Abstract
When images are stabilized on the retina, visual perception fades. During voluntary visual fixation, however, constantly occurring small eye movements, including microsaccades, prevent this fading. We previously showed that microsaccades generated bursty firing in the primary visual cortex (area V-1) in the presence of stationary stimuli. Here we examine the neural activity generated by microsaccades in the lateral geniculate nucleus (LGN), and in the area V-1 of the awake monkey, for various functionally relevant stimulus parameters. During visual fixation, microsaccades drove LGN neurons by moving their receptive fields across a stationary stimulus, offering a likely explanation of how microsaccades block fading during normal fixation. Bursts of spikes in the LGN and area V-1 were associated more closely than lone spikes with preceding microsaccades, suggesting that bursts are more reliable than are lone spikes as neural signals for visibility. In area V-1, microsaccade-generated activity, and the number of spikes per burst, was maximal when the bar stimulus centered over a receptive field matched the cell's optimal orientation. This suggested burst size as a neural code for stimuli optimality (and not solely stimuli visibility). As expected, burst size did not vary with stimulus orientation in the LGN. To address the effectiveness of microsaccades in generating neural activity, we compared activity correlated with microsaccades to activity correlated with flashing bars. Onset responses to flashes were about 7 times larger than the responses to the same stimulus moved across the cells' receptive fields by microsaccades, perhaps because of the relative abruptness of flashes.
When the visual world is stabilized on the retina, visual perception fades as a consequence of neural adaptation (1–4). But during normal vision we move our eyes involuntarily every few hundred milliseconds, even as we try to fixate our gaze on a small stimulus, preventing retinal stabilization and the associated fading of visibility. These fixational eye movements include “microsaccades,” small ballistic unidirectional eye movements that are generated at random intervals in all directions. Fixational eye movements, including microsaccades, have been correlated with stimulus visibility (5–8), and in a previous paper we showed that microsaccades increase the probability of firing in area V-1 cells by moving their receptive fields over stationary stimuli (9). Here we ask whether microsaccades might also induce an increase in neural activity at an earlier level, in the neurons of the lateral geniculate nucleus (LGN). We also ask how effective microsaccades are in generating neural activity by comparing them with previously characterized and well known visual stimuli, flashing bars. Finally, because transient neural firing (i.e., bursts of spikes) has been proposed as a neural code for the visibility of a stimulus (9–12), we also ask here whether bursts of spikes are used by the visual system to encode the salience of a stimulus. To answer this question, we compare the neural activity generated by microsaccades in the presence of different stimulus orientations, in both area V-1 and the LGN.
Methods
We recorded from neurons in the LGN and area V-1 of awake rhesus macaques. Before the experiments began, the monkeys were implanted with a head post, recording chamber, and a scleral eye coil. Standard sterile surgical techniques and animal care methods were used (9). The Harvard Medical Area Standing Committee on Animals approved all surgical and electrophysiological methods.
We trained the monkeys to fixate their gaze on a small cross within a 2° window in exchange for fruit juice (eye movements exceeding the limits of the fixation window were also recorded). Single units were recorded extracellularly with lacquer-coated electropolished tungsten electrodes (13). At the beginning of each LGN-recording session we lowered the electrode into the brain while flashing a full-field stimulus to drive the LGN responses. We identified the LGN location from stereotaxic coordinates and from the physiological properties of single units. Before the V-1 recordings we removed a small portion of the dura mater. After isolating each single LGN or V-1 cell we mapped its receptive field and determined the optimal width of the bar stimulus. In the V-1 cells, we also determined the preferred orientation by recording the responses to oriented bars. (All orientations were represented in 10-degree steps.) Light bar stimuli had a luminance of 24.3 cd/m2, and the dark monitor background was 3.8 cd/m2. (These values were opposite for dark bars over light backgrounds.) Most eye movements during fixation were about the same or larger (≈0.33°) than receptive field sizes in the LGN and area V-1, so the bars were sometimes located over the receptive fields centers, and sometimes not. Neuronal eccentricities ranged from 1° to 30°. Eye movements and spikes were sampled at 1 kHz in consecutive 2-s trials. The beginning and end of each 2-s sampling period were unknown to the animal. The bar turned on over the receptive field before data collection, and both the bar and the fixation cross persisted between trials. We identified microsaccades automatically with a computer algorithm as described (9). To distinguish microsaccades from small artifacts and larger eye movements we applied lower and upper limits of 3 arcmin and 2° to the size of microsaccades. We considered a microsaccade completed when its speed dropped below 3°/s or its direction changed by more than 15°.
