Abstract
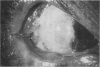
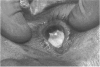
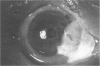
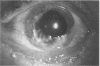
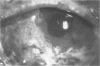
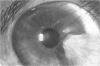
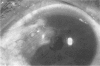
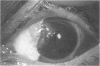
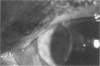
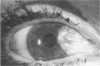
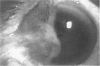
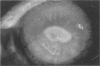
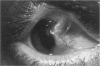
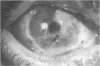
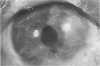
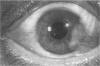
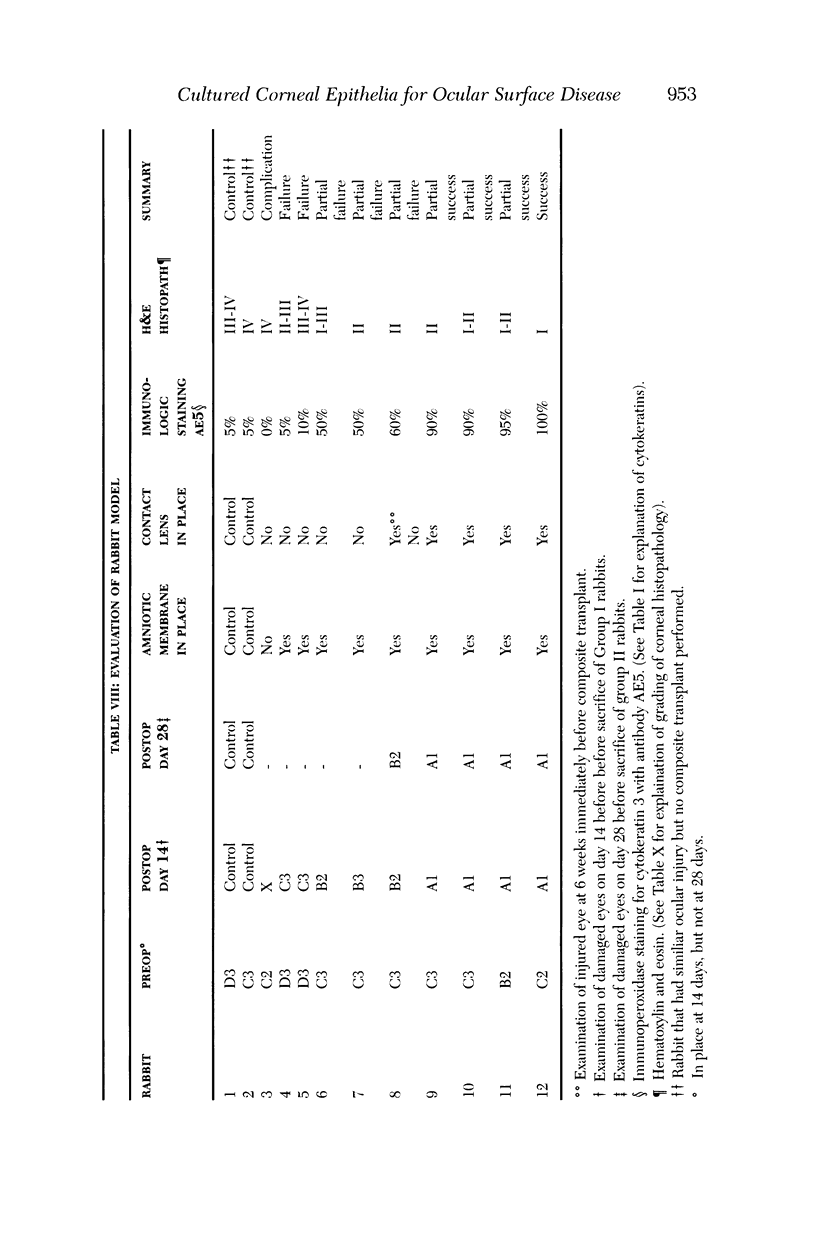
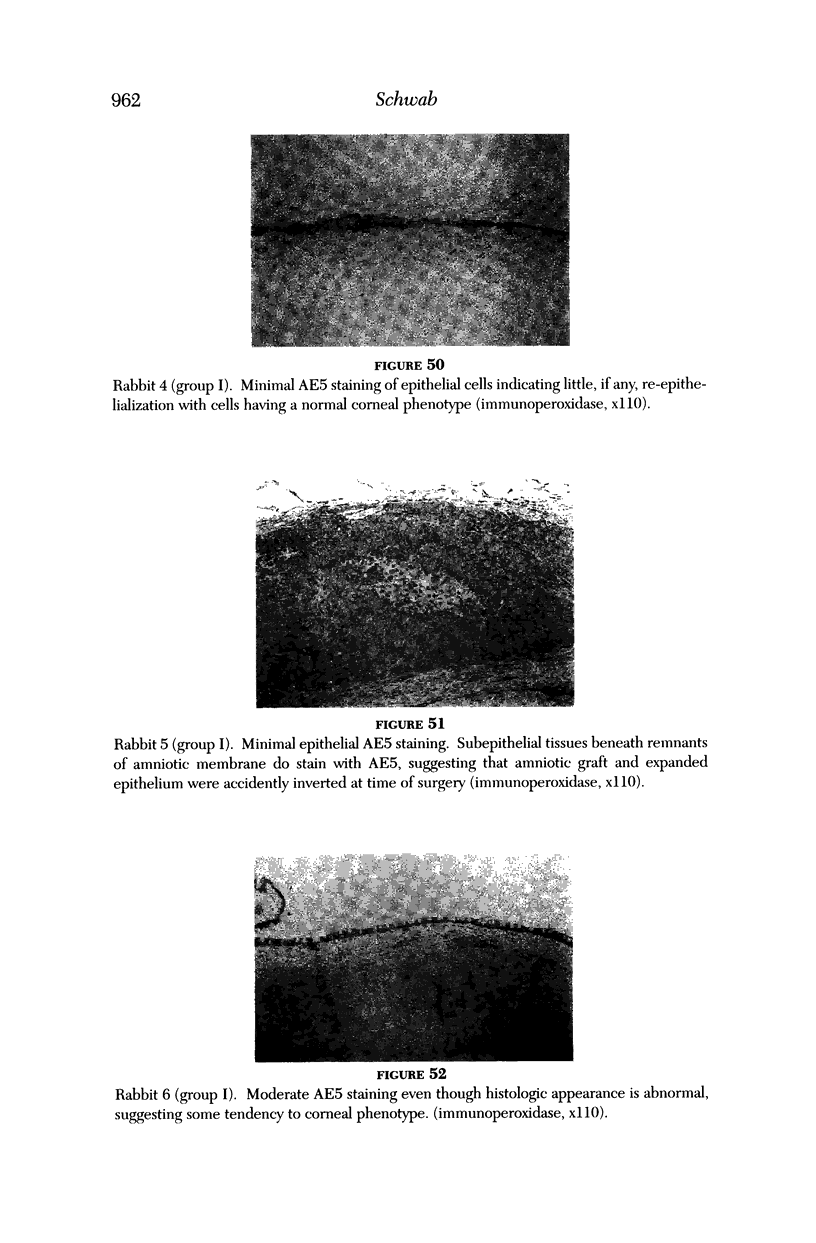
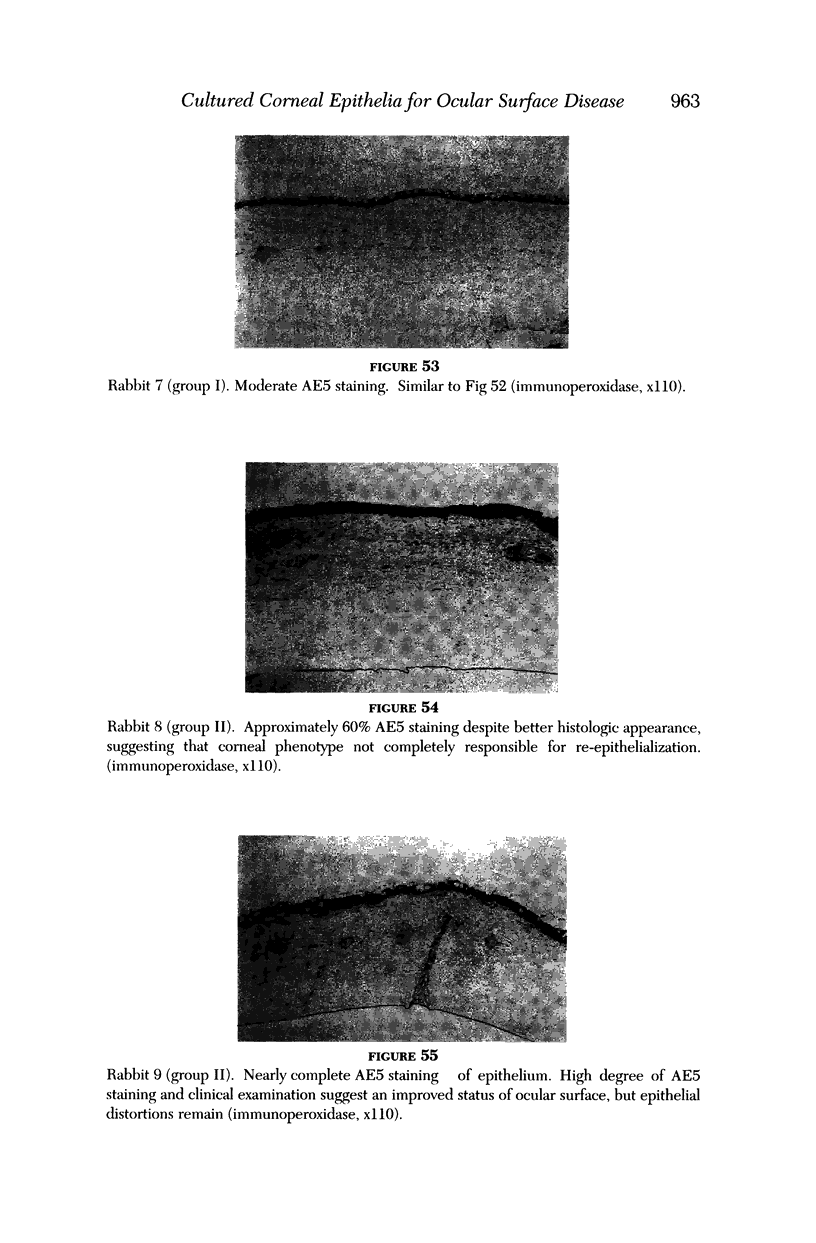
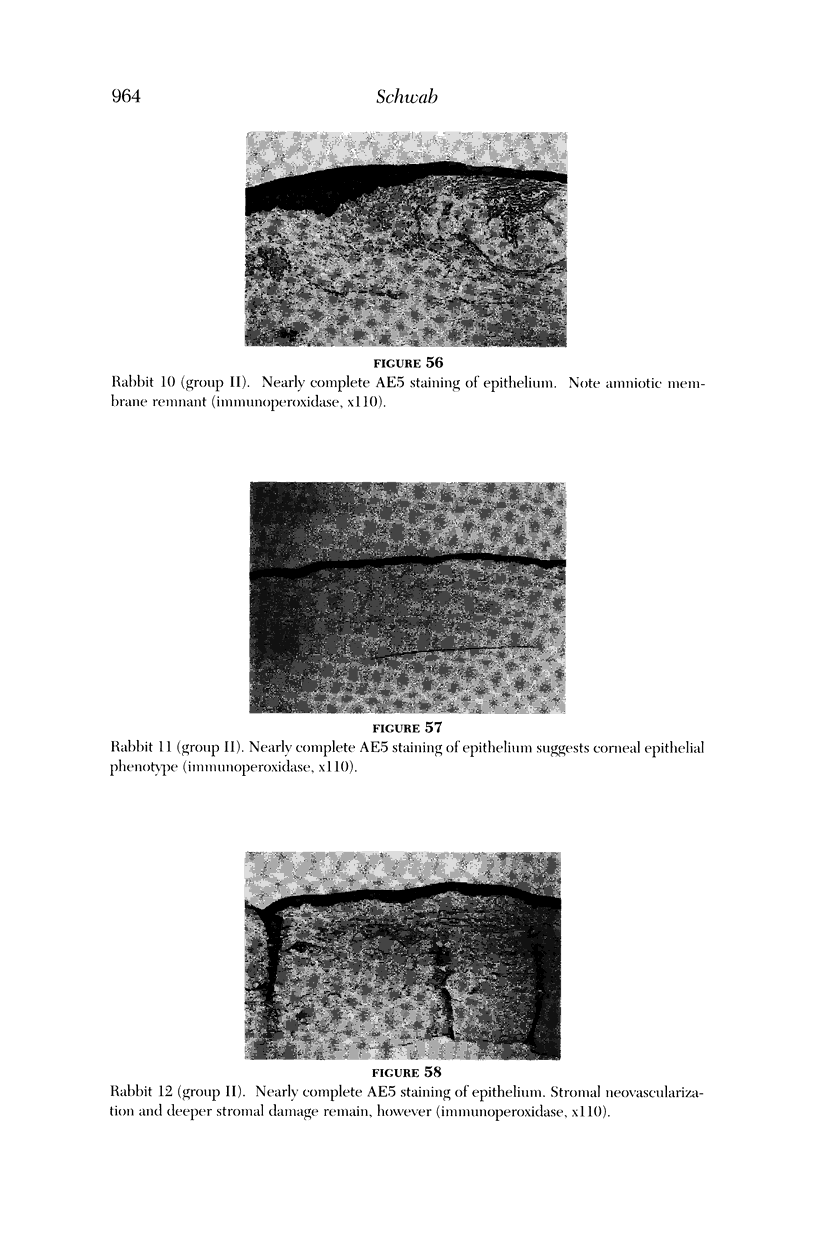
PURPOSE: To evaluate the potential efficacy for autologous and allogeneic expanded corneal epithelial cell transplants derived from harvested limbal corneal epithelial stem cells cultured in vitro for the management of ocular surface disease. METHODS: Human Subjects. Of the 19 human subjects included, 18 (20 procedures) underwent in vitro cultured corneal epithelial cell transplants using various carriers for the epithelial cells to determine the most efficacious approach. Sixteen patients (18 procedures on 17 eyes) received autologous transplants, and 2 patients (1 procedure each) received allogeneic sibling grafts. The presumed corneal epithelial stem cells from 1 patient did not grow in vitro. The carriers for the expanded corneal epithelial cells included corneal stroma, type 1 collagen (Vitrogen), soft contact lenses, collagen shields, and amniotic membrane for the autologous grafts and only amniotic membrane for the allogeneic sibling grafts. Histologic confirmation was reviewed on selected donor grafts. Amniotic membrane as carrier. Further studies were made to determine whether amniotic membrane might be the best carrier for the expanding corneal epithelial cells. Seventeen different combinations of tryspinization, sonication, scraping, and washing were studied to find the simplest, most effective method for removing the amniotic epithelium while still preserving the histologic appearance of the basement membrane of the amnion. Presumed corneal epithelial stem cells were harvested and expanded