Abstract
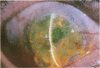
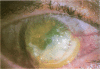
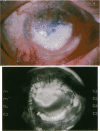
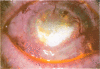
OBJECTIVE: To report the successful use of topical cyclosporin for treatment of central sterile corneal ulcers associated with rheumatoid disease. DESIGN: Retrospective, noncomparative case series. PARTICIPANTS/INTERVENTION: Five patients (7 eyes) with collagen vascular disorders presented with central, sterile corneal ulcers. An extensive medical evaluation did not reveal active underlying rheumatoid disease in any patient. Inadequate clinical response with use of topical steroids and lubricants led to corneal perforations requiring multiple tectonic procedures. Systemic immunosuppressive therapy either could not be initiated owing to a systemic contraindication or was discontinued owing to intolerance and side effects. The patients were ultimately treated with topical cyclosporin. RESULTS: Six of the 7 eyes responded favorably. An intense limbal vascularization began within 48 hours of treatment. The neovascularization progressed centrally with the simultaneous arresting of epithelial and stromal ulceration. Over a 2-week period, re-epithelization occurred with vascularization proceeding throughout the cornea. After several months, the corneal vessels attenuated, and all signs of inflammation subsided. Intrastromal bleeding with corneal blood staining occurred in 1 patient; this resolved over several months. No recurrences of corneal ulceration occurred in a mean follow-up period of 28 months (range, 7 to 60 months). None of the 5 patients have had a reactivation of their rheumatoid disease in the follow-up period. CONCLUSION: The clinical response in these patients contrasts with previous animal studies demonstrating an anti-angiogenic property of cyclosporin. We report that an immediate intense neovascularization is the first sign of a favorable clinical response. Treatment with topical cyclosporin alone may be considered in patients with sterile corneal ulcers associated with rheumatoid disease in the absence of systemic activation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens-Baumann W., Theuring S., Brewitt H. The effect of topical cyclosporin A on the rabbit cornea. A clinical and electron microscopic study. Graefes Arch Clin Exp Ophthalmol. 1986;224(6):520–524. doi: 10.1007/BF02154739. [DOI] [PubMed] [Google Scholar]
- Benelli U., Ross J. R., Nardi M., Klintworth G. K. Corneal neovascularization induced by xenografts or chemical cautery. Inhibition by cyclosporin A. Invest Ophthalmol Vis Sci. 1997 Feb;38(2):274–282. [PubMed] [Google Scholar]
- Benelli U., Ross J. R., Nardi M., Klintworth G. K. Corneal neovascularization induced by xenografts or chemical cautery. Inhibition by cyclosporin A. Invest Ophthalmol Vis Sci. 1997 Feb;38(2):274–282. [PubMed] [Google Scholar]
- Berkowitz P. J., Arentsen J. J., Felberg N. T., Laibson P. R. Presence of circulating immune complexes in patients with peripheral corneal disease. Arch Ophthalmol. 1983 Feb;101(2):242–245. doi: 10.1001/archopht.1983.01040010244012. [DOI] [PubMed] [Google Scholar]
- Bernauer W., Ficker L. A., Watson P. G., Dart J. K. The management of corneal perforations associated with rheumatoid arthritis. An analysis of 32 eyes. Ophthalmology. 1995 Sep;102(9):1325–1337. doi: 10.1016/s0161-6420(95)30867-6. [DOI] [PubMed] [Google Scholar]
- Brown S. I., Grayson M. Marginal furrows. A characteristic corneal lesion of rheumatoid arthritis. Arch Ophthalmol. 1968 May;79(5):563–567. doi: 10.1001/archopht.1968.03850040565011. [DOI] [PubMed] [Google Scholar]
- Cohen K. L. Sterile corneal perforation after cataract surgery in Sjögren's syndrome. Br J Ophthalmol. 1982 Mar;66(3):179–182. doi: 10.1136/bjo.66.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipec M., Phan T. M., Zhao T. Z., Rice B. A., Merchant A., Foster C. S. Topical cyclosporine A and corneal wound healing. Cornea. 1992 Nov;11(6):546–552. doi: 10.1097/00003226-199211000-00011. [DOI] [PubMed] [Google Scholar]
- Foster C. S., Forstot S. L., Wilson L. A. Mortality rate in rheumatoid arthritis patients developing necrotizing scleritis or peripheral ulcerative keratitis. Effects of systemic immunosuppression. Ophthalmology. 1984 Oct;91(10):1253–1263. doi: 10.1016/s0161-6420(84)34160-4. [DOI] [PubMed] [Google Scholar]
- Foster C. S. Immunosuppressive therapy for external ocular inflammatory disease. Ophthalmology. 1980 Feb;87(2):140–150. doi: 10.1016/s0161-6420(80)35272-x. [DOI] [PubMed] [Google Scholar]
- Foster C. S. Immunosuppressive therapy for external ocular inflammatory disease. Ophthalmology. 1980 Feb;87(2):140–150. doi: 10.1016/s0161-6420(80)35272-x. [DOI] [PubMed] [Google Scholar]
- Halloran P. F., Wadgymar A., Autenried P. Inhibition of MHC product induction may contribute to the immunosuppressive action of ciclosporin. Prog Allergy. 1986;38:258–268. [PubMed] [Google Scholar]