We established in a previous article (9) that bursts of spikes are better correlated with microsaccades (and therefore with visibility) than either single spikes or instantaneous firing rate. To determine the spike-grouping parameters that most optimally defined bursts, we used the correlation between the various spike groupings and microsaccades as a guide; we let perception (rather than biophysics) show us what constituted a burst. That is, because microsaccades are correlated with perception, by determining optimal parameters for grouping spikes into bursts as a function of their correlation with previous microsaccades, we let perception itself determine what a meaningful burst is. (See ref. 9 for a complete description of the burst analysis.) The factor most important to grouping spikes into bursts is the interspike interval (ISI). Thus, to measure the burst parameters that best correlated with microsaccades, we measured the ISI between all spikes in the spike train and assigned each spike uniquely to bursts of different sizes as a function of the ISI. Thus, two spikes with a given ISI (or less) were considered part of the same burst, and two spikes separated by an interval greater than that given ISI were considered to be part of two separate bursts. A burst of one spike (or a lone spike) was therefore a single spike preceded and followed by time intervals greater than the given ISI. A burst that was greater than one spike in length had its first spike at least one ISI after the previous spike, its last spike at least one ISI before the subsequent spike, and all of its internal spikes within one ISI of each other. Because the tested neurons may have had different morphologies, inputs, and biophysical properties, the burst parameters (optimal ISI, latency, and burst size) were necessarily determined individually for each neuron (instead of choosing arbitrary values for the whole population). Because we could not know a priori which ISI was optimal for a given cell, we determined the optimal ISI by recalculating the burst analysis 100 times by using each ISI between 1 and 100 ms. We then measured the peak magnitude of the correlation (for each of the 100 sets of bursts) between each burst and the presence of microsaccades, for latencies between 1 and 200 ms before the first spike of the burst. The ISI that resulted in bursts with the best correlation with previous microsaccades was thus the best ISI a postsynaptic neuron could use to determine the presence of a previous microsaccade, and therefore, to encode visibility.
Results
Our results are based on recordings from 91 LGN neurons and 319 area V-1 neurons from four monkeys. Of the V-1 cells, 258 were studied (9); however, new analyses are presented here.
Comparison Between Microsaccades and Flashing Bars.
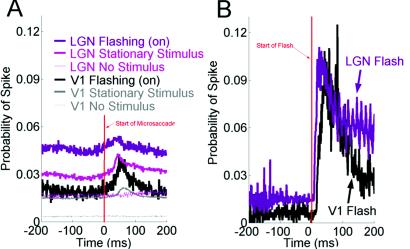
Our first experimental goal was to find out whether microsaccades modify visual activity in LGN neurons. To answer this question, we trained a rhesus macaque to fixate its gaze on a fixation cross. We then monitored the monkey's eye movements and recorded from single cells in the LGN while a bar of optimum width sat motionless over the receptive field of each cell. (Because of fixational eye movements, the bar never remained stationary over the receptive field for more than a few hundred milliseconds, see Methods.) The length of the bar always exceeded that of the receptive field. We used a white bar on a black background for on cells, and a black bar on a white background for off cells. Fig. 1A shows the correlation between microsaccades (n = 1,246,791) and spikes (n = 2,555,649) for 71 LGN and 308 V-1 neurons. The starting points of all microsaccades are aligned at zero. The thick pink (LGN) and thick gray (V-1) traces represent the average probability of a spike when the bar stimulus was stationary over the receptive field. Spike probability has a 1–1.5% increase, peaking at about 40–60 ms after the microsaccade begins. Also, spike probability dips slightly immediately before the beginning of the microsaccade (only in LGN responses; thick pink trace). We wondered if this dip was due to microsaccadic suppression [as reported by Leopold and Logothetis (14) in area V-1]. Suppression of neural activity associated with microsaccades has been proposed to explain why we are unable to perceive the visual world as shifting when microsaccades occur (even though we are perfectly able to see stimuli move when their speeds and distances are equivalent to microsaccades). To address this question, we removed the bar from the receptive field of the neuron (but left the fixation point in place, so the monkey could still fixate and make microsaccades). We then looked at the correlation between microsaccades and spikes. If microsaccadic suppression exists in the LGN, it should presumably be triggered by motor responses associated with the generation of microsaccades, regardless of whether the visual stimulus is present or not. However, we did not see either increases or decreases in spike probability correlated with microsaccades in the absence of the stimulus, either in the LGN or area V-1 (Fig. 1A, thin pink and thin gray traces). This observation indicates that neural activity induced by microsaccades in the LGN and V-1 is visual in nature; in the absence of a visual stimulus, microsaccades are not sufficient to drive visual neurons. Also, the depression in spike probability we observe in the LGN when the bar stimulus is present cannot be microsaccadic suppression, or else it would still be present in the absence of the stimulus (because the brain cannot know a priori whether the stimulus is there or not). Why then does the depression occur when the stimulus is present? The answer may be quite trivial; if spikes are generated by microsaccades during fixation, then the lowest point in spike probability will be immediately before a microsaccade.
