Abstract
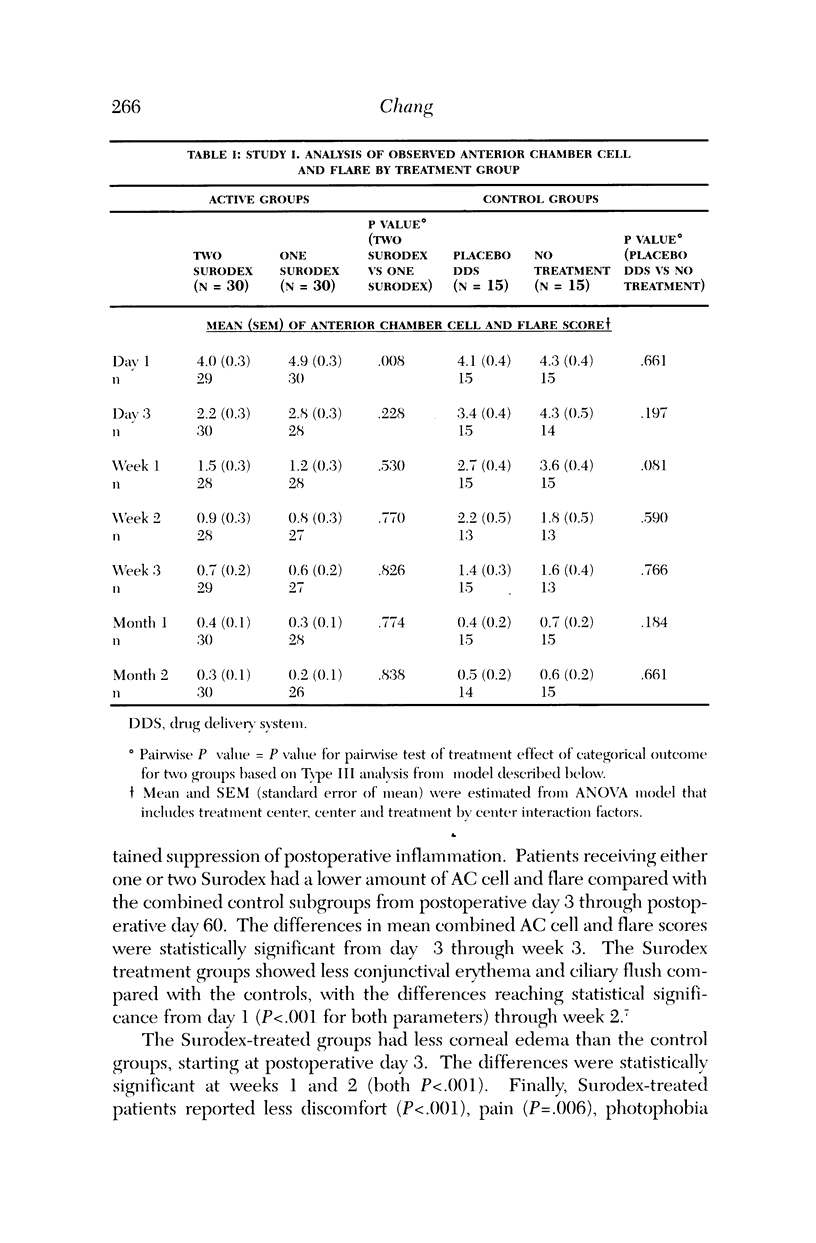
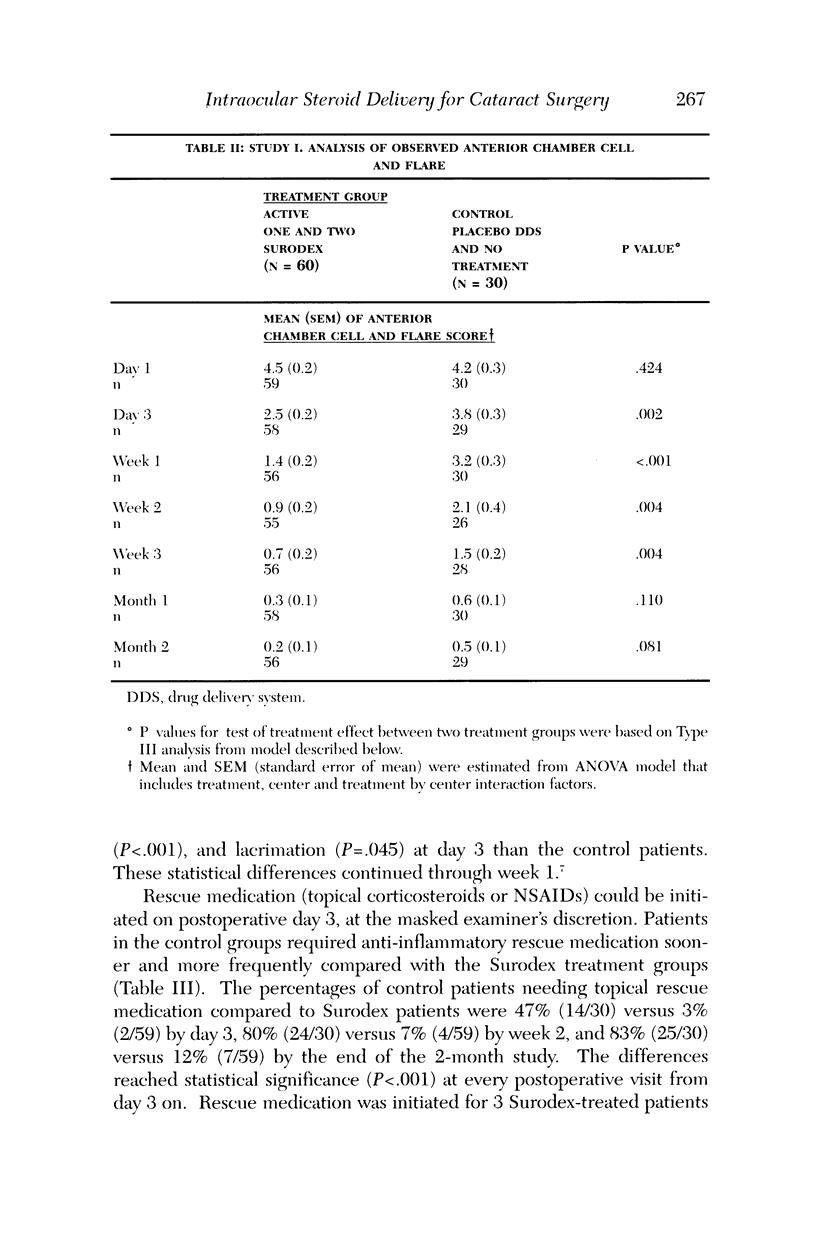
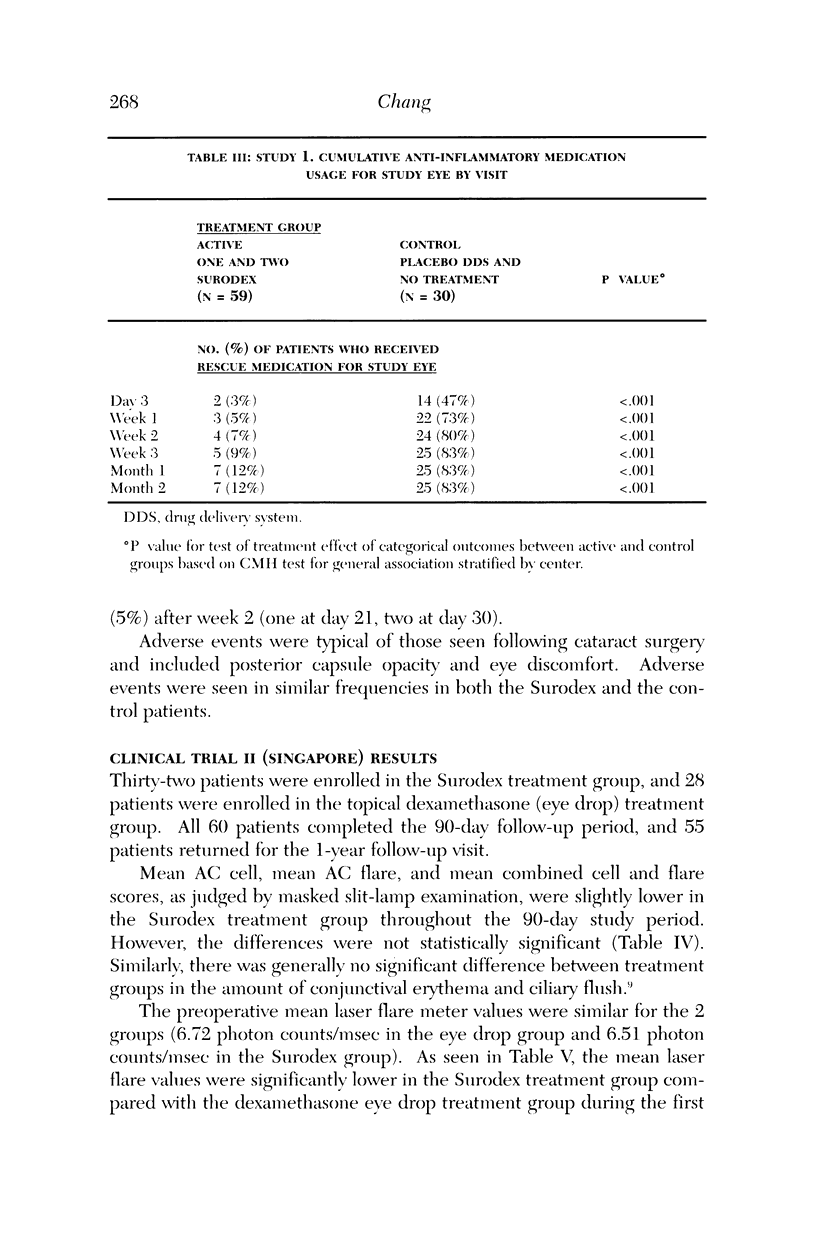
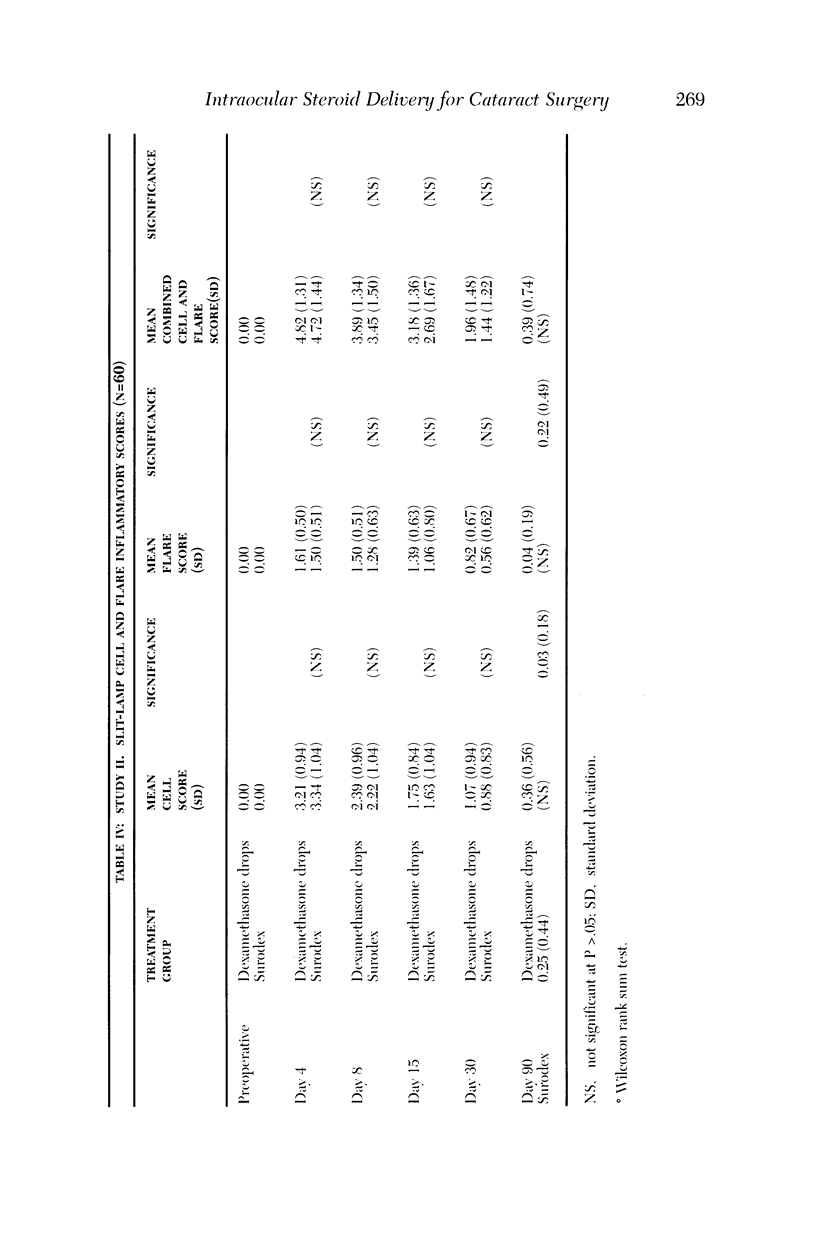
PURPOSE: To determine the safety and efficacy of an intraocular dexamethasone drug delivery system (Surodex) in the treatment of inflammation following cataract surgery. METHODS: Surodex is a biodegradable polymer that releases dexamethasone for 7 to 10 days after placement in the anterior segment. Study 1 was a prospective, randomized, double-masked Phase II clinical trial of 90 cataract surgical patients that compared treatment with Surodex to treatment with a placebo drug delivery system and to no anti-inflammatory drug treatment at all. Study 2 was a separate prospective, randomized, double-masked study of 60 cataract surgical patients that compared treatment with Surodex to topical dexamethasone (eye drop) therapy. RESULTS: In the first study, Surodex was superior to placebo in suppressing postsurgical inflammation throughout the 60-day postoperative period, as judged by masked-evaluator, slit-lamp grading of cell and flare. The differences were statistically significant from postoperative day 3 through postoperative week 3. The majority of Surodex patients did not require topical steroid by 2 weeks after surgery (93%) or by 2 months after surgery (88%). In the second study, Kowa laser flare meter readings were lower in Surodex patients throughout the 90-day postoperative period. The results were statistically significant at 4, 8, and 15 days following surgery. There were no significant adverse complications of Surodex in either study. CONCLUSION: Surodex was safe and effective in suppressing postcataract surgery inflammation and appears to be a promising alternative to topical steroids.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assil K. K., Massry G., Lehmann R., Fox K., Stewart R. Control of ocular inflammation after cataract extraction with rimexolone 1% ophthalmic suspension. J Cataract Refract Surg. 1997 Jun;23(5):750–757. doi: 10.1016/s0886-3350(97)80286-6. [DOI] [PubMed] [Google Scholar]
- Bron A., Denis P., Hoang-Xuan T. C., Boureau-Andrieux C., Crozafon P., Hachet E., Medhorn E., Akingbehin A. The effects of Rimexolone 1% in postoperative inflammation after cataract extraction. A double-masked placebo-controlled study. Eur J Ophthalmol. 1998 Jan-Mar;8(1):16–21. doi: 10.1177/112067219800800105. [DOI] [PubMed] [Google Scholar]
- Diestelhorst M., Aspacher F., Konen W., Krieglstein G. K., Hilgers R. D. Effect of dexamethasone 0.1% and prednisolone acetate 1.0% eye drops on the blood-aqueous barrier after cataract surgery: a controlled randomized fluorophotometric study. Graefes Arch Clin Exp Ophthalmol. 1992;230(5):451–453. doi: 10.1007/BF00175932. [DOI] [PubMed] [Google Scholar]