in vitro and applied to the amniotic membrane to create a composite graft. Thus, the composite graft consisted of the amniotic membrane from which the original epithelium had been removed without significant histologic damage to the basement membrane, and the expanded corneal epithelial stem cells, which had been applied to and had successfully adhered to the denuded amniotic membrane. Animal model. Twelve rabbits had the ocular surface of 1 eye damaged in a standard manner with direct removal of the presumed limbal stem cells, corneal epithelium, and related epithelium, followed by the application of n-heptanol for 60 seconds. After 6 weeks, all damaged eyes were epithelialized and vascularized. Two such treated eyes were harvested without further treatment, to be used for histologic study as damaged controls. The remaining 10 rabbits received composite grafts (consisting of amniotic membrane with expanded allogeneic rabbit corneal epithelial cell transplants) applied to the ocular surface in a standard manner followed by the application of a contact lens. At 16 days following transplantation, 5 of the rabbits were sacrificed and the corneal rims were removed for histologic study. At 28 days, the remaining rabbits were sacrificed and the previously damaged eyes were harvested for histologic and immunohistochemical study. RESULTS: Human subjects. Of the 19 total patients admitted to the study, the presumed corneal epithelial stem cells of 1 patient did not grow in vitro. Of the remaining 18 patients (20 procedures, 19 eyes), 3 patients had unsuccessful results (3 autologous procedures), 1 patient had a partially successful procedure (allogeneic procedure), and 1 patient had a procedure with an undetermined result at present (allogeneic procedure). One unsuccessful patient had entropion/trichiasis and mechanically removed the graft and eventually went into phthisis. The other 2 unsuccessful patients suffered presumed loss of autologous donor epithelium and recurrence of the ocular surface disease (pterygium). The partially successful patient receiving an allogeneic transplant had infectious keratitis delay of his re-epithelialization; he has only minimal visual improvement but has re-epithelialized. The patient receiving the second allogeneic graft lost his donor epithelium at day 4. Additional donor epithelium