Figure 1.
Comparison between microsaccades and flashes. (A) Microsaccades increase spike probabilities in the presence of a stationary bar in the LGN (thick pink trace; n = 57 neurons) and area V-1 (thick black trace; n = 308 neurons). The correlation between microsaccades and spikes disappears in the absence of visual stimulation in the LGN (thin pink trace; n = 42 neurons) and V-1 (thin gray trace; n = 37 neurons). Microsaccades increase spike probabilities in the LGN (thick purple trace; n = 48 neurons) and area V-1 (thick gray trace; n = 6 neurons) when a flashing bar is on. Starts of all microsaccades are aligned at the vertical line. (B) The probability of a spike after a flashing bar turns on is ≈7 times higher than the probability of a spike after a microsaccade when that same flashing bar is on. The same data set from A (LGN and V-1: purple and black traces) have been replotted and realigned to the flashing bar onset (vertical line).
We saw no differences between on- and off-center cells in the LGN, so we averaged their responses. The LGN data from both the stationary-bar condition and the no-stimulus condition resemble very closely the results from area V-1 (Fig. 1A, thick and thin gray traces, respectively), and confirm our prediction that microsaccade-related visual activity originates earlier than area V-1 (most probably in the retina) (9). Having shown that microsaccades drive LGN and V-1 neurons in the presence of a stationary bar, we set out to find whether microsaccades are comparable with other types of visual stimulation. We compared the probability of spikes after microsaccades with the probability of spikes after flashing bars. The purple trace in Fig. 1A shows the probability of a spike after a microsaccade when the bar stimulus is stationary and flashing cyclically in the LGN (1 s on; 1 s off). The data collected when the bar is on have been plotted. Spike probabilities are higher in this condition (Fig. 1A, purple trace; flashing bar) as compared with the nonflashing bar (Fig. 1A, thick pink trace), which presumably is due to the presence of the transient responses that are generated when the flashing bar is turned on, adding to the spike probabilities generated by microsaccades. Because the time interval between the bar turning on and the beginning of a microsaccade is random, the transient responses evoked by the bar raise equally the spike probability average of the trace at every point (in comparison with the nonflashing bar; Fig. 1A, thick pink trace). We found similar results when we flashed a bar in area V-1 (Fig. 1A, black trace).
Fig. 1B shows the same data as those shown in the purple (LGN) and black (V-1) traces in Fig. 1A, now renormalized to show the relationship between the onset of the flashing bars and spikes (as opposed to the relationship between microsaccades and spikes). Zero represents the time that the flashing bar turned on. The average probability of a spike increased by ≈8–10% after the bar was turned on, peaking at 20–30 ms after its onset for the LGN neurons, and at 40–50 ms for area V-1 neurons. Thus, within the same data set, the probability of a spike after a flashing bar is ≈7 times higher than the probability of a spike after a microsaccade (Fig. 1A, purple and back traces). For each one of the LGN and V-1 neurons recorded in this condition, flashes generated stronger visual responses than microsaccades. This difference could be caused by the abruptness of the flashing bar (which is virtually instantaneous) as compared with microsaccades, which cause more gradual changes within the receptive field (the average speed of a microsaccade is about 20–30°/s). We previously observed that larger eye movements, such as blinks and large saccades (both of which are more abrupt than microsaccades) tended to be followed by bursts that were longer than bursts that followed microsaccades (9). We therefore expect that spiking activity after flashes should be more similar to the activity after blinks than to the activity after microsaccades.
The oscillatory peaks in Fig. 1B (purple trace) were produced by the LGN cells following the flicker associated with the refresh rate of the monitor (74 Hz).
Effects of Stimulus Orientation on Visual Activity Generated by Microsaccades.