- Ferguson V. M., Spalton D. J. Recovery of the blood-aqueous barrier after cataract surgery. Br J Ophthalmol. 1991 Feb;75(2):106–110. doi: 10.1136/bjo.75.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach A. J., Dolan B. J., Donahue M. E., Faktorovich E. G., Gonzalez G. A. Comparative effects of ketorolac 0.5% or diclofenac 0.1% ophthalmic solutions on inflammation after cataract surgery. Ophthalmology. 1998 Sep;105(9):1775–1779. doi: 10.1016/S0161-6420(98)99053-4. [DOI] [PubMed] [Google Scholar]
- Heier J., Cheetham J. K., Degryse R., Dirks M. S., Caldwell D. R., Silverstone D. E., Rosenthal A. Ketorolac tromethamine 0.5% ophthalmic solution in the treatment of moderate to severe ocular inflammation after cataract surgery: a randomized, vehicle-controlled clinical trial. Am J Ophthalmol. 1999 Mar;127(3):253–259. doi: 10.1016/s0002-9394(98)00413-9. [DOI] [PubMed] [Google Scholar]
- Ichigashira N., Yamaga N. Intraocular fate of dexamethasone disodium phosphate topically applied to the eyes of rabbits. Steroids. 1978 Dec;32(5):615–628. doi: 10.1016/0039-128x(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Kass M. A., Meltzer D. W., Gordon M., Cooper D., Goldberg J. Compliance with topical pilocarpine treatment. Am J Ophthalmol. 1986 May 15;101(5):515–523. doi: 10.1016/0002-9394(86)90939-6. [DOI] [PubMed] [Google Scholar]