was reapplied, but the result is undetermined at present. Amniotic membrane as carrier. The in vitro preparation of the amniotic membrane with corneal epithelial stem cell graft overlay was successful.Histology documented removal of the amniotic epithelium and reapplication of corneal epithelial cells. Animal model. The 2 rabbits that had no reparative surgery following standard ocular surface injury had histology and immunopathology consistent with incomplete corneal epithelial stem cell failure with vascularization and scarring of the ocular surface. Light microscopy and immunohistologic staining with AE5 confirmed the conjunctival phenotype of the ocular surface repair but also documented the incomplete model. The allogeneic stern cell transplants had varying results. One rabbit had a suppurative infection and lost the graft. Reparative surgery failed in 2 of the rabbits, failed partially in 3 of the rabbits, was partially successful in 3 others, and was successful in 1 rabbit at 28 days. Histologic and immunopathologic study documented successful growth of corneal epithelium onto the recipient surface. CONCLUSIONS: 1. Presumed corneal epithelial stem cells can be harvested safely from the limbus and expanded successfully in vitro. 2. Expanded corneal epithelial cell cultures can be grown onto various carriers, but currently denuded amniotic membrane seems to be the best carrier for ocular surface repair. 3. Expanded corneal epithelial cell transplants appear to resurface damaged ocular surfaces successfully, but cellular tracking and further confirmation are required. 4. Expanded allogeneic corneal epithelial cell transplants are technically possible and may represent alternative treatment modalities for selected ocular surface problems. 5. These techniques potentially offer a new method of restoring a normal ocular surface while minimizing the threat of damage or depletion to the contralateral or sibling limbal corneal epithelial stem cells. 6. The rabbit model was probably incomplete and should be interpreted with caution. The complete eradication of all corneal epithelial stem cells from any eye is difficult, making confirmation of such work challenging. 7. The results of the rabbit model suggest that allogeneic grafts may restore a nearly normal ocular epithelial surface to certain ocular surface injuries.