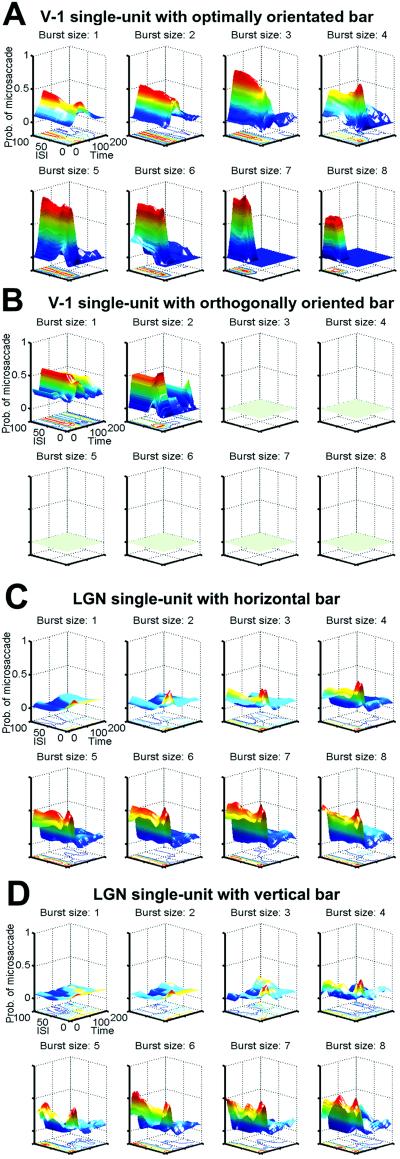
Microsaccades serve the purpose of refreshing the images of stationary stimuli on our retina, thus preventing neural adaptation and keeping the world visible. Bursts of spikes in area V-1 are better correlated than either single spikes or instantaneous firing rate, with previous microsaccades. Because bursts of spikes are better correlated with microsaccades, and microsaccades correlate with visibility, we conclude that bursts are more reliable as a neural code for visibility (9). However, bursts may not solely encode the binary property of whether a stimulus is visible or not; burst parameters such as burst size (i.e., number of spikes per burst) and rate (the internal firing rate within a burst) may moreover encode the salience and/or effectiveness of a stimulus in a graded manner. Here we address these parameters by correlating bursts of spikes to the functional properties of early visual receptive fields, such as their orientation selectivity. We compared the probability of microsaccades before bursts of different sizes for optimal and nonoptimal (orthogonal to the optimal) stimulus orientations (see Methods). Fig. 2A shows, in a single V-1 neuron, the probabilities of microsaccades preceding bursts of 1–8 spikes while a bar of optimum orientation was placed over the cell's receptive field. Fig. 2B shows, for the same V-1 neuron, the peak probability of a previous microsaccade in the presence of a bar with an orientation orthogonal to the optimal. The longer bursts were absent in this condition; only bursts of one and two spikes remained. The difference in burst distributions and reliability between the optimal and orthogonal orientation conditions suggests that bursts of spikes can be used by the brain to encode functional properties (i.e., orientation) of visual receptive fields. Because LGN neurons are not selective to specific stimulus orientations, we expected to observe similar burst distributions for vertical vs. horizontal bar orientations. Fig. 2 C and D shows the probabilities of microsaccades in the presence of a horizontal (Fig. 2C) or vertical bar (Fig. 2D) for various burst sizes in the same LGN neuron. In the LGN, as in V-1, longer bursts were again better indicators of previous microsaccades than short bursts and lone spikes. As expected, burst distribution and reliability were very similar for both orientations of the bar.
Figure 2.
Probability of microsaccades before bursts of different sizes (1–8 spikes per burst). Surface plots and the corresponding contour plots are presented for each burst size. (A) Probability of microsaccades before bursts, in the presence of an optimally oriented bar, for a single V-1 cell. (B) Same V-1 cell as in A, with orthogonal bar. Greenish flat planes for burst sizes 3–8 indicate absence of those burst sizes. (C) Microsaccade probabilities for a single LGN cell in the presence of a vertical bar. (D) Same LGN cell as C, with a horizontal bar. ISI, 1–100 ms; latency, time between the first spike in the burst and the previous microsaccade (1–200 ms).
