Abstract
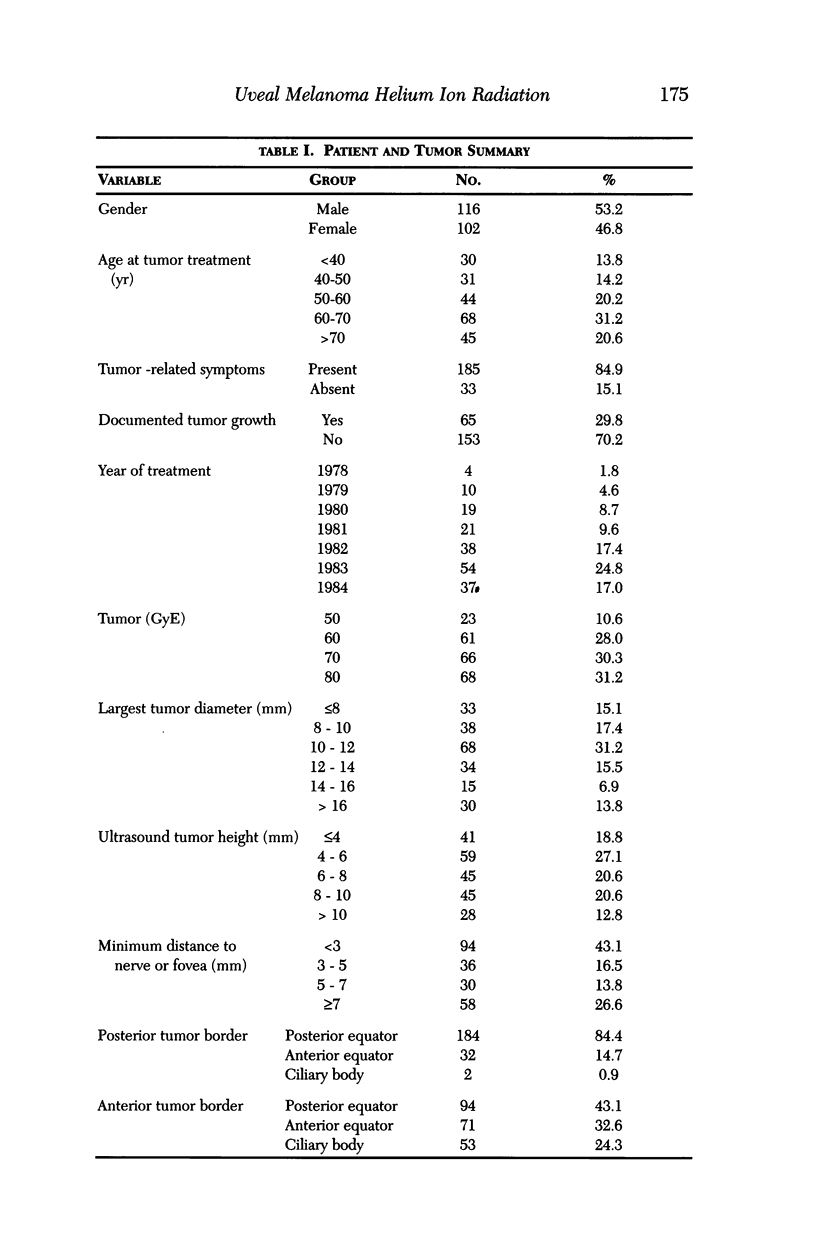
