Abstract
Exposure to an acute stressful event can enhance learning in male rats, whereas exposure to the same event dramatically impairs performance in females. Here we tested whether the presence of sex hormones during early development organizes these opposite effects of stress on learning in males vs. females. In the first experiment, males were castrated at birth whereas females were injected with testosterone. Rats were trained as adults on the hippocampal-dependent learning task of trace eyeblink conditioning. Performance in adult males that had been castrated at birth was still enhanced by exposure to an acute stressful experience. However, adult females injected with testosterone at birth responded in the opposite direction, i.e., exposure to the stressor that typically reduces performance instead enhanced their levels of conditioning. In the second experiment, exposure to testosterone was manipulated in utero by injecting pregnant females with a testosterone antagonist. After foster rearing, adult offspring were exposed to the stressor and trained on the hippocampal-dependent learning task of trace conditioning. Although performance in adult females was unaffected by antagonizing testosterone in utero, i.e., stress still reduced performance, the enhancement of conditioning after stress in adult males was prevented. Thus, the presence of sex hormones during gestation and development organizes whether and how acute stressful experience will affect the ability to acquire new information in adulthood. As with many sexual behaviors, these cognitive responses to stress appear to be masculinized by exposure to testosterone and feminized by its absence during very early development.
Exposure to sex hormones prenatally and early in development organizes the brain for many behavior patterns that manifest throughout adulthood (1–5). Reported changes in behavior tend to be sexual in nature; i.e., in the presence of ovarian hormones, adult males castrated at birth will exhibit a posture known as lordosis that normally occurs in sexually receptive females (6–8). In contrast, females that are briefly exposed to testosterone at birth do not ovulate or exhibit lordosis but will exhibit male sexual behaviors such as mounting in response to testosterone exposure. From these studies, it is generally considered that the background or default condition for reproductive behavior is feminine and the presence of testosterone in the female converts the behavior to a masculine one (9, 10).
We have reported that the exposure to a stressful and traumatic event greatly enhances new learning in adult male rats, whereas exposure to the same stressful experience impairs performance in adult female rats (11–13). The stressful event is acute, consisting of <30 min of inescapable swimming or exposure to brief intermittent tail stimulations. The learning paradigm is an associative task in which a conditioned stimulus (CS) of white noise predicts the occurrence of a periorbital eyelid stimulus, which elicits an eyeblink as an unconditioned stimulus (US). As the animal learns that the CS predicts the occurrence of the US, it elicits a conditioned eyeblink response (CR) in response to the CS. The opposite effects of stress on performance are evident during several paradigms, including trace conditioning, a hippocampal-dependent task in which the stimuli are discontiguous in time (14–16). Moreover, these opposite effects of stress are dependent on differing hormonal substrates. In males, the enhancement of learning in response to stress is dependent on the presence of the adrenal hormone, corticosterone, whereas in females, the impaired performance is dependent on the presence of the sex hormone estrogen (11, 12, 17). Thus, exposure to a stressful traumatic event can have opposite effects on an animal's ability to learn simply by virtue of its sex and these effects are mediated by different hormonal systems, at least as manipulated in adulthood.
Because of the vast literature indicating the importance of testosterone during development on subsequent sex-specific behaviors, we considered the possibility that the opposite effects of stressful experience on learning in male and female rats may be altered or even reversed by manipulating exposure to the hormone during very early development. In the first experiment, we manipulated exposure to testosterone in both sexes on the day of their birth. Specifically, we asked whether exposure to testosterone would alter the female response to that of a male, i.e., would their levels of conditioning or learning ability be enhanced by exposure to stress in adulthood? In males, we asked whether removing testosterone from males via castration on the day of birth would prevent the enhancing effect of stressful experience on learning in adulthood.
Male rats in utero experience a substantial increase in testosterone levels on approximately day 17 of gestation, which drop to female levels on approximately day 20 (18). If exposed to a testosterone antagonist in utero, they exhibit reduced male sexual behavior and preference for females. They can also exhibit lordosis upon treatment with estrogen (19–21). In a second experiment, we considered the possibility that the increase in testosterone in utero established the stress effects on conditioning in adulthood. To test this hypothesis, pregnant female rats were exposed to a testosterone antagonist for several days when testosterone levels are elevated in male pups. The offspring of the pregnant females were raised by foster mothers and the effects of stress on associative learning were evaluated when they reached adulthood.