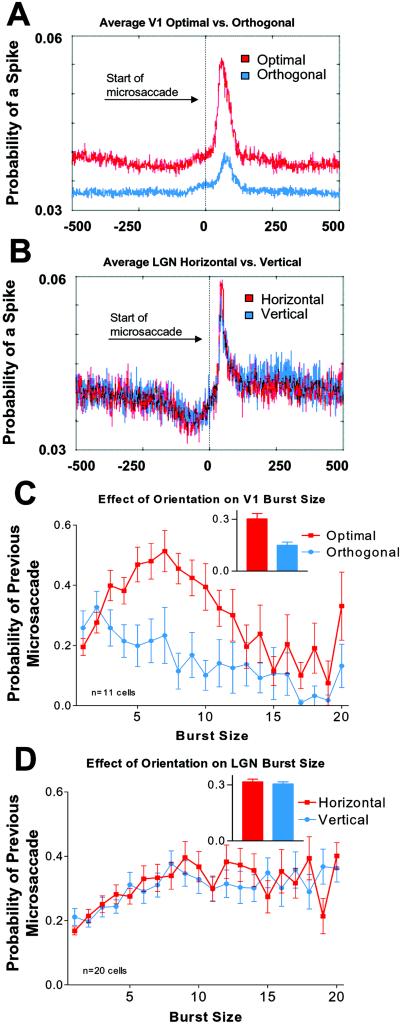
Fig. 3A shows the average probability of spikes after microsaccades in V-1, for optimal (red) and nonoptimal (blue) orientations. Microsaccade-related correlations (the difference between peak correlations and baseline for each condition) were about three times greater with optimal bars than with orthogonal bars. This result agrees with a study by Leopold and Logothetis (14), who found that microsaccade-correlated activity was greater in the presence of preferred rather than null oriented gratings, although in their case the correlation was based on the degree of suppression of activity after microsaccades in area V-1. As expected, the orientation of the bar stimulus did not affect microsaccade-generated activity in the LGN (Fig. 3B).
Figure 3.
(A) Average probability of spikes after microsaccades in V-1, for optimal (red) and orthogonal (blue) orientations (n = 11; each cell was tested in both the optimal and orthogonal conditions). (B) Average probability of spikes after microsaccades in the LGN, for horizontal (red) and vertical (blue) orientations (n = 20; each neuron was tested for both orientations). (C and D) Distribution of peak probabilities of previous microsaccades before bursts of all sizes tested. Optimal latencies and ISIs were selected for each individual neuron. (C) V-1 population with optimally (red) and orthogonally (blue) oriented bars (same neurons as in A). (D) LGN population with horizontal (red) and vertical (blue) bars (same neurons as in B). (Insets in C and D) Average probability of a microsaccade before all burst sizes, for the two different orientations tested. Error bars in C and D indicate the SEM.
Fig. 3 C and D shows the distribution of burst sizes yielding peak microsaccade probabilities across the populations of V-1 and LGN neurons. For optimally oriented bars in area V-1, the peak burst size was seven spikes (Fig. 3C). Burst sizes longer than seven spikes were not as well correlated with previous microsaccades, perhaps because in area V-1 very long bursts are correlated better with large eye movements and blinks, rather than with microsaccades (9). In the LGN, burst sizes peaked at ≈9 spikes and the correlation with previous microsaccades remained similar for longer burst sizes. (The maximum burst size tested was 20 spikes.) This similarity suggests that microsaccades may be as excitatory as larger eye movements in the LGN, at least for burst sizes of up to 20 spikes. The burst distributions for vertical and horizontal bars in the LGN were equivalent (Fig. 3D). When all burst sizes were averaged together (Insets, Fig. 3 C and D), the probability of a previous microsaccade in V-1 was ≈2 times higher for optimal bars than for orthogonal bars (Fig. 3C). In the LGN, the probability of a previous microsaccade was similar for horizontal and vertical bars (Fig. 3D).
The data for different stimuli orientations in V-1 and the LGN suggest that bursts of spikes may encode the salience (or possibly optimality) of a stimulus in both visual areas. When stimuli were similarly effective (such as with two different orientations in the LGN), the burst distributions obtained were also very similar. When both optimal and nonoptimal stimuli were presented (such as with different orientations in area V-1), then the burst distributions differed: longer burst sizes were present for optimal stimuli orientations, but not for orthogonal ones. If in area V-1 we were to present all of the intermediate orientations, we would expect to find a gradual reduction in the burst sizes as the orientation of the stimulus departed from the neuron's preferred orientation.
Discussion
The Nature of Microsaccadic Modulation in the Visual Pathway: Excitation vs. Inhibition.
The nature of the neural activity correlated with microsaccades at different levels in the visual system has been a recent subject of controversy (15). Here we show the effects of microsaccades on the activity of LGN neurons. We found a consistent excitatory effect of microsaccades on LGN neurons (both for the population data as well as for single units) in the presence of stationary or flashing bars of optimal widths. These results are consistent with our previous V-1 data (9), but not with the V-1 data reported by other groups (14, 16).