Full text
PDF











































































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adinolfi M., Akle C. A., McColl I., Fensom A. H., Tansley L., Connolly P., Hsi B. L., Faulk W. P., Travers P., Bodmer W. F. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982 Jan 28;295(5847):325–327. doi: 10.1038/295325a0. [DOI] [PubMed] [Google Scholar]
- Aitken D., Friend J., Thoft R. A., Lee W. R. An ultrastructural study of rabbit ocular surface transdifferentiation. Invest Ophthalmol Vis Sci. 1988 Feb;29(2):224–231. [PubMed] [Google Scholar]
- Akle C. A., Adinolfi M., Welsh K. I., Leibowitz S., McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981 Nov 7;2(8254):1003–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- Alldredge O. C., Krachmer J. H. Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol. 1981 Apr;99(4):599–604. doi: 10.1001/archopht.1981.03930010599002. [DOI] [PubMed] [Google Scholar]
- Behzad F., Jones C. J., Aplin J. D. The role of integrin alpha 6 beta 4 in hemidesmosomes of human amnion. Biochem Soc Trans. 1991 Nov;19(4):381S–381S. doi: 10.1042/bst019381s. [DOI] [PubMed] [Google Scholar]
- Bell E., Ehrlich H. P., Buttle D. J., Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981 Mar 6;211(4486):1052–1054. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Goretsky M. J., Greenhalgh D. G., Kagan R. J., Rieman M. T., Warden G. D. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann Surg. 1995 Dec;222(6):743–752. doi: 10.1097/00000658-199512000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewitt H. Sliding of epithelium in experimental corneal wounds. A scanning electron microscopic study. Acta Ophthalmol (Copenh) 1979;57(6):945–958. doi: 10.1111/j.1755-3768.1979.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Bron A. J. Vortex patterns of the corneal epithelium. Trans Ophthalmol Soc U K. 1973;93(0):455–472. [PubMed] [Google Scholar]
- Brown S. I., Tragakis M. P., Pearce D. B. Corneal transplantation for severe alkali burns. Trans Am Acad Ophthalmol Otolaryngol. 1972 Sep-Oct;76(5):1266–1274. [PubMed] [Google Scholar]
- Champliaud M. F., Lunstrum G. P., Rousselle P., Nishiyama T., Keene D. R., Burgeson R. E. Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment. J Cell Biol. 1996 Mar;132(6):1189–1198. doi: 10.1083/jcb.132.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Tseng S. C. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci. 1990 Jul;31(7):1301–1314. [PubMed] [Google Scholar]
- Chen W. Y., Mui M. M., Kao W. W., Liu C. Y., Tseng S. C. Conjunctival epithelial cells do not transdifferentiate in organotypic cultures: expression of K12 keratin is restricted to corneal epithelium. Curr Eye Res. 1994 Oct;13(10):765–778. doi: 10.3109/02713689409047012. [DOI] [PubMed] [Google Scholar]
- Choi Y. S., Kim J. Y., Wee W. R., Lee J. H. Effect of the application of human amniotic membrane on rabbit corneal wound healing after excimer laser photorefractive keratectomy. Cornea. 1998 Jul;17(4):389–395. doi: 10.1097/00003226-199807000-00009. [DOI] [PubMed] [Google Scholar]
- Clinch T. E., Goins K. M., Cobo L. M. Treatment of contact lens-related ocular surface disorders with autologous conjunctival transplantation. Ophthalmology. 1992 Apr;99(4):634–638. doi: 10.1016/s0161-6420(92)31925-6. [DOI] [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Copeland R. A., Jr, Char D. H. Limbal autograft reconstruction after conjunctival squamous cell carcinoma. Am J Ophthalmol. 1990 Oct 15;110(4):412–415. doi: 10.1016/s0002-9394(14)77023-0. [DOI] [PubMed] [Google Scholar]
- Coster D. J., Aggarwal R. K., Williams K. A. Surgical management of ocular surface disorders using conjunctival and stem cell allografts. Br J Ophthalmol. 1995 Nov;79(11):977–982. doi: 10.1136/bjo.79.11.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster D. J., Aggarwal R. K., Williams K. A. Surgical management of ocular surface disorders using conjunctival and stem cell allografts. Br J Ophthalmol. 1995 Nov;79(11):977–982. doi: 10.1136/bjo.79.11.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G., Cheng S. Z., Dong G., Sun T. T., Lavker R. M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989 Apr 21;57(2):201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Davanger M., Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971 Feb 19;229(5286):560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- Doran T. I., Vidrich A., Sun T. T. Intrinsic and extrinsic regulation of the differentiation of skin, corneal and esophageal epithelial cells. Cell. 1980 Nov;22(1 Pt 1):17–25. doi: 10.1016/0092-8674(80)90150-6. [DOI] [PubMed] [Google Scholar]
- Dua H. S., Forrester J. V. The corneoscleral limbus in human corneal epithelial wound healing. Am J Ophthalmol. 1990 Dec 15;110(6):646–656. doi: 10.1016/s0002-9394(14)77062-x. [DOI] [PubMed] [Google Scholar]
- Ebato B., Friend J., Thoft R. A. Comparison of central and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1987 Sep;28(9):1450–1456. [PubMed] [Google Scholar]