- Koçak I., Yalvaç I. S., Koçak A., Nurözler A., Unlü N., Kasim R., Duman S. Comparison of the anti-inflammatory effects of diclofenac and flurbiprofen eye drops after cataract extraction. Acta Ophthalmol Scand. 1998 Jun;76(3):343–345. doi: 10.1034/j.1600-0420.1998.760318.x. [DOI] [PubMed] [Google Scholar]
- Kraff M. C., Martin R. G., Neumann A. C., Weinstein A. J. Efficacy of diclofenac sodium ophthalmic solution versus placebo in reducing inflammation following cataract extraction and posterior chamber lens implantation. J Cataract Refract Surg. 1994 Mar;20(2):138–144. doi: 10.1016/s0886-3350(13)80153-8. [DOI] [PubMed] [Google Scholar]
- Krupin T., Waltman S. R., Becker B. Ocular penetration in rabbits of topically applied dexamethasone. Arch Ophthalmol. 1974 Oct;92(4):312–314. doi: 10.1001/archopht.1974.01010010322012. [DOI] [PubMed] [Google Scholar]
- Rao N. A. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans Am Ophthalmol Soc. 1990;88:797–850. [PMC free article] [PubMed] [Google Scholar]
- Roberts C. W., Brennan K. M. A comparison of topical diclofenac with prednisolone for postcataract inflammation. Arch Ophthalmol. 1995 Jun;113(6):725–727. doi: 10.1001/archopht.1995.01100060051031. [DOI] [PubMed] [Google Scholar]
