Abstract
BACKGROUND AND PURPOSE: X-linked juvenile retinoschisis (RS) provides a starting point to define clinical paradigms and understand the limitations of diagnostic molecular testing. The RS phenotype is specific, but the broad severity range is clinically confusing. Molecular diagnostic testing obviates unnecessary examinations for boys at-risk and identifies carrier females who otherwise show no clinical signs. METHODS: The XLRS1 gene has 6 exons of 26-196 base-pair size. Each exon is amplified by a single polymerase chain reaction and then sequenced, starting with exons 4 through 6, which contain mutation "hot spots." RESULTS: The 6 XLRS1 exons are sequenced serially. If alterations are found, they are compared with mutations in our > 120 XLRS families and with the > 300 mutations reported worldwide. Point mutations, small deletions, or rearrangements are identified in nearly 90% of males with a clinical diagnosis of RS. XLRS1 has very few sequence polymorphisms. Carrier-state testing produces 1 of 3 results: (1) positive, in which the woman has the same mutation as an affected male relative or known in other RS families; (2) negative, in which she lacks the mutation of her affected male relative; and (3) uninformative, in which no known mutation is identified or no information exists about the familial mutation. CONCLUSIONS: Molecular RS screening is an effective diagnostic tool that complements the clinician's skills for early detection of at-risk males. Useful outcomes of carrier testing depend on several factors: (1) a male relative with a clear clinical diagnosis; (2) a well-defined