Methods
Manipulations of Sex Hormones on the Day of Birth.
Within 24 h of birth, Sprague–Dawley male rats were castrated via incision and removal of the testes or exposed to a sham surgery, whereas female rat pups were injected once with 0.05 ml of 2.5 mg/ml (125 μg total) of testosterone propionate (Sigma) dissolved in sesame seed oil or the vehicle alone. Males and females exposed to different procedures were housed singly and on different racks when possible and provided with food and water ad libitum. When they reached adulthood (250–300 g), rats were anesthetized with sodium pentobarbital (50 mg/kg; Henry Schein, Indianapolis) and surgically implanted with four s.c. electrodes (insulated silver wire, 0.05 in. diameter; AM Systems, Carlsburg, WA) around the eyelid (16). Two delivered the eyelid stimulus and the other two recorded electromyographic activity, as a measure of eyeblinks. Rats were provided at least 5 d of recovery before training. To monitor estrous cycle, vaginal smears were obtained from females each morning through at least two cycles. Cotton-tip applicators (Fisher Scientific) were immersed in physiological saline and gently inserted into the vaginal tract to remove loose cells. Cells were rolled onto a slide and dried. They were stained with Toluidine Blue (Sigma) and classified into stages of proestrus, estrus, diestrus 1, and diestrus 2, as described (12). Females injected with testosterone propionate at birth did not cycle as adults.
Adult rats were taken directly from their home cage to the conditioning environment, which consisted of eight conditioning boxes in sound-attenuating chambers. Rats were tested in the same chamber each day of the experiment. Between rats, the bedding tray was cleaned and the chamber and conditioning boxes were disinfected with a spray cleaner (Brillianize, Laird Plastics, Avenel, NJ). Headstages were attached to the coiled cable that allowed free movement within the cage for 1 h. One hundred samples (500 ms each) of electromyography were collected and spontaneous eyeblinks during those periods were measured. Rats that were to be stressed were transferred to another room, restrained in Plexiglas tubes (Stoelting) and exposed to 30, 1 mA, 1-s tailshocks. The shocks were delivered one per minute for 30 min. The detrimental effect of stress on trace conditioning in the female is affected by stages of the estrous cycle and readily apparent when females are trained in proestrus (12, 22). To reduce variability because of stages of estrus, stressed females were exposed to the stressor during diestrus 2 and all females were trained 24 h later during proestrus. Groups were as follows: male/sham no stress, n = 7; male/sham stress, n = 9; male/castrated no stress, n = 9; male/castrated stress, n = 9; female/vehicle (veh) no stress, n = 6; female/veh sham stress, n = 8; female/testosterone (test) stress n = 7; and female/test no stress, n = 9.
All rats were returned to their home cage and 24 h later returned to the conditioning box in which the acclimation procedure had occurred. Spontaneous blink rate was again recorded (30 samples of 500 ms each). To determine whether stressor exposure or sex differences affected nonspecific responding to a white noise stimulus, rats were exposed to 10, 83–85 dB, 250-ms white noise bursts. Eyeblinks that occurred during the first 80 ms of the white noise were considered sensitized responses. Immediately thereafter, stressed and unstressed rats were presented with 600 trials of trace conditioning, with 300 trials each day for two consecutive days. Each set of 10 trials consisted of one CS-alone trial, four paired trials, one US-alone, and four paired trials in sequence. Paired trials consisted of the 250-ms CS with a 4-ms rise/fall time, followed by a 500-ms trace interval, and then a 0.7 mA, 100-ms periorbital shock US. The intertrial interval was randomized between 20 and 30 sec. Eyelid electromyography was filtered to pass 0.3–1.0 KHz and amplified (10 K) with a differential AC amplifier, and passed through a 16-bit A/D card (Keithley). Electromyographic responses were considered eyeblinks when they exceeded the maximum value during the pre-CS baseline response plus four times the SD. Eyeblinks were considered CRs when they occurred 250 ms after CS onset but before US onset (250–750 ms after CS onset) on paired trials and between 250 and 1,000 ms after CS onset on CS-alone trials. By examining responses after the offset of the CS, we avoided counting α or sensitized responses as CRs as well as short latency CRs, which are considered nonadaptive during trace conditioning (14, 16). The number of responses is presented as a percentage of eyeblinks occurring during the trace interval on paired trials and during the trace and US interval on CS-alone trials. For the first 100 trials, the percentage of CRs was calculated in blocks of 20 followed by blocks of 100 for the remaining 500 trials. Acquisition of this CR under these stimulus conditions is dependent on an intact hippocampal formation (14, 16).
