Abstract
Excitatory synapses in the brain exhibit a remarkable degree of functional plasticity, which largely reflects changes in the number of synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). However, mechanisms involved in recruiting AMPARs to synapses are unknown. Here we use hippocampal slice cultures and biolistic gene transfections to study the targeting of AMPARs to synapses. We show that AMPARs are localized to synapses through direct binding of the first two PDZ domains of synaptic PSD-95 (postsynaptic density protein of 95 kDa) to the AMPAR-associated protein, stargazin. Increasing the level of synaptic PSD-95 recruits new AMPARs to synapses without changing the number of surface AMPARs. At the same time, we show that stargazin overexpression drastically increases the number of extra-synaptic AMPARs, but fails to alter synaptic currents if synaptic PSD-95 levels are kept constant. Finally, we make compensatory mutations to both PSD-95 and stargazin to demonstrate the central role of direct interactions between them in determining the number of synaptic AMPARs.
Excitatory synapses in the brain release the transmitter glutamate, which acts primarily on two subtypes of ionotropic receptors, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) receptors. Recent studies establish that AMPA receptors (AMPARs), in contrast to NMDA receptors (NMDARs), turn over rapidly at synapses (1–4) and that synaptic plasticity involves rapid changes in synaptic AMPAR number (5–9). However, little is known about the molecular mechanisms regulating the transport and retention of AMPARs at synapses.
Previous attempts to identify proteins that retain AMPARs at the synapse have focused on PDZ domain-containing proteins that bind AMPARs directly (10–13), including GRIP, ABP, and PICK1. More recent evidence suggests that an AMPAR-binding membrane protein, stargazin, is involved in synaptic AMPAR targeting via interactions with an unknown PDZ domain-containing protein (14). This finding expands the pool of PDZ proteins that potentially regulate synaptic localization of AMPARs; however, this protein remains to be identified.
One particular synaptic PDZ domain protein, PSD-95 (postsynaptic density protein of 95 kDa)/SAP90 (15, 16), has been intensively studied for its possible role in the clustering of receptors and channels (17–19). In dissociated neurons, PSD-95 overexpression for a prolonged period during development increases the accumulation of various proteins at the synapse in a manner resembling synaptic maturation (20). However, more than 20 synaptic proteins that bind to PSD-95 have been identified, and it is unclear which of these interacting proteins might mediate various aspects of the enhanced maturation.
In this study, we show that increasing synaptic PSD-95 levels selectively increases the number of synaptic AMPARs. In contrast, overexpression of a separate synaptic protein, stargazin, massively increases the number of extra-synaptic AMPARs without affecting the synaptic response. We then perform a series of experiments with deletion constructs to identify regions crucial to the synaptic targeting of AMPARs in both proteins. Using this information, we generate compensatory mutations to both PSD-95 and stargazin to establish that a direct interaction between these two proteins localizes AMPARs to the synapse.
Methods
Plasmid Constructs.
Plasmid cDNA constructs encoding the various membrane-associated guanylate kinase (MAGUK), PSD-95 deletion, and stargazin constructs used throughout this study were made as described (14, 21). All MAGUK constructs were GFP-tagged at the C terminus unless otherwise noted. Stargazin constructs had GFP inserted in-frame at the BglII site in the C terminus. Point mutant constructs of PSD-95 were generated by using PCR. All constructs obtained via PCR were sequenced.
GST-Fusion Analysis.
GST-stargazin fusion proteins were expressed and purified as described (22). COS-7 cells were transfected with WT or mutant PSD-95, and extracts were prepared as described (22). Three micrograms of GST protein coupled to Sepharose beads was added to the soluble fraction of the COS-7 cell extracts, and the samples were incubated at 4°C for 60 min. The protein-coupled GST beads were pelleted by centrifugation and then washed five times with resuspension buffer (25 mM Tris⋅HCl, pH 7.5/150 mM NaCl/5 mM EDTA/5 mM EGTA). The beads were resuspended in 5× protein loading buffer, and samples were separated by SDS/PAGE and analyzed by Western blotting with monoclonal mouse PSD-95 antibodies.
Slice Culture Transfection and Electrophysiology.
Hippocampal slice cultures were prepared from 6- to 11-day-old rats as described (23, 24). Transfections were carried out 4–6 days later with the Helios Gene Gun (Bio-Rad), using 1.0-μm gold particles coated with DNA per the manufacturer's protocol. Bullets coated with two cDNAs reliably yielded coexpression in >90% of transfected cells when dsRed and PSD-95 GFP were cotransfected in preliminary experiments.