- Ebato B., Friend J., Thoft R. A. Comparison of limbal and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci. 1988 Oct;29(10):1533–1537. [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Friend J., Kinoshita S., Thoft R. A., Eliason J. A. Corneal epithelial cell cultures on stromal carriers. Invest Ophthalmol Vis Sci. 1982 Jul;23(1):41–49. [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Fujishima H., Shimazaki J., Tsubota K. Temporary corneal stem cell dysfunction after radiation therapy. Br J Ophthalmol. 1996 Oct;80(10):911–914. doi: 10.1136/bjo.80.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNDERSEN T. Surgical treatment of bullous keratopathy. Arch Ophthalmol. 1960 Aug;64:260–267. doi: 10.1001/archopht.1960.01840010262013. [DOI] [PubMed] [Google Scholar]
- Geggel H. S., Friend J., Thoft R. A. Collagen gel for ocular surface. Invest Ophthalmol Vis Sci. 1985 Jun;26(6):901–905. [PubMed] [Google Scholar]
- Gipson I. K., Grill S. M. A technique for obtaining sheets of intact rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1982 Aug;23(2):269–273. [PubMed] [Google Scholar]
- Guo M., Grinnell F. Basement membrane and human epidermal differentiation in vitro. J Invest Dermatol. 1989 Sep;93(3):372–378. [PubMed] [Google Scholar]
- HANNA C., BICKNELL D. S., O'BRIEN J. E. Cell turnover in the adult human eye. Arch Ophthalmol. 1961 May;65:695–698. doi: 10.1001/archopht.1961.01840020697016. [DOI] [PubMed] [Google Scholar]
- HARTMAN D. C. Use of free grafts in correction of recurrent pterygia, pseudopterygia and symblepharon. Calif Med. 1951 Oct;75(4):279–280. [PMC free article] [PubMed] [Google Scholar]
- Haik B. G., Zimny M. L. Scanning electron microscopy of corneal wound healing in the rabbit. Invest Ophthalmol Vis Sci. 1977 Sep;16(9):787–796. [PubMed] [Google Scholar]
- Harris T. M., Berry E. R., Pakurar A. S., Sheppard L. B. Biochemical transformation of bulbar conjunctiva into corneal epithelium: an electrophoretic analysis. Exp Eye Res. 1985 Nov;41(5):597–605. doi: 10.1016/0014-4835(85)90032-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Dykes P. J., Marks R. Retinoid-induced inhibition of growth and reduction of spreading of human epidermal cells in culture. Br J Dermatol. 1985 Jun;112(6):637–646. doi: 10.1111/j.1365-2133.1985.tb02331.x. [DOI] [PubMed] [Google Scholar]
- Herman W. K., Doughman D. J., Lindstrom R. L. Conjunctival autograft transplantation for unilateral ocular surface diseases. Ophthalmology. 1983 Sep;90(9):1121–1126. doi: 10.1016/s0161-6420(83)80056-6. [DOI] [PubMed] [Google Scholar]
- Huang A. J., Tseng S. C. Development of monoclonal antibodies to rabbit ocular mucin. Invest Ophthalmol Vis Sci. 1987 Sep;28(9):1483–1491. [PubMed] [Google Scholar]
- Jenkins C., Tuft S., Liu C., Buckley R. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye (Lond) 1993;7(Pt 5):629–633. doi: 10.1038/eye.1993.145. [DOI] [PubMed] [Google Scholar]
- Kasper M., Moll R., Stosiek P., Karsten U. Patterns of cytokeratin and vimentin expression in the human eye. Histochemistry. 1988;89(4):369–377. doi: 10.1007/BF00500639. [DOI] [PubMed] [Google Scholar]
- Kaye D. B. Epithelial response in penetrating keratoplasty. Am J Ophthalmol. 1980 Mar;89(3):381–387. doi: 10.1016/0002-9394(80)90008-2. [DOI] [PubMed] [Google Scholar]
- Kenyon K. R., Tseng S. C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989 May;96(5):709–723. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- Kenyon K. R., Wagoner M. D., Hettinger M. E. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 1985 Nov;92(11):1461–1470. doi: 10.1016/s0161-6420(85)33831-9. [DOI] [PubMed] [Google Scholar]
- Kim J. C., Tseng S. C. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995 Sep;14(5):473–484. [PubMed] [Google Scholar]
- Kinoshita S., Friend J., Kiorpes T. C., Thoft R. A. Keratin-like proteins in corneal and conjunctival epithelium are different. Invest Ophthalmol Vis Sci. 1983 May;24(5):577–581. [PubMed] [Google Scholar]
- Kinoshita S., Friend J., Thoft R. A. Biphasic cell proliferation in transdifferentiation of conjunctival to corneal epithelium in rabbits. Invest Ophthalmol Vis Sci. 1983 Aug;24(8):1008–1014. [PubMed] [Google Scholar]
- Kinoshita S., Friend J., Thoft R. A. Sex chromatin of donor corneal epithelium in rabbits. Invest Ophthalmol Vis Sci. 1981 Sep;21(3):434–441. [PubMed] [Google Scholar]
- Kinoshita S., Kiorpes T. C., Friend J., Thoft R. A. Limbal epithelium in ocular surface wound healing. Invest Ophthalmol Vis Sci. 1982 Jul;23(1):73–80. [PubMed] [Google Scholar]
- Kolega J., Manabe M., Sun T. T. Basement membrane heterogeneity and variation in corneal epithelial differentiation. Differentiation. 1989 Oct;42(1):54–63. doi: 10.1111/j.1432-0436.1989.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Kruse F. E., Chen J. J., Tsai R. J., Tseng S. C. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Invest Ophthalmol Vis Sci. 1990 Sep;31(9):1903–1913. [PubMed] [Google Scholar]