After training, rats were overdosed with pentobarbital and cardiac blood was collected for later solid-phase RIA (Coat-A-Count, Diagnostic Products, Los Angeles) of corticosterone, estradiol, and testosterone.
Manipulations of Testosterone in Utero.
Pregnant Sprague–Dawley rats were injected s.c. with cyproterone acetate (CA; Sigma), a testosterone antagonist (23). They were injected with 10 mg of CA in 0.1 ml of saline or saline alone (veh) from day 10 to day 19 of pregnancy. Litters were surgically removed on day 22 of gestation and placed with foster mothers whose newborns were removed. Offspring were weaned 28 days later and raised to adulthood (19), as in the previous experiment. As before, females were selected in diestrus 2 and exposed to the acute stressor (or not stressed) and trained 24 h later during proestrus. Groups were: male/veh no stress, n = 9; male/veh stress, n = 7; male/CA no stress, n = 12; male/CA stress, n = 12; female/veh no stress, n = 8; female/veh stress, n = 8; female/CA stress, n = 8; and female/CA no stress, n = 8. All rats were trained with 600 trials of trace conditioning across 2 d, after which blood was collected for RIA.
Results
Manipulations of Sex Hormones at Birth Reverse the Stress Effect on Learning in Females.
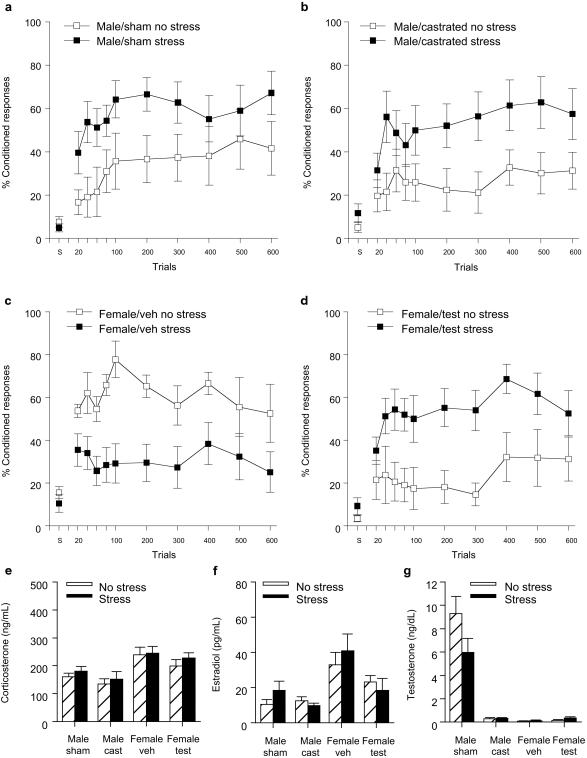
Performance (percentage of CRs) over six blocks of 100 trials of trace conditioning was analyzed with stressor exposure (stress vs. no stress) and hormonal manipulation (castration or sham surgery in males and testosterone or vehicle injection in females) as independent variables. In males, there was an overall effect of stress on the percentage of CRs emitted during exposure to the trace conditioning paradigm in the stressed vs. unstressed groups [F (1, 30) = 7.68; P < 0.01] (Fig. 1 a and b). Exposure to the acute stressor of brief inescapable tailshocks increased the percentage of CRs irrespective of whether the male was castrated on the day of birth or not. The percentage of CRs also increased across blocks of 100 trials in stressed and unstressed groups of males [F (3, 150) = 3.72; P < 0.005], suggesting that learning occurred in these groups. Neither exposure to the stressor nor castration altered spontaneous blinking (P values > 0.05) or induced sensitized responses to a white noise stimulus (P values > 0.05). These results suggest that the stressor exposure did not enhance overall arousal or nonspecific responding to environmental stimuli.
