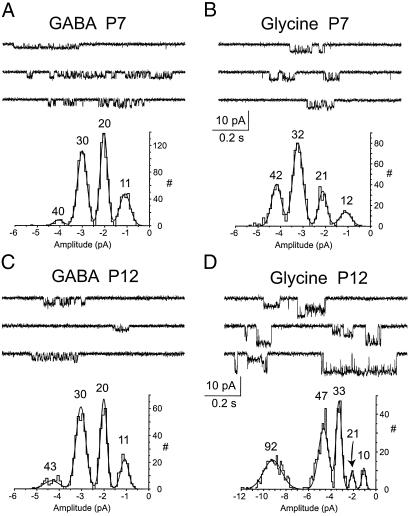
It is possible that the currents we have measured by using presynaptic whole-cell recording and the effects on EPSCs result from activation of receptors present on the preterminal axon rather than the synapse itself (18). To determine whether at least some of these receptors were located on the nerve terminals themselves, outside-out patches were obtained from calyces and solutions containing 10 μM GABA or glycine were alternately applied to each patch. In the best cases, patches lasted only long enough to test each solution at one potential (−100 mV), and patches were discarded if any events were observed in control solution. Three patches were obtained from calyces from P5–7 rats and 1 each was obtained from a P11 and P12 rat. In all of the patches, GABA application resulted in single-channel openings of variable amplitude, ranging from 11 to 42 pS. Fig. 4 A and C illustrates examples of individual traces and amplitude histograms of the currents. Gaussian fits to the histograms gave peaks at 11, 20, 30, and 40 pS, with the 20- and 30-pS currents generating 35 ± 6% and 40 ± 7% of the recorded events. With glycine, the three patches in P5–7 animals generated openings similar to those observed with GABA, having chord conductance values between 11 and 42 pS, and most of the events generated by 21- and 31-pS openings (21 ± 6% and 41 ± 7%, respectively; see Fig. 4B). However, the two patches from older animals also contained glycine-evoked openings of 91 and 92 pS (40% and 30% of the events) (Fig. 4D). These values are similar to those observed previously for GABA- and glycine-gated channels in rat (5, 19). These data directly demonstrate that GABAA and glycine receptors are expressed on the presynaptic membrane.
Figure 4.
Single GABAA and glycine channels from presynaptic membrane. (A–D) Sample records and amplitude histograms for patches from the indicated ages exposed to 10 μM GABA or glycine. Histograms were fitted with sums of four to five Gaussian curves. Means of component were used to estimate channel chord conductance (given in pS above each peak), assuming a reversal potential of 0 mV.
Receptor Expression in Presynaptic Cell Bodies.
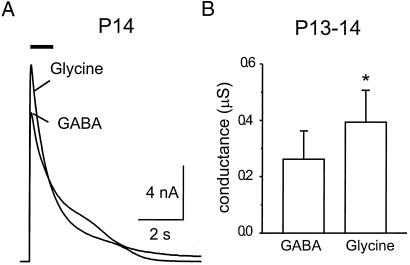
We next asked whether the apparent loss of GABAA receptors in the calyx reflected a change in the targeting of receptors to the axon terminal or simply a global change in expression over the entire cell. To explore this question, recordings of GABA and glycine sensitivity were made from globular bushy cells of the cochlear nucleus, the parent-cell bodies of the calyces (see Methods). As shown in Fig. 5, bushy cells of P13–14 animals produced large responses to pressure-applied GABA and glycine, with glycine responses only 34% larger than the GABA-evoked currents. Thus, despite the observation that calyceal glycine responses were nearly 6-fold larger than GABA responses, bushy cells abundantly express GABAA receptors, consistent with results from molecular characterization of receptor expression in adult rat (20).
Figure 5.
GABA and glycine responses from somata of globular bushy cells of the ventral cochlear nucleus. (A) In one neuron, current evoked by 1 mM GABA was slightly smaller than that evoked by 1 mM glycine. (B) Average data for seven bushy cells. Glycine responses were significantly larger than GABA responses (P < 0.003). Current–voltage relations were obtained for each cell, and the conductance was determined by linear regression.
Discussion
We report here direct measurements of GABAA receptors in a vertebrate central synapse. Presynaptic GABAA receptors also are thought to mediate inhibition of primary afferent terminals in the spinal cord (21) and of vasopressin release in the secretory terminals of the posterior pituitary (22). Yet, like glycine receptors, GABAA receptors potentiate glutamate release from the calyx, probably via a Cl-mediated depolarization (10). It is likely that whether GABA inhibits or facilitates release depends on the types of Ca2+ channel expressed in the synapse, the sensitivity of the release apparatus to Ca2+, and the reversal potential for [Cl−] (10).
These experiments suggest that presynaptic ionotropic receptors are subject to multiple developmental signals that dramatically alter receptor expression during synaptic maturation. After the first 2 postnatal weeks, glycine receptors are expressed in the cell bodies of bushy cells, consistent with studies documenting increased expression of α1 glycine receptor subunit in the rat auditory system (23, 24). The number of functional GABAA receptors declined sharply in the calyces but remained high in bushy cells, despite the apparent absence of GABA-ergic transmission onto those cells in mature animals (25, 26). Thus, the changing expression of GABAA receptors in terminals is not simply a by-product of grossly reduced expression levels in the whole cell but, instead, reflects a selective mechanism for controlling membrane proteins in the axon/terminal compartment.