- Kruse F. E., Tseng S. C. Retinoic acid regulates clonal growth and differentiation of cultured limbal and peripheral corneal epithelium. Invest Ophthalmol Vis Sci. 1994 Apr;35(5):2405–2420. [PubMed] [Google Scholar]
- Kruse F. E., Völcker H. E. Stem cells, wound healing, growth factors, and angiogenesis in the cornea. Curr Opin Ophthalmol. 1997 Aug;8(4):46–54. doi: 10.1097/00055735-199708000-00007. [DOI] [PubMed] [Google Scholar]
- Kuckelkorn R., Redbrake C., Schrage N. F., Reim M. Keratoplastik mit 11-12 mm Durchmesser zur Versorgung von schwerstverätzten Augen. Ophthalmologe. 1993 Dec;90(6):683–687. [PubMed] [Google Scholar]
- Kurpakus M. A., Stock E. L., Jones J. C. The role of the basement membrane in differential expression of keratin proteins in epithelial cells. Dev Biol. 1992 Apr;150(2):243–255. doi: 10.1016/0012-1606(92)90239-d. [DOI] [PubMed] [Google Scholar]
- Kuwabara T., Perkins D. G., Cogan D. G. Sliding of the epithelium in experimental corneal wounds. Invest Ophthalmol. 1976 Jan;15(1):4–14. [PubMed] [Google Scholar]
- LEBLOND C. P. CLASSIFICATION OF CELL POPULATIONS ON THE BASIS OF THEIR PROLIFERATIVE BEHAVIOR. Natl Cancer Inst Monogr. 1964 May;14:119–150. [PubMed] [Google Scholar]
- Lajtha L. G. Stem cell concepts. Differentiation. 1979;14(1-2):23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Lane E. B., Alexander C. M. Use of keratin antibodies in tumor diagnosis. Semin Cancer Biol. 1990 Jun;1(3):165–179. [PubMed] [Google Scholar]
- Lauweryns B., van den Oord J. J., De Vos R., Missotten L. A new epithelial cell type in the human cornea. Invest Ophthalmol Vis Sci. 1993 May;34(6):1983–1990. [PubMed] [Google Scholar]
- Lauweryns B., van den Oord J. J., Missotten L. The transitional zone between limbus and peripheral cornea. An immunohistochemical study. Invest Ophthalmol Vis Sci. 1993 May;34(6):1991–1999. [PubMed] [Google Scholar]
- Lavker R. M., Dong G., Cheng S. Z., Kudoh K., Cotsarelis G., Sun T. T. Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-synthesizing keratinocytes. Invest Ophthalmol Vis Sci. 1991 May;32(6):1864–1875. [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Tseng S. C. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997 Mar;123(3):303–312. doi: 10.1016/s0002-9394(14)70125-4. [DOI] [PubMed] [Google Scholar]
- Lemp M. A., Mathers W. D. Corneal epithelial cell movement in humans. Eye (Lond) 1989;3(Pt 4):438–445. doi: 10.1038/eye.1989.65. [DOI] [PubMed] [Google Scholar]
- Leone C. R., Jr Mucous membrane grafting for cicatricial entropion. Ophthalmic Surg. 1974 Summer;5(2):24–28. [PubMed] [Google Scholar]
- Levin P. S., Dutton J. J. Polytef (polytetrafluoroethylene) alloplastic grafting as a substitute for mucous membrane. Arch Ophthalmol. 1990 Feb;108(2):282–285. doi: 10.1001/archopht.1990.01070040134047. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Lee C. W., Morakis D. J. New method for preparing large surfaces of intact human basement membrane for tumor invasion studies. Cancer Lett. 1980 Dec;11(2):141–152. doi: 10.1016/0304-3835(80)90105-6. [DOI] [PubMed] [Google Scholar]
- Ljubimov A. V., Burgeson R. E., Butkowski R. J., Michael A. F., Sun T. T., Kenney M. C. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995 Apr;72(4):461–473. [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Thulin G., Madri J. A., Barrett C., Warshaw J. B. An acellular human amnionic membrane model for in vitro culture of type II pneumocytes: the role of the basement membrane in cell morphology and function. J Cell Physiol. 1984 Oct;121(1):215–225. doi: 10.1002/jcp.1041210127. [DOI] [PubMed] [Google Scholar]
- Mann I. A STUDY OF EPITHELIAL REGENERATION IN THE LIVING EYE. Br J Ophthalmol. 1944 Jan;28(1):26–40. doi: 10.1136/bjo.28.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann I., Pullinger B. D. A Study of Mustard Gas Lesions of the Eyes of Rabbits and Men: (Section of Ophthalmology). Proc R Soc Med. 1942 Jan;35(3):229–244.7. [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997 Apr 4;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Mattax J. B., McCulley J. P. Corneal surgery following alkali burns. Int Ophthalmol Clin. 1988 Spring;28(1):76–82. doi: 10.1097/00004397-198802810-00011. [DOI] [PubMed] [Google Scholar]
- Modesti A., Scarpa S., D'Orazi G., Simonelli L., Caramia F. G. Localization of type IV and V collagens in the stroma of human amnion. Prog Clin Biol Res. 1989;296:459–463. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Morgan S., Murray A. Limbal autotransplantation in the acute and chronic phases of severe chemical injuries. Eye (Lond) 1996;10(Pt 3):349–354. doi: 10.1038/eye.1996.72. [DOI] [PubMed] [Google Scholar]
- Naumann G. O., Lang G. K., Rummelt V., Wigand M. E. Autologous nasal mucosa transplantation in severe bilateral conjunctival mucus deficiency syndrome. Ophthalmology. 1990 Aug;97(8):1011–1017. doi: 10.1016/s0161-6420(90)32471-5. [DOI] [PubMed] [Google Scholar]