Figure 1.
Castration at birth did not alter the effect of stress on trace conditioning in adult males but exposure to testosterone on the day of birth reversed the effect of stress on trace conditioning in adult females. (a) Levels of conditioning [percentage of (%) CRs] in stressed and unstressed intact males. CRs were measured as eyeblink responses to the CS emitted during the trace interval. Data are presented as a mean percentage of CRs ± SE that occurred over 600 trials of training. Spontaneous blinks (s) are presented as a percentage of 30 samples in which a blink occurred. (b) Levels of conditioning (% CRs) in stressed and unstressed males castrated within 24 h of birth. (c) Levels of conditioning (% CRs) in stressed and unstressed females injected with a vehicle within 24 h of birth and trained during proestrus. (d) Levels of conditioning (% CRs) in stressed and unstressed female rats injected with testosterone within 24 h of birth and trained during proestrus. (e–g) Hormone levels in the above groups >48 h after stressor exposure and minutes after the end of training.
In females, exposure to testosterone on the day of birth altered and indeed reversed the stress effect on conditioning in adulthood. There was a significant interaction between exposure to the stressor or not and treatment with testosterone or the vehicle [F (1, 26) = 15.92; P < 0.0005] (Fig. 1 c and d). Post hoc analysis revealed that exposure to the stressor reduced the percentage of CRs emitted in adult females that had been injected with the vehicle (P < 0.05) but increased the percentage of CRs emitted in females that had been injected with testosterone on the day of their birth (P = 0.01). Thus, the effect of stress on conditioning in adult females was reversed by exposure to testosterone shortly after birth, and they displayed a behavioral response similar in direction to that observed in adult males. Collapsing across groups of females, the percentage of CRs increased over sessions of training (P < 0.05), suggesting that, as a whole, females were learning the association. However, those injected with the vehicle (Fig. 1c) did not exhibit an increase in percentage of responses across blocks of trials. It is noted that these females were in estrus on the second day of training (trials 301–600), a stage when performance is reportedly reduced (22). Again, neither treatment nor stress altered spontaneous blinking (P values > 0.05) or induced sensitized responses to a white noise stimulus (P values > 0.05).
In addition to sex differences in the response to the stressor, there were sex differences in overall performance, but only during the initial trials of training. Analyzing unstressed groups alone, there was an interaction between exposure to testosterone and sex on percentage of CRs emitted during the initial 100 trials (analyzed in sets of 20) [F (1, 25) = 6.98; P = 0.01]. Post hoc analysis revealed that untreated females emitted a greater percentage of CRs than both unstressed males and unstressed females treated with testosterone (P values ≤ 0.005). Because females were in proestrus on the first day of training, these results are consistent with previous observations that females in proestrus outperform males under unstressed conditions (12) and moreover indicate that exposure to testosterone shortly after birth can eliminate the enhanced responding observed in cycling females during this stage. Recall that females treated with testosterone at birth do not ovulate and therefore were not in proestrus at the time of training.
Hormone levels were measured for all groups after training. As reported previously (24, 25), corticosterone levels were elevated in females relative to males [F (1, 56) = 21.28; P < 0.0001] (Fig. 1e). They were not affected by stressor exposure, but this effect was expected because blood sampling occurred >48 h after stressor cessation (P > 0.05). Estradiol levels were higher in females than in males [F (1, 56) = 17.23; P < 0.0001] but reduced in females treated with testosterone shortly after birth (P < 0.05) (Fig. 1f). Also expected, testosterone levels were higher in males exposed to the sham surgery than in males that were castrated at birth or in any of the four groups of females (P values < 0.001) (Fig. 1g).
Manipulations of Testosterone in Utero Prevent the Stress Effect on Learning in Males.