Recordings were made from transfected cells 1–4 days after transfection, using 2–3 MΩ glass electrodes filled with an internal solution consisting of 115 mM CsMeSO3, 20 mM CsCl, 10 mM Hepes, 2.5 mM MgCl2, 4 mM Na2-ATP, 0.4 mM Na-GTP, 10 mM Na-phosphocreatine, 0.6 mM EGTA, and 0.1 mM spermine, pH 7.2. External perfusion medium consisted of 119 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgSO4, 2.7 mM MgCl2, 1 mM NaH2PO4, 26.2 mM NaHCO3, and 11 mM glucose, saturated with 95% O2 and 5% CO2, and included 100 μM picrotoxin, 20 μM bicuculline, and 5–20 μM 2-Cl adenosine to block inhibition and suppress epileptiform activity. Transfected pyramidal cells were identified by using fluorescence microscopy. A bipolar tungsten stimulating electrode was placed in stratum radiatum approximately 100 μm from the cells. Recording electrodes were used to first establish cell-attached connections with both a transfected cell and an immediately adjacent untransfected cell under visual guidance (×40, differential interference contrast optics). Both cells were broken into simultaneously, and stimulation intensity was slowly increased until excitatory postsynaptic currents (EPSCs) were elicited from both cells. In PSD-95 experiments, lower levels of stimulation were generally used to allow adequate clamping of the larger AMPAR EPSCs in transfected cells. Likewise, in experiments involving stargazinΔC, higher stimulation was required to obtain a measurable AMPAR EPSC. A total of 50–100 trials were obtained at 0.33 Hz while holding the cells at −70 mV, followed by 50–100 trials at +40 mV. Series resistances typically ranged from 8 to 12 MΩ; a cell pair was discarded if the series resistances differed substantially between the two cells. AMPA-mediated whole-cell currents were obtained from two cells simultaneously by using a 10-min bath perfusion of AMPA in the presence of 1 μM Tetrodotoxin. All statistics were obtained by using paired t tests.
Fixation and Confocal Microscopy.
Slice cultures were fixed for use in confocal microscopy with 4% paraformaldehyde/4% sucrose in PBS overnight at 4°C. Images were obtained by compiling Z-stacks of images made at ×100 with 0.5-μm thick sections, using a Bio-Rad Confocal system attached to a Nikon microscope.
Results
Control of Synaptic AMPARs by PSD-95.
We used organotypic slice cultures in conjunction with biolistic transfections to express GFP-tagged proteins in hippocampal pyramidal neurons. All experiments involved simultaneous patch-clamp recordings from a transfected cell and a neighboring untransfected cell. A stimulating electrode placed in stratum radiatum activated excitatory afferents. The relative response magnitudes evoked by activating the same presynaptic afferents were directly compared, allowing assessment of the effects of protein expression in the postsynaptic neuron on synaptic currents. A typical experiment, in this case with GFP-tagged PSD-95, is shown in Fig. 1. Confocal microscopy of fixed slices (Fig. 2A) allowed for high-resolution localization of PSD-95 to dendritic spines, the contact site for excitatory synapses.
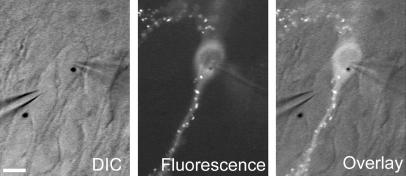
Figure 1.
Slice culture recording configuration. Each experiment involved simultaneous whole-cell voltage-clamp recordings from both a transfected cell (PSD-95 GFP in this example, cell on right, gold particle in nucleus) and an immediately adjacent untransfected cell (left). (Left) The differential interference contrast (DIC) transmitted light image. (Center) Clusters of PSD-95 GFP in the spines of the transfected cell when viewed under fluorescence. (Right) The two images are overlaid. (Scale bar: 20 μm.)
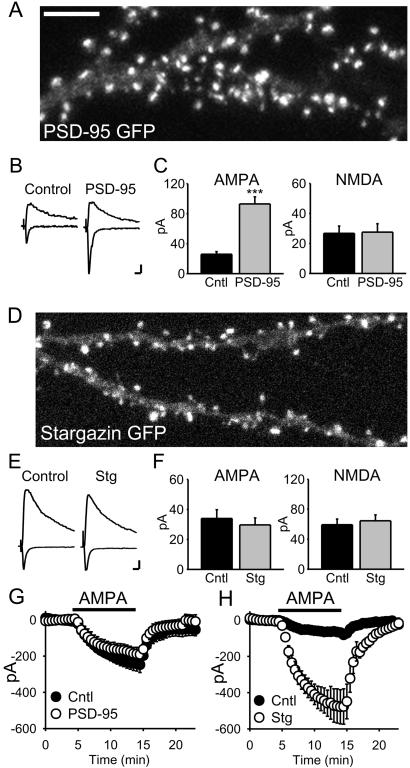
Figure 2.