This might occur in several ways. Possibly, bushy cells express developmentally regulated targeting proteins that escort particular substrates, such as GABAA receptors, to the axon. Alternatively, there may be a developmental switch in GABAA subtypes, such that both are found in somatic membrane but only one can be targeted to the axon. In single-channel recordings, we found a variety of conductance states induced by GABA. The range suggests the possibility of multiple subtypes. It has been shown that the addition of γ- or δ-subunits shifts GABAA receptor conductance from 20 to 30 pS (27). Presynaptic GABAA receptors may be heterogeneous with respect to these additional subunits.
Finally, it may be that the loss of GABAA receptors occurs because receptor insertion at the synapse decreases or internalization increases. However, it is interesting that the changes we observed in receptor expression coincide with changes in Ca2+ channel expression in the calyx (28), just preceding the maturation of hearing. Enhancement of average intraterminal Ca2+ levels might trigger down-regulation of GABAA receptor (29) and enhancement of glycine receptor activity (30, 31). Molecular and biophysical analysis of GABAA receptor expression during development will be required to clarify this issue. By contrast, the effectiveness of GABAB receptors in reducing glutamate release is unchanged during this period. Thus, whatever the mechanisms responsible for regulation of presynaptic GABAA receptors, GABA shifts from having mixed effects on neonatal transmission to being purely inhibitory, whereas glycine takes over as a facilitator of release.
The appearance in P11–12 terminals of high-conductance glycine channel events is consistent with the presence of glycine receptor complexes that do not contain the β-subunit (32). The β-subunit is characteristic of synaptic glycine receptors and mediates localization through tethering to gephyrin (33). In developing spinal cord, a shift was observed from high- to low-conductance glycine channels, opposite from what we have seen (5). It is not clear that presynaptic glycine or GABAA receptors require localization to any particular region of the terminal; that they are found in patches suggests that they are at least distributed over the outer face of the synapse. Thus, the presence of receptors lacking β-subunits is not maladaptive. Indeed, given their higher channel conductance and higher reported glycine affinity (32), homomeric glycine receptors may be ideal for sensing the diffusion of glycine from distant sources.
During the first 1.5 weeks after birth, glycine receptor mRNA and protein expression increases in the cochlear nuclei (24, 34). This increase parallels the presynaptic glycine receptor localization that we have observed and coincides with an increase in glycine immunoreactivity of the glycinergic MNTB neurons themselves (35). During this time, striking changes take place in the pharmacology of inhibition in targets of the MNTB, lateral superior olive, and medial superior olive, in which slow GABA-ergic transmission abates and glycinergic transmission is strengthened (11, 13, 35). Thus, the change in expression of presynaptic receptors takes place in the context of other shifts in receptors and transmitters mediating synaptic transmission.
What is the functional significance of the reciprocal control of GABAA and glycine receptors at pre- and postsynaptic membrane? In animals older than P10, presynaptic depolarization induced by glycine spillover enhances EPSC amplitude. Perhaps, GABAA receptors are needed in young animals because these receptors tend to have slower gating kinetics and, so, may provide additional current and higher sensitivity to weaker synapses. Another possibility, however, is that GABAA receptors serve a trophic role in the growth cone and young synapse, sensing ambient GABA, which could enhance axonal outgrowth. Such a model has been proposed for somatodendritic GABAA receptors in migrating neurons (36, 37). Here, GABAA receptor activation leads to weak depolarization and calcium influx through voltage-dependent Ca channels. In this view, loss of calyceal GABAA receptors reflects the maturation from growth cone to a stabilized nerve terminal.
Regulation of EPSC amplitude by GABA and glycine depends on their depolarizing action on the calyx. It is well known that GABAA and glycine receptor activation in neonatal and embryonic brain is depolarizing because of elevated intracellular [Cl−] (38). Recently, it was suggested that activation of GABAA receptors, and the attendant elevation of intracellular Ca2+, enhances expression of the Cl− transporter KCC2, thus leading to reduced [Cl−]i and the development of hyperpolarizing inhibition (39). It is of interest, then, that depolarizing presynaptic glycine responses remain even at ages in which glycine is hyperpolarizing in somatodendritic regions of bushy cells (10, 26). The average enhancement of EPSC amplitude induced by isoguvacine and glycine was the same, and glycine was effective even in the oldest animals we have used (P15). Thus, it seems possible that KCC2 is excluded from the axon and that nerve terminal Ca2+ does not contribute to the regulation of KCC2 levels.
Acknowledgments
We thank Gautam Awatramani and Thanos Tzounopoulos for comments. This work was supported by National Institutes of Health Grant DC04450 (to L.O.T.) and by Grant TW05406-01 from the Fogarty International Center, National Institutes of Health (to R.T.).