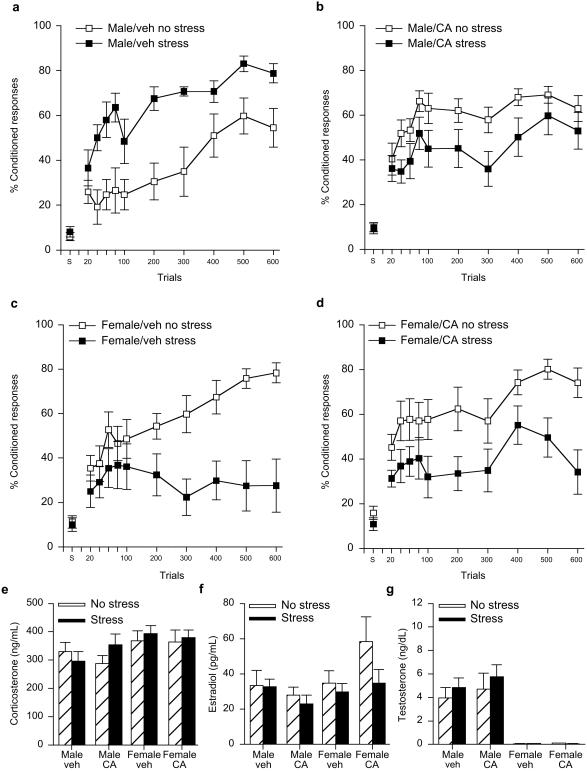
Performance (percentage of CRs) over six blocks of conditioning trials was analyzed with sex (male vs. female), stressor exposure (stress vs. no stress) and exposure to testosterone antagonist (CA or veh) as independent variables (Fig. 2 a–d). Exposure to the antagonist in utero differentially affected conditioning in males vs. females depending on whether they were stressed or not. Thus, there was a three-way interaction among the independent variables [F (1, 64) = 9.65; P < 0.005]. Post hoc analysis revealed that females exposed to either vehicle or CA in utero and stressed in adulthood emitted a reduced percentage of CRs than those that were unstressed (P = 0.001; P = 0.02) (Fig. 2 c and d). Thus, exposure to the testosterone antagonist in utero did not alter the response to stress in the adult females. On the other hand, exposure to the antagonist did alter the response to stress in the adult males. Those that were exposed to the vehicle in utero and stressed in adulthood emitted a greater percentage of CRs than those left unstressed in adulthood (P = 0.01), whereas those exposed to the antagonist CA in utero and stressed as adults performed similarly to those that were left unstressed (P = 0.07) (Fig. 2 a and b). These results suggest that preventing the actions of testosterone in utero ameliorates the stress-induced enhancement of conditioning typically observed in the adult male rat.
Figure 2.
Treatment of pregnant females with the antitestosterone CA prevented the stress effect on trace conditioning in male but not female offspring when trained as adults. (a) Levels of conditioning [percentage of (%) CRs] in stressed and unstressed adult males exposed to the vehicle in utero and trained as adults. Data are presented as a mean percentage of CRs ± SE that occurred over 600 trials of training. Spontaneous blinks (s) are presented as a percentage of 30 samples in which a blink occurred. (b) Levels of conditioning (% CRs) in stressed and unstressed males exposed to CA in utero and trained as adults. (c) Levels of conditioning (% CRs) in stressed and unstressed females exposed to the vehicle in utero and trained in proestrus as adults. (d) Levels of conditioning (% CRs) in stressed and unstressed female rats exposed to CA in utero and trained during proestrus as adults. (e–g) Hormone levels in the above groups >48 h after stress and minutes after the end of training.
There also were sex differences in conditioning itself; unstressed females that were trained in proestrus emitted a greater percentage of CRs than unstressed males during training [F (1, 33) = 6.22; P < 0.05]. The enhanced responding in females relative to males occurred regardless of whether they were exposed to the testosterone antagonist in utero (P > 0.05). With respect to learning, male groups increased the percentage of CRs across blocks of 100 trials irrespective of stressor exposure or treatment [F (5, 180) = 10.18; P < 0.000001]. In females, unstressed groups increased their responding across blocks of trials (P < 0.001), whereas those exposed to the stressor in adulthood did not (female/veh stress, P = 0.48; female/CA stress, P = 0.06). Thus, all groups exhibited learning during training except females exposed to the stressful event.