PSD-95 and stargazin have differential effects on synaptic and surface AMPAR number. (A) Confocal image of a pyramidal cell expressing PSD-95 GFP showing its localization to synaptic spines. (Scale bar: 5 μm.) (B) Averaged EPSCs recorded simultaneously from a pair of cells, showing the responses at −70 mV and +40 mV for a PSD-95-transfected cell and a neighboring untransfected (control) cell. (Scale bars: 10 pA, 20 ms.) (C) Bar graph representations of data from the PSD-95 transfections. AMPAR EPSCs are significantly enhanced (P < 1 × 10−6, n = 27 pairs), whereas NMDAR EPSCs are unchanged (P = 0.81, n = 23 pairs). (D) Confocal image of a cell expressing stargazin-GFP shows that it also localizes to synaptic spines (same scale as A). (E and F) Overexpression of stargazin has no effect on evoked synaptic responses (AMPAR EPSCs, n = 26 pairs, P = 0.48; NMDAR EPSCs, n = 14 pairs, P = 0.99). (G) PSD-95-expressing cells do not show a change in the response to bath-applied AMPA (1 μM, n = 3 pairs). (H) Overexpression of stargazin dramatically increases responses to bath application of AMPA (500 nM, n = 7 pairs, P = 0.003).
In all experiments we measured the amplitude of the AMPAR-mediated EPSC at −70 mV and measured the amplitude of the NMDAR-mediated EPSC at +40 mV and at a latency when the AMPAR EPSC had fully decayed (60 ms). Representative traces of averaged AMPAR and NMDAR EPSCs simultaneously recorded from a control and transfected cell (Fig. 2B) show that PSD-95 dramatically enhanced the AMPAR EPSC, whereas the NMDAR EPSC was unchanged. Data from 27 such experiments are summarized in bar graphs (Fig. 2C), demonstrating the marked and specific enhancement of AMPAR-mediated synaptic transmission by PSD-95. This enhancement occurred within 12 h of transfection, which correlated with the time course of synaptic clustering of transfected PSD-95 in slice culture.
The enhancement of the AMPAR EPSC observed here occurred without a change in the NMDAR EPSC, implying no increase in glutamate release. This conclusion was supported by the lack of change in paired-pulse facilitation, a sensitive assay for changes in release probability (untransfected = 1.75 ± 0.17; PSD-95 GFP = 1.83 ± 0.25; n = 5). In a number of pairs we simultaneously recorded the response to bath-applied AMPA, which activated both synaptic and extra-synaptic AMPARs. No enhancement was detected in neurons expressing PSD-95 (Fig. 2G), even though in these same cells the AMPAR EPSCs were strongly enhanced.
Control of Surface AMPARs by Stargazin.
As PSD-95 does not interact directly with AMPARs, we performed a similar set of experiments with the putative adaptor protein, stargazin. Transfected stargazin-GFP showed a similar punctate expression pattern, with most of the puncta present in dendritic spines (Fig. 2D). In striking contrast to PSD-95, however, overexpression of stargazin did not affect AMPAR or NMDAR EPSCs (Fig. 2 E and F).
Remarkably, we found that stargazin caused a 5-fold increase in the response to bath-applied AMPA (Fig. 2G), suggesting a dramatic increase in the number of surface AMPARs. The increased response to bath-applied AMPA was not caused by an effect of stargazin on AMPAR desensitization, as it was still observed in the presence of cyclothiazide, which blocks desensitization (100 nM AMPA + 100 μM cyclothiazide; untransfected = 377 ± 78 pA versus transfected = 1210 ± 227 pA; n = 7, P = 0.01). The effect of stargazin was specific to AMPARs, because responses to bath-applied NMDA (5 μM) were unaffected (untransfected = 496 ± 65 pA versus transfected = 419 ± 147 pA; n = 3). This result indicates that, in contrast to synaptic AMPARs, stargazin is limiting for expression of extra-synaptic surface AMPARs.
AMPAR Recruitment to Synapses Involves Synaptic PDZ Interactions.