- Holland E. J., Olsen T. W., Ketcham J. M., Florine C., Krachmer J. H., Purcell J. J., Lam S., Tessler H. H., Sugar J. Topical cyclosporin A in the treatment of anterior segment inflammatory disease. Cornea. 1993 Sep;12(5):413–419. doi: 10.1097/00003226-199309000-00008. [DOI] [PubMed] [Google Scholar]
- Jayson M. I., Easty D. L. Ulceration of the cornea in rheumatoid arthritis. Ann Rheum Dis. 1977 Oct;36(5):428–432. doi: 10.1136/ard.36.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayson M. I., Jones D. E. Scleritis and rheumatoid arthritis. Ann Rheum Dis. 1971 Jul;30(4):343–347. doi: 10.1136/ard.30.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaan G., Ozden O. Therapeutic use of topical cyclosporine. Ann Ophthalmol. 1993 May;25(5):182–186. [PubMed] [Google Scholar]
- Kaswan R. L., Salisbury M. A., Ward D. A. Spontaneous canine keratoconjunctivitis sicca. A useful model for human keratoconjunctivitis sicca: treatment with cyclosporine eye drops. Arch Ophthalmol. 1989 Aug;107(8):1210–1216. doi: 10.1001/archopht.1989.01070020276038. [DOI] [PubMed] [Google Scholar]
- Kervick G. N., Pflugfelder S. C., Haimovici R., Brown H., Tozman E., Yee R. Paracentral rheumatoid corneal ulceration. Clinical features and cyclosporine therapy. Ophthalmology. 1992 Jan;99(1):80–88. doi: 10.1016/s0161-6420(92)32006-8. [DOI] [PubMed] [Google Scholar]
- Kervick G. N., Pflugfelder S. C., Haimovici R., Brown H., Tozman E., Yee R. Paracentral rheumatoid corneal ulceration. Clinical features and cyclosporine therapy. Ophthalmology. 1992 Jan;99(1):80–88. doi: 10.1016/s0161-6420(92)32006-8. [DOI] [PubMed] [Google Scholar]
- Krachmer J. H., Laibson P. R. Corneal thinning and perforation in Sjögren's syndrome. Am J Ophthalmol. 1974 Dec;78(6):917–920. doi: 10.1016/0002-9394(74)90801-0. [DOI] [PubMed] [Google Scholar]
- Lipman R. M., Epstein R. J., Hendricks R. L. Suppression of corneal neovascularization with cyclosporine. Arch Ophthalmol. 1992 Mar;110(3):405–407. doi: 10.1001/archopht.1992.01080150103037. [DOI] [PubMed] [Google Scholar]
- Lipman R. M., Epstein R. J., Hendricks R. L. Suppression of corneal neovascularization with cyclosporine. Arch Ophthalmol. 1992 Mar;110(3):405–407. doi: 10.1001/archopht.1992.01080150103037. [DOI] [PubMed] [Google Scholar]
- McGavin D. D., Williamson J., Forrester J. V., Foulds W. S., Buchanan W. W., Dick W. C., Lee P., MacSween R. N., Whaley K. Episcleritis and scleritis. A study of their clinical manifestations and association with rheumatoid arthritis. Br J Ophthalmol. 1976 Mar;60(3):192–226. doi: 10.1136/bjo.60.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels M. L., Cobo L. M., Caldwell D. S., Rice J. R., Haynes B. F. Rheumatoid arthritis and sterile corneal ulceration. Analysis of tissue immune effector cells and ocular epithelial antigens using monoclonal antibodies. Arthritis Rheum. 1984 Jun;27(6):606–614. doi: 10.1002/art.1780270602. [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Sato K., Goto M., Sasano M., Natsuyama M., Inoue K., Nishioka K. Augmented interleukin-1 production and HLA-DR expression in the synovium of rheumatoid arthritis patients. Possible involvement in joint destruction. Arthritis Rheum. 1988 Apr;31(4):480–486. doi: 10.1002/art.1780310404. [DOI] [PubMed] [Google Scholar]
- Pfister R. R., Murphy G. E. Corneal ulceration and perforation associated with Sjögren's syndrome. Arch Ophthalmol. 1980 Jan;98(1):89–94. doi: 10.1001/archopht.1980.01020030091006. [DOI] [PubMed] [Google Scholar]
- Radtke N., Meyers S., Kaufman H. E. Sterile corneal ulcers after cataract surgery in keratoconjunctivitis sicca. Arch Ophthalmol. 1978 Jan;96(1):51–52. doi: 10.1001/archopht.1978.03910050015003. [DOI] [PubMed] [Google Scholar]
- Tauber J., Sainz de la Maza M., Hoang-Xuan T., Foster C. S. An analysis of therapeutic decision making regarding immunosuppressive chemotherapy for peripheral ulcerative keratitis. Cornea. 1990 Jan;9(1):66–73. [PubMed] [Google Scholar]
- Watson P. G., Hayreh S. S. Scleritis and episcleritis. Br J Ophthalmol. 1976 Mar;60(3):163–191. doi: 10.1136/bjo.60.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierhut M., Thiel H. J., Weidle E. G., Waetjen R., Pleyer U. Topical treatment of severe corneal ulcers with cyclosporin A. Graefes Arch Clin Exp Ophthalmol. 1989;227(1):30–35. doi: 10.1007/BF02169821. [DOI] [PubMed] [Google Scholar]
- Zierhut M., Thiel H. J., Weidle E. G., Waetjen R., Pleyer U. Topical treatment of severe corneal ulcers with cyclosporin A. Graefes Arch Clin Exp Ophthalmol. 1989;227(1):30–35. doi: 10.1007/BF02169821. [DOI] [PubMed] [Google Scholar]
- von Domarus D., Böhnke M., Meissner M., Burk R. Regeneration artefizieller Hornhautwunden unter Cyclosporin-A-Augentropfen. Fortschr Ophthalmol. 1986;83(6):647–649. [PubMed] [Google Scholar]