Corticosterone levels were elevated in females relative to males [F (1, 64) = 5.75; P < 0.05] but did not differ as consequence of stressor exposure, which was delivered >48 h earlier (P > 0.05) (Fig. 2e). Overall, corticosterone levels were higher in the second than the first experiment, a trend that may simply reflect differences in assay sensitivities. However, because all rats in the second experiment were reared by foster mothers, the higher levels could reflect the stress of foster rearing, maternal separation or injections to the biological mother during their gestation (26–28). This same trend was observed for the estradiol levels in the second experiment, which were relatively high. Across conditions, however, estradiol levels were higher in females than in males [F (1, 64) = 3.79; P = 0.05] (Fig. 2f) and testosterone levels were higher in males than in females [F (1, 63) = 50.15; P < 0.000001]. Treatment with the antagonist CA in utero did not alter testosterone levels in the adult males (P > 0.05) (Fig. 2g).
Discussion
Stress and sex hormones have significant activational effects on learning processes in adulthood (29–32), but their contribution during early development to adult learning is less well understood. Here we present data from two studies which indicate that the presence of sex hormones in utero and at birth can determine how well an animal will perform a hippocampal-dependent task of trace conditioning and how it will respond to an acute stressful event in adulthood. In the first experiment, adult male rats exposed to an acute stressful event of brief intermittent tailshocks outperformed males not exposed to the stressful event, whereas adult females exposed to the stressful event were impaired compared to unstressed females. Adult males that were castrated at birth behaved similarly to those exposed to a sham surgery, i.e., their responding was enhanced after exposure to the stressful event. However, females injected with testosterone on the day of their birth behaved similarly to males as adults, i.e., their performance was enhanced after exposure to the stressful event rather than impaired. In a second experiment, we observed that males treated with a testosterone antagonist in utero were not affected by exposure to the stressful event in adulthood, i.e., stress did not increase the percentage of conditioned responses as it did in the males treated with a vehicle of saline. In both experiments there were sex differences in conditioning itself with unstressed females in proestrus emitting more responses than unstressed males. This sex difference in learning was not evident, however when comparing females that were exposed to testosterone at birth to unstressed males; levels of conditioning were similar. These effects were not an indirect effect of stress or sex differences on arousal or nonspecific responding, at least to the extent that neither condition altered the spontaneous blinking behavior or induced sensitized responses to the conditioning stimuli. In combination, these data indicate that exposure to testosterone in utero determines whether or not acute stressful experience will affect the formation of trace memories in adult males. Moreover, the absence of testosterone shortly after birth organizes the ability to acquire trace memories in adulthood and dictates its modulation by stressful experience in females. These effects are generally consistent with those of sexual behaviors, which become masculinized by exposure to testosterone in utero and feminized by its absence shortly after birth (3, 9, 33, 34). Our data suggest that sexually dimorphic effects of emotional experience on cognitive behaviors in adulthood are similarly organized by a relatively brief exposure to testosterone during development.
We previously determined that the detrimental effect of stressful experience on learning in adult females is dependent on the presence of ovarian hormones. Specifically, ovariectomy and treatment with an estrogen antagonist tamoxifen prevented the impairment after stress (11). However, neither treatment reversed the effect of stress on learning; they simply eliminated the impairment. The present findings are thus unique in demonstrating a reversal in direction of the stress effect on trace conditioning in females. One injection of testosterone at birth significantly reduced adult levels of estrogen, prevented the establishment of an estrous cycle, and altered the stress effect on performance to one similar in direction to males.
In both experiments, exposure to the stressful event prevented the acquisition of the CR in the control groups of females. Thus, one could propose that stressor exposure prevented learning in adult females. Interestingly, those injected with testosterone at birth and stressed in adulthood did emit a greater percentage of CRs across trials, suggesting that exposure to testosterone imposed learning in these females. There are several alternative explanations. We have previously observed that unstressed females in proestrus exhibit high levels of conditioning relative to females in other stages. Thus, for those females that were still learning the response, the transition into estrus on the second day of training (trials 301–600) may have affected their performance. It also is possible that the stressed females would have acquired the response if they were provided additional trials of training. It is noted that there was no evidence that sex differences, exposure to the stressful event, or the hormonal manipulations altered nonspecific responding such as by increasing spontaneous blinking or inducing sensitized responses to the CS. In previous studies, we also found no evidence that sex differences or exposure to the stressor altered pain sensitivity or general activity 24 h after the stressor and at the time of training (11). Responses to the US are being examined.