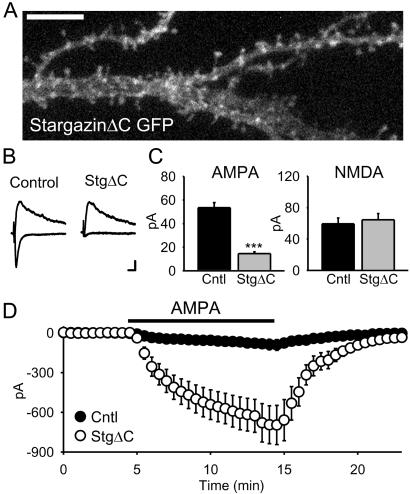
To gain insights into the mechanisms of PSD-95 and stargazin function, we analyzed a series of mutant and deletion constructs. Removing the PDZ binding site of stargazin by deleting the last 4 aa at its C terminus (stargazinΔC) reduced both its clustering (Fig. 3A) and the AMPAR EPSC (Fig. 3 B and C). This effect was selective for the AMPAR EPSC, as the NMDAR EPSC did not change. Intriguingly, when these same cells were exposed to bath-applied AMPA, they showed a massive enhancement of the AMPAR-mediated response (Fig. 3D) similar to that seen with stargazin (Fig. 2H). Thus, stargazinΔC had two opposing actions: it simultaneously depleted the synapse of AMPARs while loading the extra-synaptic membrane with AMPARs (Fig. 3 C and D). Together, these results highlight the critical role of stargazin and, in particular, its PDZ-binding C terminus, in controlling delivery of AMPARs to the synapse.
Figure 3.
The stargazin PDZ-binding region is required for synaptic, but not extra-synaptic, AMPAR trafficking. (A) Confocal image of a pyramidal cell expressing stargazinΔC-GFP shows its diffuse localization. (Scale bar: 5 μm.) (B and C) Overexpression of stargazinΔC strongly reduces AMPAR EPSCs (n = 54 pairs, P < 1 × 10 −9) while having no effect on NMDAR EPSCs (n = 37 pairs, P = 0.43). (Scale bars, traces: 10 pA, 20 ms.) (D) In contrast, stargazinΔC still dramatically increases responses to bath application of 500 nM AMPA (n = 9 pairs, P = 0.0006).
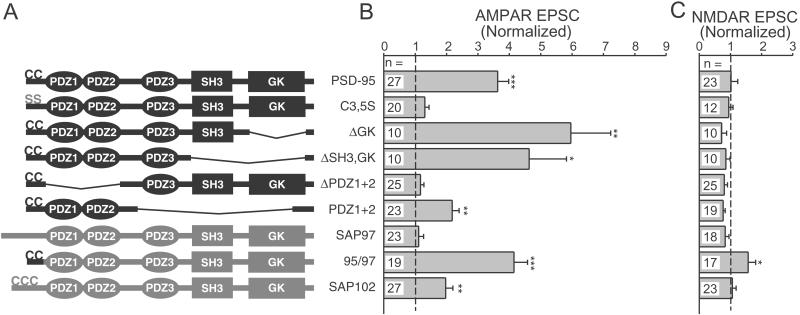
We also analyzed PSD-95 deletion constructs to identify regions critical to PSD-95 function. For each of the constructs illustrated in Fig. 4A we examined the degree of synaptic clustering and the degree of enhancement of AMPAR and NMDAR EPSCs. Our results with clustering (data not shown) generally agreed with previous findings (21) and, importantly, we found that the level of clustering correlated with the degree of enhancement of the AMPAR EPSC. The N-terminal palmitoylation of PSD-95 that is essential for its synaptic clustering was also necessary for enhancement of synaptic responses, as the PSD-95 C3,5S mutant did not enhance AMPAR EPSCs (Fig. 4B). Removing the guanylate kinase (GK), Src homology 3, and third PDZ domains of PSD-95 did not disrupt the robust enhancement of the AMPAR EPSC (Fig. 4B), suggesting that the first two PDZ domains were sufficient. Deletion of the first two PDZ domains completely abolished the enhancement (Fig. 4B). We were unable to observe any effects of PSD-95 overexpression on NMDAR EPSCs (Fig. 4C). These results demonstrate the importance of PSD-95 palmitoylation and the involvement of the first two PDZ domains in the selective enhancement of the AMPAR EPSC.
Figure 4.
PSD-95 palmitoylation and PDZ domains are needed to enhance synaptic AMPAR EPSCs. (A) Diagrams showing the domain structure of the various PSD-95 and MAGUK constructs. SH3, Src homology 3; GK, guanylate kinase. (B) Summary graph showing the effects of overexpressing the various constructs on the evoked AMPAR EPSC in simultaneously recorded pairs. For each construct, the normalized response was obtained by dividing the average AMPAR EPSC amplitude in the transfected cells by the average amplitude in paired, untransfected cells. Statistically significant values are marked with asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001); the number of paired recordings is listed for each construct. (C) Summary graph showing the effects of these constructs on the NMDAR EPSC.