In a previous experiment (9) we found that microsaccades consistently increased neural activity in V-1 cells. We therefore speculated that microsaccades should enhance both temporal and spatial summation by synchronizing the bursting activity of neurons with neighboring receptive fields, as was also suggested by Leopold and Logothetis (14). Greschner et al. (17) have recently shown that, in fact, fixational eye movements not only increase the activity of retinal ganglion cells, but also their synchronicity, and suggested that the brain may use this synchronization of retinal firing to improve the estimation of stimulus features. Bair and O'Keefe (18) also found that microsaccades had a strong and transient effect on the activity of MT neurons. The sign of the effect (activation vs. suppression) depended on the direction of the microsaccade in relation to the preferred direction of the neuron, and also on the ongoing activity of the neuron. They suggested microsaccades activate directional neurons in area MT simply by providing visual motion signals. Other groups have found much less uniform effects of microsaccades on the spiking activity of the early visual system. Snodderly et al. (16) reported three different groups of neurons in area V-1, according to their responses to eyes movements during fixation: microsaccade-activated cells, drift-activated cells, and mixed cells (which were activated by both drift and microsaccades). However, Greschner et al. (17) have more recently tested the role of slow drift-like eye movements vs. faster (but smaller magnitude), periodic tremor-like fixational movements in the isolated turtle retina, and found that only fast fixational movements reliably increased the activity of single and multiple units. It is possible, therefore, that the drift-activated neurons in the study of Snodderly et al. are in fact activated by visual tremor (and not by drifts) during fixation. It is conceivable that, because Snodderly et al. sampled eye positions at just 100 Hz, the smaller microsaccades were not detected, and the duration of the detected microsaccades was consistently underestimated. (Microsaccades occur 3–5 times per s, and the average duration of a microsaccade is only 20–30 ms.) We therefore sampled our eye positions at 1 kHz to record all microsaccades. The neural activity that Snodderly et al. attributed to drifts (which occur in between microsaccades) could possibly have been caused by undiscovered microsaccades.
Microsaccadic Suppression.
Because we can see small shifts in position that are the size of microsaccades, why does the visual world remain stable during microsaccades? One possible mechanism is “microsaccadic suppression,” that is, the suppression of neural firing associated with the occurrence of a microsaccade. It is clear that in long-range saccades, psychophysical and physiological correlates of saccadic suppression have been shown (19–23). In microsaccades, the brain location of the neural correlates of microsaccadic suppression is controversial. Zuber et al. (24) and Zuber and Stark (25) suggested a common motor-to-sensory feedback mechanism for both saccadic and microsaccadic suppression, probably located at the level of the brainstem ocular motor nuclei. Murakami and Cavanagh (26) suggested that the compensation mechanism for fixational eye movements is based solely on visual motion signals. They proposed an extrastriate contribution for the retinal-slip compensation during fixation, possibly at the level of area MT (27).
Leopold and Logothetis (14) found that the overall effect of microsaccades in area V-1 was suppression of activity. We were surprised by this result, because in our experimental conditions the correlation between microsaccades and bursty neuronal firing seemed evident by listening to the activity of neurons in the audio monitor, even before any data analysis. Leopold and Logothetis observed enhancement of firing more often downstream, however, in areas V-2 and V-4 (IT activity did not reflect any changes). It is possible that the discrepancy between Leopold and Logothetis' results and ours is due to task-specific differences between the two studies. In our experiments, we tried to replicate normal visual fixation behavior; the monkeys were rewarded for fixating loosely (within a 2° window) for long periods of time, and the beginning and end of each trial were unknown to the animal. We recorded and analyzed eye movements even when the animal fixated outside the window. In Leopold and Logothetis' experiments, the monkeys were required to fixate much more precisely (within a 0.8° window) while performing a demanding discrimination task, and the trial ended immediately when the animal varied from this window. Thus, their animals received feedback (in the form of less reward) for poor fixation and it is possible that the suppression they found in most V-1 cells after microsaccades was due to top-down or task-dependent (i.e., attentional) influences that are not present during the more relaxed fixation conditions in our study. Task dependency of microsaccadic suppression may be further implicated, because they reported that the amount of suppression decreased as microsaccade size increased.
In our study, we cannot correlate microsaccade size to the probability of a spike because we used a single bar of light as our stimulus. Presumably, many microsaccades began when the receptive field of the cell was away from the stimulus, and the probability of the receptive field overlapping the stimulus at some point during each microsaccade goes up as a function of microsaccade size. If we found that larger microsaccades tended to correlate better with activity, it would therefore amount to a trivial result. Leopold and Logothetis instead restricted their analysis to those microsaccades in which the receptive field overlapped a grating stimulus at all times during the microsaccade. Because spatial frequency of the grating was not optimized for each cell, however, it is possible that only certain portions of the grating were excitatory to the cell, and that the differential activation that they found correlated with microsaccade size was a reflection of the probability that those critical parts of the stimulus crossed the receptive field, rather than an increased role of saccadic suppression for very small eye movements.