- Sanders D. R., Kraff M. Steroidal and nonsteroidal anti-inflammatory agents. Effect on postsurgical inflammation and blood-aqueous humor barrier breakdown. Arch Ophthalmol. 1984 Oct;102(10):1453–1456. doi: 10.1001/archopht.1984.01040031173012. [DOI] [PubMed] [Google Scholar]
- Tan D. T., Chee S. P., Lim L., Lim A. S. Randomized clinical trial of a new dexamethasone delivery system (Surodex) for treatment of post-cataract surgery inflammation. Ophthalmology. 1999 Feb;106(2):223–231. doi: 10.1016/S0161-6420(99)90060-X. [DOI] [PubMed] [Google Scholar]
- Tan D. T., Chee S. P., Lim L., Lim A. S. Randomized clinical trial of a new dexamethasone delivery system (Surodex) for treatment of post-cataract surgery inflammation. Ophthalmology. 1999 Feb;106(2):223–231. doi: 10.1016/S0161-6420(99)90060-X. [DOI] [PubMed] [Google Scholar]
- Watson D., Noble M. J., Dutton G. N., Midgley J. M., Healey T. M. Penetration of topically applied dexamethasone alcohol into human aqueous humor. Arch Ophthalmol. 1988 May;106(5):686–687. doi: 10.1001/archopht.1988.01060130748037. [DOI] [PubMed] [Google Scholar]
- Winfield A. J., Jessiman D., Williams A., Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990 Aug;74(8):477–480. doi: 10.1136/bjo.74.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]