PSD-95 is a member of a family of MAGUK proteins, which also includes the AMPAR-binding protein SAP97 (25). Overexpressed SAP97 had no significant effect on either AMPAR or NMDAR EPSCs (Fig. 4 B and C). SAP97 lacks N-terminal palmitoylation and exhibits a diffuse localization when overexpressed (21). Insertion of the PSD-95 palmitoylation motif onto the N terminus of SAP97 resulted in a high degree of clustering (21) and enhanced AMPAR EPSCs (Fig. 4B). This 95/97 construct was the only one to cause a significant increase in the NMDAR EPSC (Fig. 4C); the functional significance of this is unclear. SAP102 overexpression also selectively enhanced the AMPAR EPSC (Fig. 4 B and C).
A Direct Interaction Between Stargazin and PSD-95 Mediates the Synaptic Delivery of AMPARs.
Might the effects of PSD-95 and stargazin be interdependent? We designed two types of experiments to address this possibility. First, if AMPARs are localized to synapses through the interaction of stargazin with PSD-95, overexpressing the C terminus of stargazin should depress AMPAR EPSCs and interfere with the AMPAR-enhancing effect of PSD-95 overexpression. Indeed, expression of a GFP-tagged stargazin C terminus significantly depressed AMPAR EPSCs (untransfected average = 58.3 ± 6.0 pA, transfected = 34.3 ± 5.2 pA, n = 47 pairs, P = 0.002) and significantly attenuated the enhancement caused by PSD-95 (GFP + PSD-95 = 202 ± 13% increase, n = 15 pairs; GFP-StarCterm + PSD-95 = 72 ± 19% increase, n = 14 pairs, P < 0.05). Although these results supported our model, expression of the C terminus of stargazin could have displaced other ligands that bind PSD-95 and/or perturbed interactions involving other PDZ domain-containing proteins. Indeed, this construct depressed NMDAR EPSCs (untransfected average = 71.4 ± 14.6 pA, transfected = 45.9 ± 9.7 pA, n = 16 pairs, P = 0.009), confirming that this approach lacked specificity.
To determine more decisively whether stargazin and PSD-95 interact to control AMPAR localization, we generated compensatory mutations in the PDZ domains of PSD-95 and the C terminus of stargazin (26). Crystallographic and NMR structures of PSD-95 PDZ domains (27–29) identified key residues that should interact with the stargazin C terminus. Conserved histidine residues in the PSD-95 PDZ domains (which are class I) are predicted to form hydrogen bonds with the threonine at the −2 position in the C terminus of stargazin, as is typical of most class I PDZ domains and their ligands (30). Class II PDZ domains often contain a valine at this position and bind ligands with a large hydrophobic residue, such as tyrosine or phenylalanine, at their −2 position (31). Thus, we made mutations in both stargazin and PSD-95 to convert the PDZ/ligand interaction from a class I to a class II (26). In the case of stargazin, we mutated the −2 position threonine to phenylalanine (=Stargazin T321F), and for PSD-95, we mutated histidines 130 and 225 (corresponding to PDZs 1 and 2, respectively) to valines (=PSD-95 H130V or H225V).
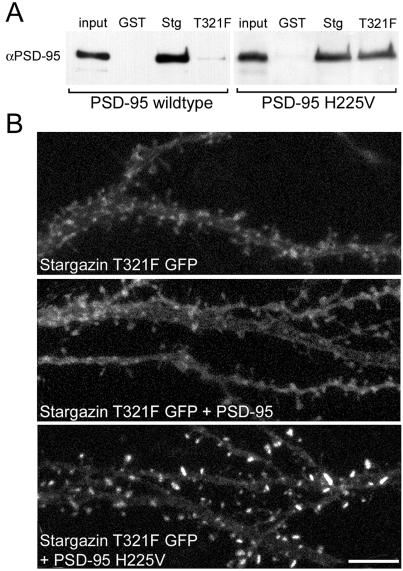
The PSD-95/stargazin mutants were first examined biochemically by using GST-fusion constructs terminating with the last 12 aa of either WT or T321F stargazin. The T321F point mutation disrupted stargazin binding to PSD-95 (Fig. 5A). By contrast, this mutant stargazin bound PSD-95 bearing a compensatory mutation (Fig. 5A).
Figure 5.