inheritance pattern; (3) high disease penetrance; (4) size and organization of the gene; and (5) the types of disease-associated mutations. Ethical questions include molecular diagnostic testing of young at-risk females before the age of consent, the impact of this information on the emotional health of the patient and family, and issues of employability and insurance coverage.
Full text
PDF




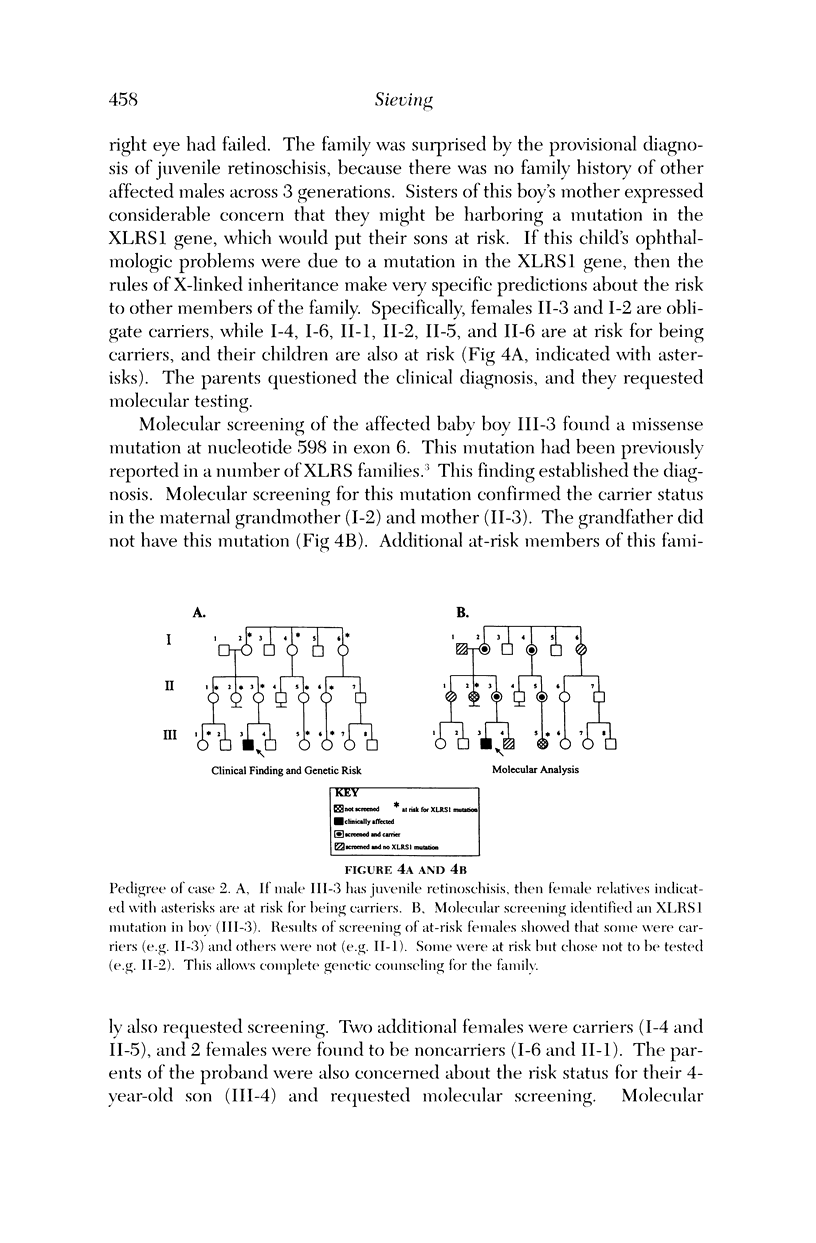
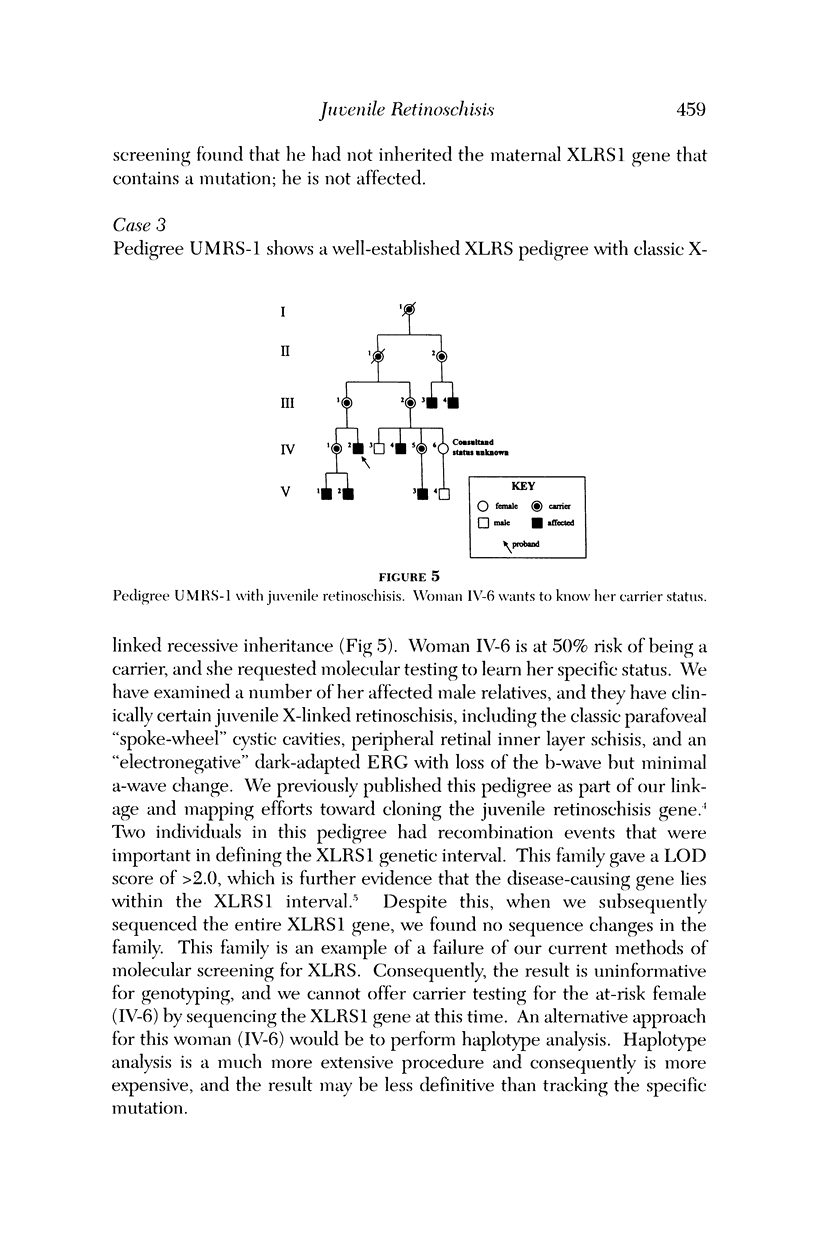










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cremers F. P., van de Pol D. J., van Driel M., den Hollander A. I., van Haren F. J., Knoers N. V., Tijmes N., Bergen A. A., Rohrschneider K., Blankenagel A. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Genet. 1998 Mar;7(3):355–362. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- Elias S., Annas G. J. Generic consent for genetic screening. N Engl J Med. 1994 Jun 2;330(22):1611–1613. doi: 10.1056/NEJM199406023302213. [DOI] [PubMed] [Google Scholar]
- George N. D., Yates J. R., Moore A. T. Clinical features in affected males with X-linked retinoschisis. Arch Ophthalmol. 1996 Mar;114(3):274–280. doi: 10.1001/archopht.1996.01100130270007. [DOI] [PubMed] [Google Scholar]
- Handyside A. H., Lesko J. G., Tarín J. J., Winston R. M., Hughes M. R. Birth of a normal girl after in vitro fertilization and preimplantation diagnostic testing for cystic fibrosis. N Engl J Med. 1992 Sep 24;327(13):905–909. doi: 10.1056/NEJM199209243271301. [DOI] [PubMed] [Google Scholar]