We observed a very small amount of suppression of firing correlated with microsaccades for the average responses in the LGN cells. However, this suppression disappeared in the absence of visual stimulation. We can therefore conclude that this suppression is not true microsaccadic suppression, but the consequence of neural adaptation after microsaccade-generated neural firing. The average responses of area V-1 neurons did not show a suppressive effect correlated with microsaccades. At the single-unit level, we saw a clear suppression of firing associated with microsaccade onset in only 10 of 308 cells tested in V-1. In seven of these cells, suppression was followed by a period of excitation. It is unlikely that this very small percentage of neurons showing suppression in V-1 can account for the stability of the visual world during microsaccades (as the vast majority of cells in V-1 show excitation but not suppression). Thus, we expect that the neural correlates of microsaccadic suppression occur at some level downstream of area V-1.
Bursts as a Possible Neural Code for Stimulus Optimality.
In 1996, Livingstone et al. (28) recorded from area V-1 neurons in the awake monkey during free-viewing and found that bursts encoded the optimal orientation of stimuli better than single spikes. Their study was dedicated to comparing the coding efficiency of bursts vs. single spikes, and therefore no systematic analysis was performed to evaluate the contribution of possibly relevant burst parameters (such as burst size or ISI). Several other studies have shown that neurons in cat area 17 respond with both greater burst frequency and number of spikes per burst when presented with stimuli having optimal orientations, rather than nonoptimal orientations (29–31). However, these studies were performed in anesthetized animals, making it unclear whether anesthesia effects played a role in the biophysics of the neurons, and only very specific arbitrary burst parameters were analyzed (i.e., two or more spikes with ISIs of ≤8 ms). Our study controls for these earlier problems in that it parametrically examines the correlation of burst distributions across all relevant burst sizes and ISIs, for both optimal and orthogonal orientations, in the awake primate. Our results show that both the number of spikes per burst and their correlation to previous microsaccades are much greater for optimal orientations than for nonoptimal orientations. By relating the different burst parameters to the probability of previous microsaccades our study has the further benefit that it correlates the function of bursts to perception. Because microsaccades are correlated with visibility, the burst (or spike) parameters that correlate most reliably with microsaccades are therefore the most reliable indicators of visibility. Our current results suggest that bursts may not only encode the visibility of a stimulus, but also the functional properties of cells at the different levels of processing along the visual pathway. Orientation selectivity is a functional property of V-1-receptive fields but not LGN-receptive fields, so it is not surprising that we found that the parameters of bursts changed with orientation in V-1, but not in the LGN. We expect that other receptive field properties should also have an effect on the number of spikes per burst. For instance, in a directionally selective V-1 cell, burst sizes should be greater for the preferred vs. the nonpreferred direction, whereas varying stimulus directions should not lead to different burst sizes in the LGN and in nondirectionally selective cells in V-1. We expect that contrast and brightness would also induce varying burst sizes both in the LGN and V-1 cells (i.e., the larger the contrast, the larger the number of spikes per burst). Thus, bursts of spikes would not be encoding specific visual characteristics (such as orientation vs. brightness), but would instead convey the salience or optimality of a given type of stimulus.
Acknowledgments
We thank Tamara Chuprina, Yanai Duran, Lyn Feeney, David Freeman, Michael and Timothy Lafratta, and Frederic Russo for their excellent technical assistance. We also thank Dr. David Leopold, Dr. Nikos Logothetis, and Xoana G. Troncoso for discussion and comments on the manuscript. This work was supported by research (to D.H.H.) and training grants (to S.L.M.) from the National Eye Institute, startup awards from Fight for Sight (to S.M.-C. and S.L.M.), and an International Appointment award (to S.L.M.) from the Medical Research Council (United Kingdom).