Compensatory mutations to PSD-95 and stargazin reconstitute binding and clustering. (A) GST-fusion proteins show that stargazin with a point mutation in its PDZ-binding region (T321F) does not bind WT PSD-95, but does bind PSD-95 bearing a compensatory point mutation (H225V) in its second PDZ domain. (B) Confocal images of pyramidal cells expressing stargazin T321F GFP. Stargazin T321F GFP alone expressed diffusely (Top). Coexpression of untagged PSD-95 did not alter the diffuse localization of stargazin T321F (Middle). Cotransfection of an untagged compensatory PSD95 (PSD-95 H225V) caused robust synaptic localization of stargazin T321F GFP (Bottom). (Scale bar: 5 μm.)
We then tested the effects of these mutations on protein function in neurons. When stargazin T321F was transfected into neurons alone, it had a diffuse, membrane-associated pattern (Fig. 5B Top), reminiscent of the expression pattern of stargazinΔC (Fig. 3A). The expression pattern of stargazin T321F was unaffected by cotransfection with PSD-95 (Fig. 5B Middle). In striking contrast, cotransfection of PSD-95 with a compensatory mutation in PDZ1 (data not shown) or PDZ2 (Fig. 5B Bottom) restored the synaptic localization of the stargazin mutant. This finding demonstrated that an appropriate, synaptically localized PSD-95 PDZ domain is sufficient for the synaptic targeting of stargazin.
We next examined whether the reconstitution of synaptic clustering was associated with changes in synaptic transmission. Transfection of stargazin T321F strongly depressed AMPAR EPSCs, without any change in the NMDAR EPSC (Fig. 6A). Again, this result was identical to that obtained with stargazinΔC, as would be predicted if the mutation interfered with the binding of stargazin to its endogenous PDZ partner. In addition, coexpression of stargazin T321F with WT PSD-95 prevented the typical enhancement of the AMPAR EPSC, and the AMPAR EPSC was actually depressed (Fig. 6B). By contrast, coexpression of stargazin T321F with PSD-95 bearing a compensatory mutation reversed the depressant effect of stargazin T321F. AMPAR EPSCs were significantly enhanced (Fig. 6C), in a manner reminiscent of the overexpression of WT PSD-95 (Fig. 2 B and C). This occurred both for PSD-95 constructs bearing a compensatory point mutation in PDZ1 (untransfected AMPAR average = 31.9 ± 3.6 pA; star T321F + PSD-95 H130V transfected = 71.5 ± 9.7 pA; n = 21 pairs, P = 0.001) or PDZ2 (untransfected AMPAR average = 21.4 ± 2.9 pA; star T321F+ PSD-95 H225V transfected = 50.2 ± 6.1 pA; n = 14 pairs, P = 0.001), with neither causing a change in the NMDAR EPSC. These data show that direct interactions between PSD-95 and stargazin mediate the synaptic targeting of AMPARs.
Figure 6.
PSD-95 and stargazin directly interact to mediate synaptic AMPAR delivery. (A) Stargazin T321F overexpression dramatically reduces AMPAR EPSCs (n = 14 pairs, P < 0.00001) but does not change NMDAR EPSCs (n = 13 pairs, P = 0.52). (B) Coexpression of stargazin T321F with PSD-95 still results in a significant reduction of the AMPAR EPSC (n = 30 pairs, P = 0.035). (C) Coexpression of stargazin T321F with a PSD-95 construct bearing a compensatory mutation rescues and significantly enhances the AMPAR EPSC. PSD-95 mutants bearing a complementary mutation in either PDZ1 (H130V) or PDZ2 (H225V) were cotransfected with the stargazin mutant. In the bar graphs, results from both cotransfections were combined as the results were identical (see text) (AMPAR EPSCs, P = 0.00001, n = 35 pairs; NMDAR EPSCs, P = 0.43, n = 31 pairs). (Scale bars: 10 pA, 20 ms.)
Discussion
This study used hippocampal slice cultures in conjunction with biolistics to address the roles of PSD-95 and stargazin in controlling synaptic AMPAR localization. We demonstrate that overexpressing PSD-95 selectively enhances the AMPAR EPSC, with no change in the NMDAR EPSC. Results obtained in dissociated cultures during development suggested that PSD-95 expression required a week or more to cause changes in synaptic structure (20). However, the time course of this effect in mature neurons implies that increased levels of synaptic PSD-95 specifically recruit AMPARs to synapses, which is clearly not an effect on synaptic development. Overexpression of PSD-95 does not alter responses to exogenously applied AMPA, even though in these same cells synaptic AMPAR-mediated responses are greatly enhanced. Thus, PSD-95 appears to shift extra-synaptic AMPARs to the synapse, with no net change in total surface AMPARs. However, if synaptic AMPARs represent only a small fraction of the response to exogenous AMPA, it is possible that insertion of new receptors could contribute to this effect.