- Pawar H., Bingham E. L., Hiriyanna K., Segal M., Richards J. E., Sieving P. A. X-linked juvenile retinoschisis: localization between (DXS1195, DXS418) and AFM291wf5 on a single YAC. Hum Hered. 1996 Nov-Dec;46(6):329–335. doi: 10.1159/000154373. [DOI] [PubMed] [Google Scholar]
- Pawar H., Bingham E. L., Hiriyanna K., Segal M., Richards J. E., Sieving P. A. X-linked juvenile retinoschisis: localization between (DXS1195, DXS418) and AFM291wf5 on a single YAC. Hum Hered. 1996 Nov-Dec;46(6):329–335. doi: 10.1159/000154373. [DOI] [PubMed] [Google Scholar]
- Rothenberg K., Fuller B., Rothstein M., Duster T., Ellis Kahn M. J., Cunningham R., Fine B., Hudson K., King M. C., Murphy P. Genetic information and the workplace: legislative approaches and policy changes. Science. 1997 Mar 21;275(5307):1755–1757. doi: 10.1126/science.275.5307.1755. [DOI] [PubMed] [Google Scholar]
- Sauer C. G., Gehrig A., Warneke-Wittstock R., Marquardt A., Ewing C. C., Gibson A., Lorenz B., Jurklies B., Weber B. H. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet. 1997 Oct;17(2):164–170. doi: 10.1038/ng1097-164. [DOI] [PubMed] [Google Scholar]
- Shattuck-Eidens D., McClure M., Simard J., Labrie F., Narod S., Couch F., Hoskins K., Weber B., Castilla L., Erdos M. A collaborative survey of 80 mutations in the BRCA1 breast and ovarian cancer susceptibility gene. Implications for presymptomatic testing and screening. JAMA. 1995 Feb 15;273(7):535–541. [PubMed] [Google Scholar]
- Sieving P. A., Bingham E. L., Roth M. S., Young M. R., Boehnke M., Kuo C. Y., Ginsburg D. Linkage relationship of X-linked juvenile retinoschisis with Xp22.1-p22.3 probes. Am J Hum Genet. 1990 Oct;47(4):616–621. [PMC free article] [PubMed] [Google Scholar]