Abbreviations
- LGN
lateral geniculate nucleus
- ISI
interspike interval
References
- 1.Troxler D. In: Ophthalmologische Bibliothek. Himly K, Schmidt J A, editors. II, Part 2. Jena, Germany: Springer; 1804. pp. 1–53. [Google Scholar]
- 2.Riggs L A, Ratliff F. J Opt Soc Am. 1952;42:872–873. [Google Scholar]
- 3.Ditchburn R W, Ginsborg B L. Nature. 1952;170:36–37. doi: 10.1038/170036a0. [DOI] [PubMed] [Google Scholar]
- 4.Yarbus A L. Eye Movements and Vision. New York: Plenum; 1967. [Google Scholar]
- 5.Gerrits H J, Vendrik A J. Vision Res. 1974;14:175–180. doi: 10.1016/0042-6989(74)90098-4. [DOI] [PubMed] [Google Scholar]
- 6.Sansbury R V, Skavenski A A, Haddad G M, Steinman R M. J Opt Soc Am. 1973;63:612–614. doi: 10.1364/josa.63.000612. [DOI] [PubMed] [Google Scholar]
- 7.Ditchburn R W. Vision Res. 1980;20:271–272. doi: 10.1016/0042-6989(80)90112-1. [DOI] [PubMed] [Google Scholar]
- 8.Gerrits H J M, Stassen H P W, van Erning L J T O. In: Sensory Experience, Adaptation, and Perception. Spillmann L, Wooten B R, editors. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984. pp. 439–459. [Google Scholar]
- 9.Martinez-Conde S, Macknik S L, Hubel D H. Nat Neurosci. 2000;3:251–258. doi: 10.1038/72961. [DOI] [PubMed] [Google Scholar]
- 10.Macknik S L, Livingstone M S. Nat Neurosci. 1998;1:144–149. doi: 10.1038/393. [DOI] [PubMed] [Google Scholar]
- 11.Gawne T J, Martin J M. J Neurophysiol. 2000;84:2691–2694. doi: 10.1152/jn.2000.84.5.2691. [DOI] [PubMed] [Google Scholar]
- 12.Ramcharan E J, Gnadt J W, Sherman S M. Visual Neurosci. 2001;18:253–258. doi: 10.1017/s0952523801182106. [DOI] [PubMed] [Google Scholar]
- 13.Hubel D H. Science. 1956;156:549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- 14.Leopold D A, Logothetis N K. Exp Brain Res. 1998;123:341–345. doi: 10.1007/s002210050577. [DOI] [PubMed] [Google Scholar]
- 15.Ross J, Morrone M C, Goldberg M E, Burr D C. Trends Neurosci. 2001;24:113–121. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- 16.Snodderly D M, Kagan I, Gur M. Visual Neurosci. 2001;18:259–277. doi: 10.1017/s0952523801182118. [DOI] [PubMed] [Google Scholar]
- 17.Greschner M, Bongard M, Rujan P, Ammermuller J. Nature. 2002;5:341–347. doi: 10.1038/nn821. [DOI] [PubMed] [Google Scholar]
- 18.Bair W, O'Keefe L P. Visual Neurosci. 1998;15:779–786. doi: 10.1017/s0952523898154160. [DOI] [PubMed] [Google Scholar]
- 19.Dodge R. Psychol Rev. 1900;7:454–465. [Google Scholar]
- 20.Wurtz R H. Science. 1968;162:1148–1150. doi: 10.1126/science.162.3858.1148. [DOI] [PubMed] [Google Scholar]
- 21.Wurtz R H. J Neurophysiol. 1969;32:987–994. doi: 10.1152/jn.1969.32.6.987. [DOI] [PubMed] [Google Scholar]
- 22.Macknik S L, Fisher B D, Bridgeman B. Vision Res. 1991;31:2057–2064. doi: 10.1016/0042-6989(91)90163-y. [DOI] [PubMed] [Google Scholar]
- 23.Bridgeman B B, Macknik S L. Psychol Res. 1995;58:163–168. doi: 10.1007/BF00419631. [DOI] [PubMed] [Google Scholar]
- 24.Zuber B L, Crider A, Stark L. Q Prog Rep Res Lab Electr MIT. 1964;74:244–249. [Google Scholar]
- 25.Zuber B L, Stark L. Exp Neurol. 1966;16:65–79. doi: 10.1016/0014-4886(66)90087-2. [DOI] [PubMed] [Google Scholar]
- 26.Murakami I, Cavanagh P. Nature. 1998;395:798–801. doi: 10.1038/27435. [DOI] [PubMed] [Google Scholar]
- 27.Murakami I, Cavanagh P. Vision Res. 2001;41:173–186. doi: 10.1016/s0042-6989(00)00237-6. [DOI] [PubMed] [Google Scholar]
- 28.Livingstone M, S, Freeman D C, Hubel D H. Cold Spring Harbor Symp Quant Biol. 1996;61:27–37. [PubMed] [Google Scholar]
- 29.Cattaneo A, Maffei L, Morrone C. Proc R Soc London Ser B. 1981;212:279–297. doi: 10.1098/rspb.1981.0039. [DOI] [PubMed] [Google Scholar]
- 30.Cattaneo A, Maffei L, Morrone C. Exp Brain Res. 1981;43:115–118. doi: 10.1007/BF00238819. [DOI] [PubMed] [Google Scholar]
- 31.Debusk B C, Debruyn E J, Snider R K, Kabara J F, Bonds A B. J Neurophysiol. 1997;78:199–213. doi: 10.1152/jn.1997.78.1.199. [DOI] [PubMed] [Google Scholar]