- Noguchi Y., Uchida Y., Endo T., Ninomiya H., Nomura A., Sakamoto T., Goto Y., Haraoka S., Shimokama T., Watanabe T. The induction of cell differentiation and polarity of tracheal epithelium cultured on the amniotic membrane. Biochem Biophys Res Commun. 1995 May 16;210(2):302–309. doi: 10.1006/bbrc.1995.1661. [DOI] [PubMed] [Google Scholar]
- Paton D., Milauskas A. T. Indications, surgical technique, and results of thin conjunctival flaps on the cornea: a review of 122 consecutive cases. Int Ophthalmol Clin. 1970 Summer;10(2):329–345. [PubMed] [Google Scholar]
- Pellegrini G., Traverso C. E., Franzi A. T., Zingirian M., Cancedda R., De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997 Apr 5;349(9057):990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- Pfister R. R. Corneal stem cell disease: concepts, categorization, and treatment by auto- and homotransplantation of limbal stem cells. CLAO J. 1994 Jan;20(1):64–72. [PubMed] [Google Scholar]
- Pfister R. R. The healing of corneal epithelial abrasions in the rabbit: a scanning electron microscope study. Invest Ophthalmol. 1975 Sep;14(9):648–661. [PubMed] [Google Scholar]
- Poirier R. H., Fish J. R. Lamellar keratoplasty for recurrent pterygium. Ophthalmic Surg. 1976 Winter;7(4):38–41. [PubMed] [Google Scholar]
- Prabhasawat P., Barton K., Burkett G., Tseng S. C. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997 Jun;104(6):974–985. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- Prabhasawat P., Tseng S. C. Impression cytology study of epithelial phenotype of ocular surface reconstructed by preserved human amniotic membrane. Arch Ophthalmol. 1997 Nov;115(11):1360–1367. doi: 10.1001/archopht.1997.01100160530001. [DOI] [PubMed] [Google Scholar]
- RUSCH H. P. Carcinogenesis; a facet of living processes. Cancer Res. 1954 Jul;14(6):407–417. [PubMed] [Google Scholar]
- Redbrake C., Buchal V. Keratoplastik mit Skleraring nach schwersten Verätzungen des vorderen Augenabschnittes. Klin Monbl Augenheilkd. 1996 Mar;208(3):145–151. doi: 10.1055/s-2008-1035188. [DOI] [PubMed] [Google Scholar]
- Reeh M. J. Corneoscleral lamellar transplant for recurrent pterygium. Arch Ophthalmol. 1971 Sep;86(3):296–297. doi: 10.1001/archopht.1971.01000010298011. [DOI] [PubMed] [Google Scholar]
- Roat M. I., Thoft R. A. Ocular surface epithelial transplantation. Int Ophthalmol Clin. 1988 Summer;28(2):169–174. doi: 10.1097/00004397-198802820-00011. [DOI] [PubMed] [Google Scholar]
- Rodrigues M., Ben-Zvi A., Krachmer J., Schermer A., Sun T. T. Suprabasal expression of a 64-kilodalton keratin (no. 3) in developing human corneal epithelium. Differentiation. 1987;34(1):60–67. doi: 10.1111/j.1432-0436.1987.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Roper-Hall M. J. Thermal and chemical burns. Trans Ophthalmol Soc U K. 1965;85:631–653. [PubMed] [Google Scholar]
- SUGAR H. S. THE USE OF GUNDERSEN FLAPS IN THE TREATMENT OF BULLOUS KERATOPATHY. Am J Ophthalmol. 1964 Jun;57:977–983. doi: 10.1016/0002-9394(64)91044-x. [DOI] [PubMed] [Google Scholar]
- Sabolinski M. L., Alvarez O., Auletta M., Mulder G., Parenteau N. L. Cultured skin as a 'smart material' for healing wounds: experience in venous ulcers. Biomaterials. 1996 Feb;17(3):311–320. doi: 10.1016/0142-9612(96)85569-4. [DOI] [PubMed] [Google Scholar]
- Schermer A., Galvin S., Sun T. T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986 Jul;103(1):49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. S., Friend J., Thoft R. A. Corneal re-epithelialization from the conjunctiva. Invest Ophthalmol Vis Sci. 1981 Jul;21(1 Pt 1):135–142. [PubMed] [Google Scholar]
- Sharma A., Coles W. H. Kinetics of corneal epithelial maintenance and graft loss. A population balance model. Invest Ophthalmol Vis Sci. 1989 Sep;30(9):1962–1971. [PubMed] [Google Scholar]
- Shimazaki J., Yang H. Y., Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997 Dec;104(12):2068–2076. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- Shimazaki J., Yang H. Y., Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997 Dec;104(12):2068–2076. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- Shimazaki J., Yang H. Y., Tsubota K. Limbal autograft transplantation for recurrent and advanced pterygia. Ophthalmic Surg Lasers. 1996 Nov;27(11):917–923. [PubMed] [Google Scholar]
- TROENSEGAARD-HANSEN E. Amniotic grafts in chronic skin ulceration. Lancet. 1950 May 6;1(6610):859–860. doi: 10.1016/s0140-6736(50)90693-3. [DOI] [PubMed] [Google Scholar]
- Tan D. T., Ficker L. A., Buckley R. J. Limbal transplantation. Ophthalmology. 1996 Jan;103(1):29–36. doi: 10.1016/s0161-6420(96)30737-9. [DOI] [PubMed] [Google Scholar]
- Thoft R. A. Conjunctival transplantation as an alternative to keratoplasty. Ophthalmology. 1979 Jun;86(6):1084–1092. doi: 10.1016/s0161-6420(79)35433-1. [DOI] [PubMed] [Google Scholar]
- Thoft R. A., Friend J. Biochemical transformation of regenerating ocular surface epithelium. Invest Ophthalmol Vis Sci. 1977 Jan;16(1):14–20. [PubMed] [Google Scholar]