Abbreviations
- GABA
γ-aminobutyric acid
- MNTB
medial nucleus of the trapezoid body
- IPSC
inhibitory postsynaptic current
- EPSC
excitatory postsynaptic current
- P
postnatal day
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mammen A L, Huganir R L, O'Brien R J. J Neurosci. 1997;17:7351–7358. doi: 10.1523/JNEUROSCI.17-19-07351.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien R J, Mammen A L, Blackshaw S, Ehlers M D, Rothstein J D, Huganir R L. J Neurosci. 1997;17:7339–7350. doi: 10.1523/JNEUROSCI.17-19-07339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson M J, Cohen M W. J Physiol (London) 1977;268:757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuromi H, Kidokoro Y. Dev Biol. 1984;103:53–61. doi: 10.1016/0012-1606(84)90006-x. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- 6.Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B. Nature. 1986;321:406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- 7.MacDermott A B, Role L W, Siegelbaum S A. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 8.Rossi D J, Hamann M. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- 9.Kullmann D M. Prog Brain Res. 2000;125:339–351. doi: 10.1016/S0079-6123(00)25023-1. [DOI] [PubMed] [Google Scholar]
- 10.Turecek R, Trussell L O. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- 11.Kotak V C, Korada S, Schwartz I R, Sanes D H. J Neurosci. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandler K, Friauf E. J Neurosci. 1995;15:6890–6904. doi: 10.1523/JNEUROSCI.15-10-06890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith A J, Owens S, Forsythe I D. J Physiol (London) 2000;529:681–698. doi: 10.1111/j.1469-7793.2000.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willard F H, Ryugo D K. In: The Auditory Psychobiology of the Mouse. Willott J F, editor. Springfield, IL: Thomas; 1983. pp. 201–304. [Google Scholar]
- 15.Borst J G, Helmchen F, Sakmann B. J Physiol (London) 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi T, Kajikawa Y, Tsujimoto T. J Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacson J S. J Neurophysiol. 1998;80:1571–1576. doi: 10.1152/jn.1998.80.3.1571. [DOI] [PubMed] [Google Scholar]
- 18.Pouzat C, Marty A. J Neurosci. 1999;19:1675–1690. doi: 10.1523/JNEUROSCI.19-05-01675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bormann J, Hamill O P, Sakmann B. J Physiol (London) 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frostholm A, Rotter A. Brain Res Bull. 1986;16:189–203. doi: 10.1016/0361-9230(86)90033-x. [DOI] [PubMed] [Google Scholar]
- 21.Rudomin P. J Physiol (London) 2000;528:1. doi: 10.1111/j.1469-7793.2000.t01-1-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S J, Jackson M B. J Neurophysiol. 1995;73:1135–1144. doi: 10.1152/jn.1995.73.3.1135. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, Kuriyama H, Altschuler R A. Hear Res. 1995;91:7–18. doi: 10.1016/0378-5955(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 24.Piechotta K, Weth F, Harvey R J, Friauf E. J Comp Neurol. 2001;438:336–352. [PubMed] [Google Scholar]
- 25.Lim R, Alvarez F J, Walmsley B. J Physiol (London) 2000;525:447–459. doi: 10.1111/j.1469-7793.2000.t01-1-00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickesberg R E, Oertel D. J Neurosci. 1990;10:1762–1768. doi: 10.1523/JNEUROSCI.10-06-01762.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher J L, Macdonald R L. J Physiol (London) 1997;505:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasaki S, Takahashi T. J Physiol (London) 1998;509:419–423. doi: 10.1111/j.1469-7793.1998.419bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyons H R, Land M B, Gibbs T T, Farb D H. J Neurochem. 2001;78:1114–1126. doi: 10.1046/j.1471-4159.2001.00501.x. [DOI] [PubMed] [Google Scholar]
- 30.Kirsch J, Betz H. Nature. 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- 31.Fucile S, De Saint Jan D, de Carvalho L P, Bregestovski P. Neuron. 2000;28:571–583. doi: 10.1016/s0896-6273(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 32.Bormann J, Rundstrom N, Betz H, Langosch D. EMBO J. 1994;12:3729–3737. doi: 10.1002/j.1460-2075.1993.tb06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer G, Kirsch J, Betz H, Langosch D. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 34.Friauf E, Hammerschmidt B, Kirsch J. J Comp Neurol. 1997;385:117–134. doi: 10.1002/(sici)1096-9861(19970818)385:1<117::aid-cne7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Korada S, Schwartz I R. J Comp Neurol. 1999;409:664–681. doi: 10.1002/(sici)1096-9861(19990712)409:4<664::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 36.Owens D F, Liu X, Kriegstein A R. J Neurophysiol. 1999;82:570–583. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- 37.Maric D, Liu Q Y, Maric I, Chaudry S, Chang Y H, Smith S V, Sieghart W, Fritschy J M, Barker J L. J Neurosci. 2001;21:2343–2360. doi: 10.1523/JNEUROSCI.21-07-02343.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherubini E, Gaiarsa J L, Ben-Ari Y. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 39.Ganguly K, Kiss L, Poo M. Nat Neurosci. 2000;3:1018–1026. doi: 10.1038/79838. [DOI] [PubMed] [Google Scholar]