Remarkably, we found that expression of either stargazin or stargazinΔC greatly increased the response to exogenously applied AMPA, indicating a large increase in the number of extra-synaptic AMPARs. This result contrasts with our previous data in dissociated neuronal cell cultures (14), where stargazin did not affect whole-cell AMPA currents. A difference in the trafficking of AMPARs between dissociated and slice cultured pyramidal cells has been noted (24), as the slice culture preparation retains many more physiological properties of the intact hippocampus. The differential regulation of synaptic and extra-synaptic AMPARs is particularly striking for stargazinΔC, where in the same cell the AMPAR EPSC was severely depressed whereas the extra-synaptic response was greatly enhanced. These results indicate that dramatic changes in the expression of extra-synaptic AMPARs can go undetected at the synapse (see also ref. 9).
What might account for the privileged access to the postsynaptic membrane? Perhaps a limited number of positions or slots are available for AMPARs at the synapse (7). We propose that PSD-95 and other MAGUKs play the role of slot at the postsynaptic membrane and via their interaction with stargazin bring AMPARs to the synapse. Thus, even when the number of surface AMPARs is increased by overexpressing stargazin, the amount of synaptic MAGUK protein imposes a limit on the number of synaptic AMPARs.
To determine whether a direct interaction between PSD-95 and stargazin can control synaptic AMPAR number we mutated both PSD-95 and stargazin in a complementary manner, allowing them to interact uniquely with each other. A point mutation in the PDZ-binding region of stargazin created a dominant negative construct that reversed the enhancement normally observed with WT PSD-95. However, when this mutant was coexpressed with PSD-95 constructs bearing compensatory point mutations that reconstituted stargazin binding, the enhancement was fully rescued. This finding identifies the interaction between PSD-95 (or other synaptic MAGUKs) and stargazin as a crucial aspect in the function of both proteins.
We propose the following model: through an association with stargazin, AMPARs that are localized to intracellular compartments are brought to the cell surface. These surface AMPAR/stargazin complexes are retained at the synapse when stargazin binds a synaptic MAGUK PDZ domain and otherwise potentially serve as a reserve pool of receptors. The extra-synaptic reserve pool could serve as the source of new synaptic AMPARs to be added during long-term potentiation. Consistent with this suggestion, GluR1 knockout mice, which lack extrasynaptic AMPARs, have severe deficits in long-term potentiation (9).
Such a model relies heavily on the central role of PSD-95 or other MAGUKs in controlling the number of synaptic AMPARs. This view is supported by recent results showing that acute removal of PSD-95 from the synapse with 2-bromopalmitate depletes synaptic AMPARs (32) and suggests that PSD-95 interactions with stargazin are both necessary and sufficient for the synaptic localization of AMPARs. That synaptic AMPARs appear to be unaltered in PSD-95 mutant mice (33) suggests that other MAGUKs, such as SAP102, which we show is functionally similar to PSD-95, can also mediate synaptic AMPAR targeting. Similarly, hippocampal AMPARs appear normally localized in the stargazer mutant mouse (34), presumably because other stargazin isoforms (such as γ-3 or γ-4) are present. It will be important to elucidate the mechanisms that might regulate the amount of synaptic MAGUK protein, as well as those that potentially modulate the binding of stargazin to both AMPARs and PDZ domains, as they could have profound effects on surface and synaptic AMPAR trafficking.
An alternative model is that the PSD-95/stargazin complex delivers AMPARs to the synapse, at which point AMPARs are transferred to other clustering proteins, such as GRIP/ABP or PICK1. This model would be consistent with data suggesting that GluR1 and GluR2 constructs lacking C-terminal PDZ-binding motifs are delivered to synapses, but not retained (35, 36). However, as the AMPAR EPSC correlates with the amount of synaptic MAGUK protein, another mechanism would have to regulate the binding or number of these secondary AMPAR binding proteins. Also, the localizations of the various stargazin GFP constructs at synapses, and their correlation with AMPAR-mediated transmission, suggests stargazin remains associated with AMPARs at the synapse. Regardless of the exact model, the present results demonstrate the critical role played by the stargazin/PSD-95 interaction in controlling the number of synaptic AMPARs.