- Thoft R. A., Friend J., Murphy H. S. Ocular surface epithelium and corneal vascularization in rabbits. I. The role of wounding. Invest Ophthalmol Vis Sci. 1979 Jan;18(1):85–92. [PubMed] [Google Scholar]
- Thoft R. A., Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983 Oct;24(10):1442–1443. [PubMed] [Google Scholar]
- Thoft R. A. Indications for conjunctival transplantation. Ophthalmology. 1982 Apr;89(4):335–339. doi: 10.1016/s0161-6420(82)34784-3. [DOI] [PubMed] [Google Scholar]
- Thoft R. A., Sugar J. Graft failure in keratoepithelioplasty. Cornea. 1993 Jul;12(4):362–365. doi: 10.1097/00003226-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Trelford J. D., Trelford-Sauder M. The amnion in surgery, past and present. Am J Obstet Gynecol. 1979 Aug 1;134(7):833–845. doi: 10.1016/0002-9378(79)90957-8. [DOI] [PubMed] [Google Scholar]
- Tsai R. J., Sun T. T., Tseng S. C. Comparison of limbal and conjunctival autograft transplantation in corneal surface reconstruction in rabbits. Ophthalmology. 1990 Apr;97(4):446–455. doi: 10.1016/s0161-6420(90)32575-7. [DOI] [PubMed] [Google Scholar]
- Tsai R. J., Sun T. T., Tseng S. C. Comparison of limbal and conjunctival autograft transplantation in corneal surface reconstruction in rabbits. Ophthalmology. 1990 Apr;97(4):446–455. doi: 10.1016/s0161-6420(90)32575-7. [DOI] [PubMed] [Google Scholar]
- Tsai R. J., Tseng S. C. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994 Sep;13(5):389–400. doi: 10.1097/00003226-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Tseng S. C. Concept and application of limbal stem cells. Eye (Lond) 1989;3(Pt 2):141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Hatchell D., Tierney N., Huang A. J., Sun T. T. Expression of specific keratin markers by rabbit corneal, conjunctival, and esophageal epithelia during vitamin A deficiency. J Cell Biol. 1984 Dec;99(6):2279–2286. doi: 10.1083/jcb.99.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S. C., Hirst L. W., Farazdaghi M., Green W. R. Goblet cell density and vascularization during conjunctival transdifferentiation. Invest Ophthalmol Vis Sci. 1984 Oct;25(10):1168–1176. [PubMed] [Google Scholar]
- Tseng S. C., Hirst L. W., Farazdaghi M., Green W. R. Inhibition of conjunctival transdifferentiation by topical retinoids. Invest Ophthalmol Vis Sci. 1987 Mar;28(3):538–542. [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Satake Y., Ohyama M., Toda I., Takano Y., Ono M., Shinozaki N., Shimazaki J. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol. 1996 Jul;122(1):38–52. doi: 10.1016/s0002-9394(14)71962-2. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Satake Y., Shimazaki J. Treatment of severe dry eye. Lancet. 1996 Jul 13;348(9020):123–123. doi: 10.1016/s0140-6736(96)24028-0. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Toda I., Saito H., Shinozaki N., Shimazaki J. Reconstruction of the corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995 Oct;102(10):1486–1496. doi: 10.1016/s0161-6420(95)30841-x. [DOI] [PubMed] [Google Scholar]
- Vastine D. W., Stewart W. B., Schwab I. R. Reconstruction of the periocular mucous membrane by autologous conjunctival transplantation. Ophthalmology. 1982 Sep;89(9):1072–1081. doi: 10.1016/s0161-6420(82)34681-3. [DOI] [PubMed] [Google Scholar]
- Verspaget H. W., Elmgreen J., Weterman I. T., Peña A. S., Riis P., Lamers C. B. Impaired activation of the neutrophil oxidative metabolism in chronic inflammatory bowel disease. Scand J Gastroenterol. 1986 Nov;21(9):1124–1130. doi: 10.3109/00365528608996432. [DOI] [PubMed] [Google Scholar]
- Wei Z. G., Sun T. T., Lavker R. M. Rabbit conjunctival and corneal epithelial cells belong to two separate lineages. Invest Ophthalmol Vis Sci. 1996 Mar;37(4):523–533. [PubMed] [Google Scholar]
- Wei Z. G., Wu R. L., Lavker R. M., Sun T. T. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci. 1993 Apr;34(5):1814–1828. [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. A., Brereton H. M., Aggarwal R., Sykes P. J., Turner D. R., Russ G. R., Coster D. J. Use of DNA polymorphisms and the polymerase chain reaction to examine the survival of a human limbal stem cell allograft. Am J Ophthalmol. 1995 Sep;120(3):342–350. doi: 10.1016/s0002-9394(14)72164-6. [DOI] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Eichner R., Nelson W. G., Sun T. T. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske J. D., Bukusoglu G., Yankauckas M. A. Characterization of a potential marker of corneal epithelial stem cells. Invest Ophthalmol Vis Sci. 1992 Jan;33(1):143–152. [PubMed] [Google Scholar]
- van Herendael B. J., Oberti C., Brosens I. Microanatomy of the human amniotic membranes. A light microscopic, transmission, and scanning electron microscopic study. Am J Obstet Gynecol. 1978 Aug 15;131(8):872–880. doi: 10.1016/s0002-9378(16)33135-0. [DOI] [PubMed] [Google Scholar]
- van der Linden P. J., de Goeij A. F., Dunselman G. A., Erkens H. W., Evers J. L. Endometrial cell adhesion in an in vitro model using intact amniotic membranes. Fertil Steril. 1996 Jan;65(1):76–80. [PubMed] [Google Scholar]