In males, castration at birth did not alter the effect of stress on learning in adulthood. Because they were virtually devoid of testosterone in adulthood, yet their performance was enhanced by stress, the presence of male levels of testosterone is not necessary for observing the stress effects on learning in males. However, blocking access to testosterone in utero prevented the male response to stress yet left testosterone levels intact during adulthood. These data are consistent with others indicating that exposure to the testosterone antagonist CA alters sex-specific responses through its interactions with testosterone, although it does have progestin activity, (19, 35–38). This type of response has been observed for sexual and affiliative behaviors although less often for overall learning ability or emotional responses (27, 39–42). In most studies, the behavior was altered by castration shortly after birth and/or in utero but not preferentially in utero. Our data suggest that the enhancement of conditioning after stressful experience is preferentially established by exposure to testosterone before birth.
The means whereby exposure to sex hormones during development alters neuronal plasticity in adulthood to achieve different levels of learning and responses to stress are unknown. As discussed, hormone levels in adulthood are only moderately informative in this regard (Figs. 1 e–g and 2 e–g). Because the effects of stress on learning persist for several days, one might assume that anatomical changes are involved, especially considering the number of organizational changes that have been reported (1, 10). We recently investigated a potential role for dendritic spines, whose density is heavily influenced by the presence of sex hormones and which are potential substrates for associative memory formation (43, 44). Exposure to the stressful experience of intermittent tailshocks that enhances trace conditioning also increases dendritic spine density in adult males, whereas the opposite effect occurs in females during proestrus: stress impairs performance and reduces spine density (45). These effects were observed in the hippocampal formation, a brain region necessary for learning the trace conditioned response used in the present studies (14–16, 46, 47). If the presence of spines is associated with memory formation, one could propose that exposure to the stressful event differentially alters the availability of dendritic spines in males vs. females and thereby alters the opportunity for new learning. If such a hypothesis were valid, then these effect of stress on spine density should be similarly dependent on organizational effects of sex hormones, that is, reversed by exposure to testosterone in females at birth and prevented by antagonism of testosterone during gestation in males. Whatever the mechanism, it is clear from the present data that male and female animals raised for the most part in social isolation can respond in opposite directions to the same environmental event, and these responses are organized by the presence of testosterone very early in development.
Acknowledgments
We thank Drs. B. Campbell, R. Gandelman, and L. Matzel for comments on a previous version of this manuscript and M. Slomovits for technical assistance. This work was supported by National Institute of Mental Health (59970) and National Alliance for Research on Schizophrenia and Depression (to T.J.S.).
Abbreviations
- CS
conditioned stimulus
- CA
cyproterone acetate
- US
unconditioned stimulus
- CR
conditioned response
- veh
vehicle
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Arnold A P, Breedlove S M. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 2.Balthazart J, Tlemcani O, Ball G F. Horm Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- 3.Arnold A. Horm Behav. 1996;30:495–505. doi: 10.1006/hbeh.1996.0053. [DOI] [PubMed] [Google Scholar]
- 4.Kendrick A M, Schlinger B A. Horm Behav. 2000;30:600–610. doi: 10.1006/hbeh.1996.0063. [DOI] [PubMed] [Google Scholar]
- 5.Williams C L, Barnett A M, Meck W H. Behav Neurosci. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- 6.Pfaff D, Frohlich J, Morgan M. Trends Neurosci. 2002;25:45–50. doi: 10.1016/s0166-2236(00)02084-1. [DOI] [PubMed] [Google Scholar]
- 7.Feder H H, Whalen R E. Science. 1965;147:306–307. [PubMed] [Google Scholar]