Acknowledgments
This research was supported by grants from the National Institutes of Health (to D.S.B. and R.A.N.), the Christopher Reeves Paralysis Foundation (to D.S.B.), the Human Frontier Research Program (to D.S.B.), and the Bristol-Myers Squibb Company (to R.A.N.). R.A.N. is a member of the Keck Center for Integrative Neuroscience and the Silvo Conte Center for Neuroscience Research. D.S.B. is an Established Investigator for the American Heart Association. E.S. is supported by the Medical Scientist Training Program.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
AMPA receptor
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptor
- PSD-95
postsynaptic density protein of 95 kDa
- EPSC
excitatory postsynaptic current
- MAGUK
membrane-associated guanylate kinase
References
- 1.Scannevin R H, Huganir R L. Nat Rev Neurosci. 2000;1:133–141. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- 2.Lüscher C, Xia H, Beattie E C, Carroll R C, von Zastrow M, Malenka R C, Nicoll R A. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 3.Sheng M. Proc Natl Acad Sci USA. 2001;98:7058–7061. doi: 10.1073/pnas.111146298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziff E B. Ann NY Acad Sci. 1999;868:465–473. doi: 10.1111/j.1749-6632.1999.tb11315.x. [DOI] [PubMed] [Google Scholar]
- 5.Malenka R C, Nicoll R A. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 6.Carroll R C, Beattie E C, von Zastrow M, Malenka R C. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 7.Malinow R, Mainen Z F, Hayashi Y. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Man H, Ju W, Trimble W S, MacDonald J F, Wang Y T. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 9.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser K M, Koster H J, Borchardt T, Worley P, et al. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 10.Dong H, O'Brien R J, Fung E T, Lanahan A A, Worley P F, Huganir R L. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava S, Osten P, Vilim F S, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff J G, et al. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- 12.Xia J, Zhang X, Staudinger J, Huganir R L. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 13.Daw M I, Chittajallu R, Bortolotto Z A, Dev K K, Duprat F, Henley J M, Collingridge G L, Isaac J T. Neuron. 2000;28:873–886. doi: 10.1016/s0896-6273(00)00160-4. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Chetkovich D M, Petralia R S, Sweeney N T, Kawasaki Y, Wenthold R J, Bredt D S, Nicoll R A. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 15.Cho K O, Hunt C A, Kennedy M B. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 16.Kistner U, Wenzel B M, Veh R W, Cases-Langhoff C, Garner A M, Appeltauer U, Voss B, Gundelfinger E D, Garner C C. J Biol Chem. 1993;268:4580–4583. [PubMed] [Google Scholar]
- 17.Kornau H-C, Seeburg P H, Kennedy M B. Curr Opin Neurobiol. 1997;7:368–373. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- 18.Sheng M, Sala C. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Garner C C, Nash J, Huganir R L. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- 20.El-Husseini A E, Schnell E, Chetkovich D M, Nicoll R A, Bredt D S. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 21.Craven S E, El-Husseini A E, Bredt D S. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 22.McGee A W, Bredt D S. J Biol Chem. 1999;274:17431–17436. doi: 10.1074/jbc.274.25.17431. [DOI] [PubMed] [Google Scholar]
- 23.Stoppini L, Buchs P A, Muller D. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 24.Shi S H, Hayashi Y, Petralia R S, Zaman S H, Wenthold R J, Svoboda K, Malinow R. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 25.Leonard A S, Davare M A, Horne M C, Garner C C, Hell J W. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- 26.Kaech S M, Whitfield C W, Kim S K. Cell. 1998;94:761–771. doi: 10.1016/s0092-8674(00)81735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle D A, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- 28.Tochio H, Hung F, Li M, Bredt D S, Zhang M. J Mol Biol. 2000;295:225–237. doi: 10.1006/jmbi.1999.3350. [DOI] [PubMed] [Google Scholar]
- 29.Piserchio A, Pellegrini M, Mehta S, Blackman S M, Garcia E P, Marshall J, Mierke D F. J Biol Chem. 2002;277:6967–6973. doi: 10.1074/jbc.M109453200. [DOI] [PubMed] [Google Scholar]
- 30.Songyang Z, Fanning A S, Fu C, Xu J, Marfatia S M, Chishti A H, Crompton A, Chan A C, Anderson J M, Cantley L C. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 31.Daniels D L, Cohen A R, Anderson J M, Brunger A T. Nat Struct Biol. 1998;5:317–325. doi: 10.1038/nsb0498-317. [DOI] [PubMed] [Google Scholar]
- 32.El-Husseini A E, Schnell E, Dakoji S, Sweeney N T, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll R A, Bredt D S. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 33.Migaud M, Charlesworth P, Dempster M, Webster L C, Watabe A M, Makhinson M, He Y, Ramsay M F, Morris R G, Morrison J H, et al. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. J Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi Y, Shi S H, Esteban J A, Piccini A, Poncer J C, Malinow R. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 36.Osten P, Khatri L, Perez J L, Kohr G, Giese G, Daly C, Schulz T W, Wensky A, Lee L M, Ziff E B. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]