- 8.Phoenix C H, Goy R W, Gerall A A, Young W C. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 9.Jost A, Vigier B, Prepin J, Perchellet J P. Recent Prog Horm Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- 10.Becker J B, Breedlove S M, Crews D. Behavioral Endocrinology. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- 11.Wood G E, Shors T J. Proc Natl Acad Sci USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood G E, Beylin A V, Shors T J. Behav Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- 13.Shors T J, Weiss C, Thompson R F. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- 14.Solomon P R, van der Schaaf E R, Thompson R F, Weisz D. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- 15.Clark R E, Squire L R. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 16.Beylin A V, Talk A C, Gandhi C C, Wood G E, Matzel L D, Shors T J. Neurobiol Learn Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- 17. Beylin, A. V. & Shors, T. J. (2002) Horm. Behav., in press. [DOI] [PMC free article] [PubMed]
- 18.Weisz J, Ward I L. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 19.Vega Matuszczyk J, Larsson K. Horm Behav. 1995;29:191–206. doi: 10.1006/hbeh.1995.1014. [DOI] [PubMed] [Google Scholar]
- 20.Habert R, Picon R. J Steroid Biochem. 1984;21:193–198. doi: 10.1016/0022-4731(84)90383-2. [DOI] [PubMed] [Google Scholar]
- 21.Nadler R D. Horm Behav. 1969;1:53–63. [Google Scholar]
- 22.Shors T J, Lewczyk C, Paczynski M, Mathew P R, Pickett J. NeuroReport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- 23.Dohler K D, Coquelin A, Davis F, Hines M, Shryne J E, Sickmoller P M, Jarzab B, Gorski R A. Neuroendocrinology. 1986;42:443–448. doi: 10.1159/000124484. [DOI] [PubMed] [Google Scholar]
- 24.Shors T J, Pickett J, Wood G E, Paczynski M. Stress. 1999;3:163–171. doi: 10.3109/10253899909001120. [DOI] [PubMed] [Google Scholar]
- 25.Viau V, Meaney M J. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 26.Levine S. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- 27.McCormick C M, Mahoney E. Horm Behav. 1999;35:90–101. doi: 10.1006/hbeh.1998.1500. [DOI] [PubMed] [Google Scholar]
- 28.Vallee M, Maccari S, Dellu F, Simon H, Moal M, Mayo W. Eur J Neurosci. 1999;11:2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 29.McEwen B S. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 30.Sapolsky R M. Science. 1996;97:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 31.de Kloet E R, Oitzl M S, Joels M. Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 32.Rissman E F, Heck A L, Shupnik M A, Gustafsson J A. Proc Natl Acad Sci USA. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamond M, Binstock T, Kohl J V. Horm Behav. 1996;30:333–353. doi: 10.1006/hbeh.1996.0040. [DOI] [PubMed] [Google Scholar]
- 34.Breedlove S M, Cooke B M, Jordan C L. Brain Behav Evol. 1999;54:8–14. doi: 10.1159/000006607. [DOI] [PubMed] [Google Scholar]
- 35.McEwen B S, Lieberburg I, Chaptal C, Davis P G, Krey L C, MacLusky N J, Roy E J. Horm Behav. 1979;13:269–281. doi: 10.1016/0018-506x(79)90044-8. [DOI] [PubMed] [Google Scholar]
- 36.Whalen R E. Arch Sexual Behav. 1984;13:497–502. doi: 10.1007/BF01541432. [DOI] [PubMed] [Google Scholar]
- 37.Dukes M, Furr B J, Hughs L R, Tucker H, Woodburn J R. Steroids. 2000;65:725–731. doi: 10.1016/s0039-128x(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 38.Perakis A, Stylianopoulou F. J Endocrinol. 1986;108:281–285. doi: 10.1677/joe.0.1080281. [DOI] [PubMed] [Google Scholar]
- 39.Beatty W W. Horm Behav. 1979;12:112–163. doi: 10.1016/0018-506x(79)90017-5. [DOI] [PubMed] [Google Scholar]
- 40.Roberts R L, Zullo A, Gustafson E A, Carter C S. Horm Behav. 1996;30:576–582. doi: 10.1006/hbeh.1996.0060. [DOI] [PubMed] [Google Scholar]
- 41.Williams C L, Meck W H. Psychoneuroendocrinology. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- 42.Krzanowska E K, Ogawa S, Pfaff D, Bodnar R J. Behav Brain Res. 2002;929:1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- 43.Gould E, Woolley C S, Frankfurt M, McEwen B S. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woolley C S, Gould E, Frankfurt M, McEwen B S. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shors T J, Chua C, Falduto J. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss C, Bouwmeester H, Power J M, Disterhoft J F. Behav Brain Res. 1999;99:123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 47.McEchron M D, Bouwmeester H, Tseng W, Weiss C, Disterhoft J F. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]