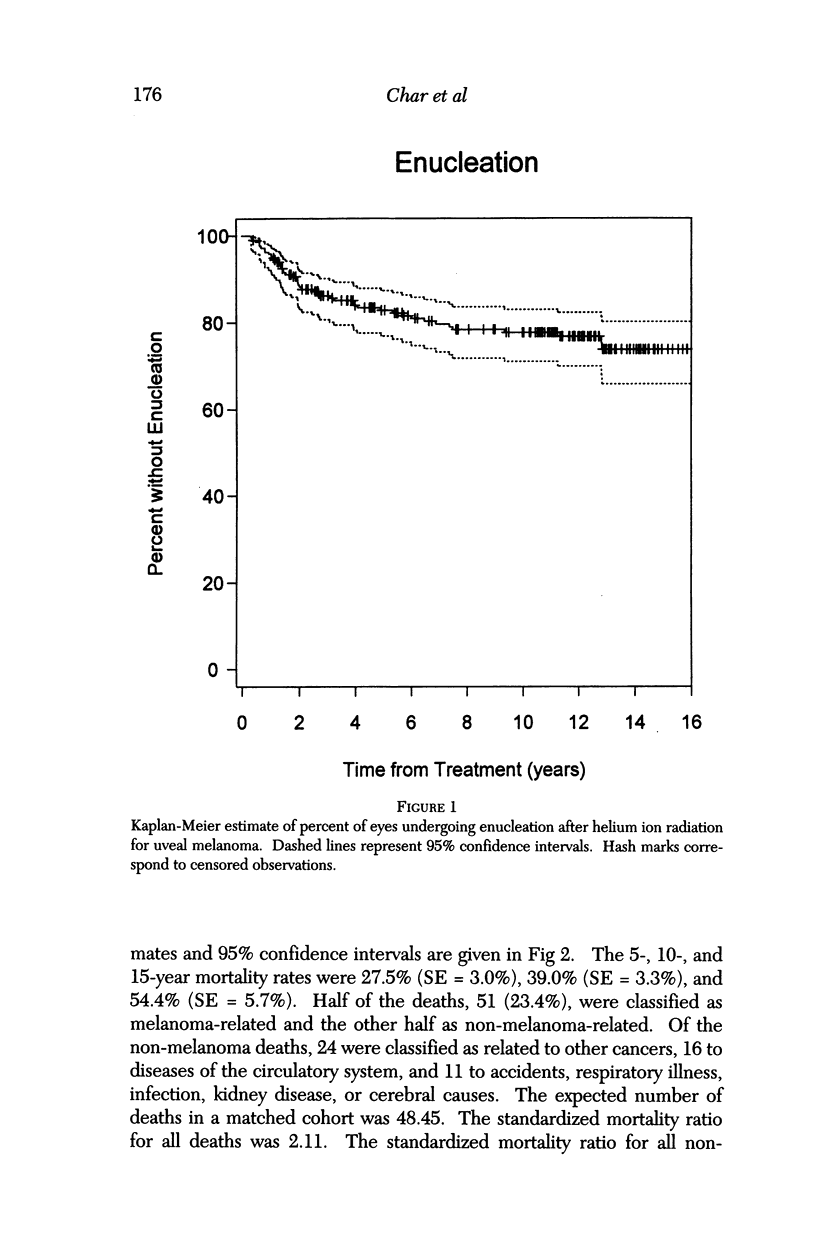
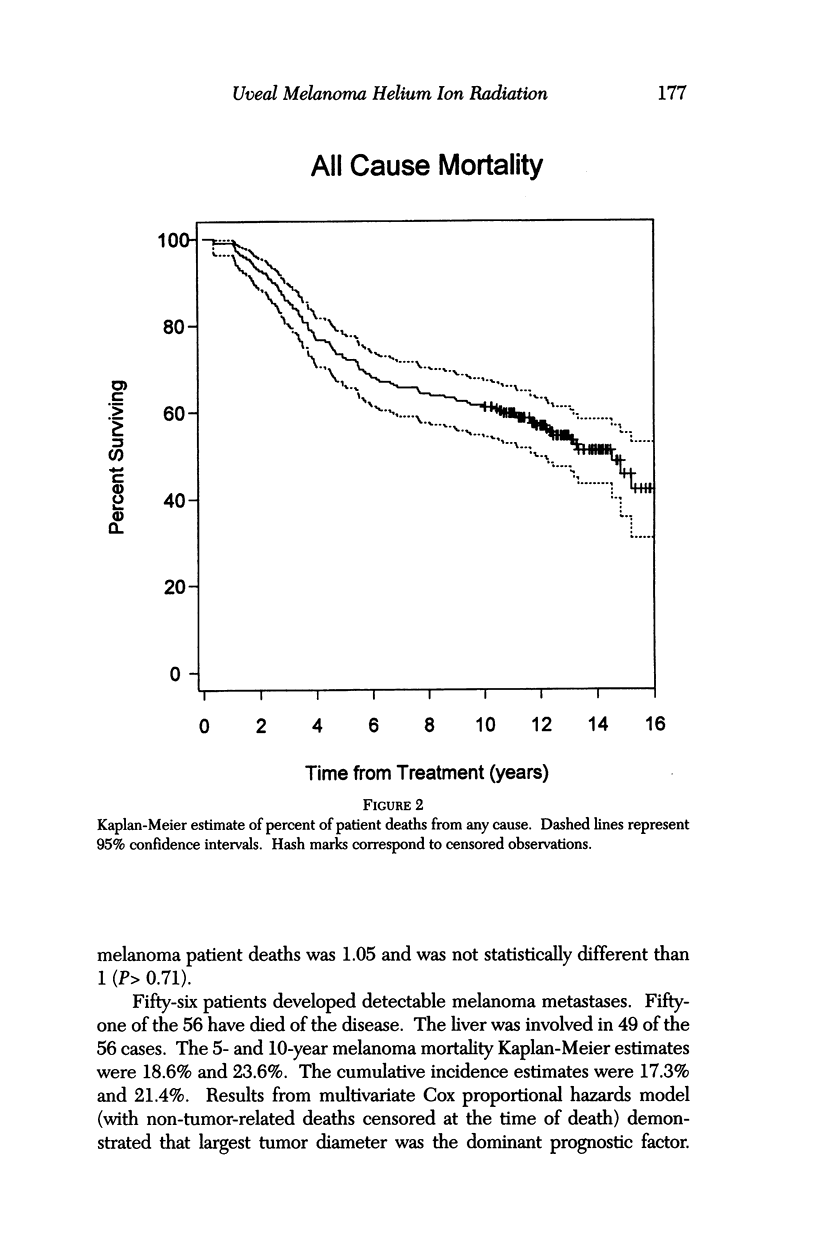
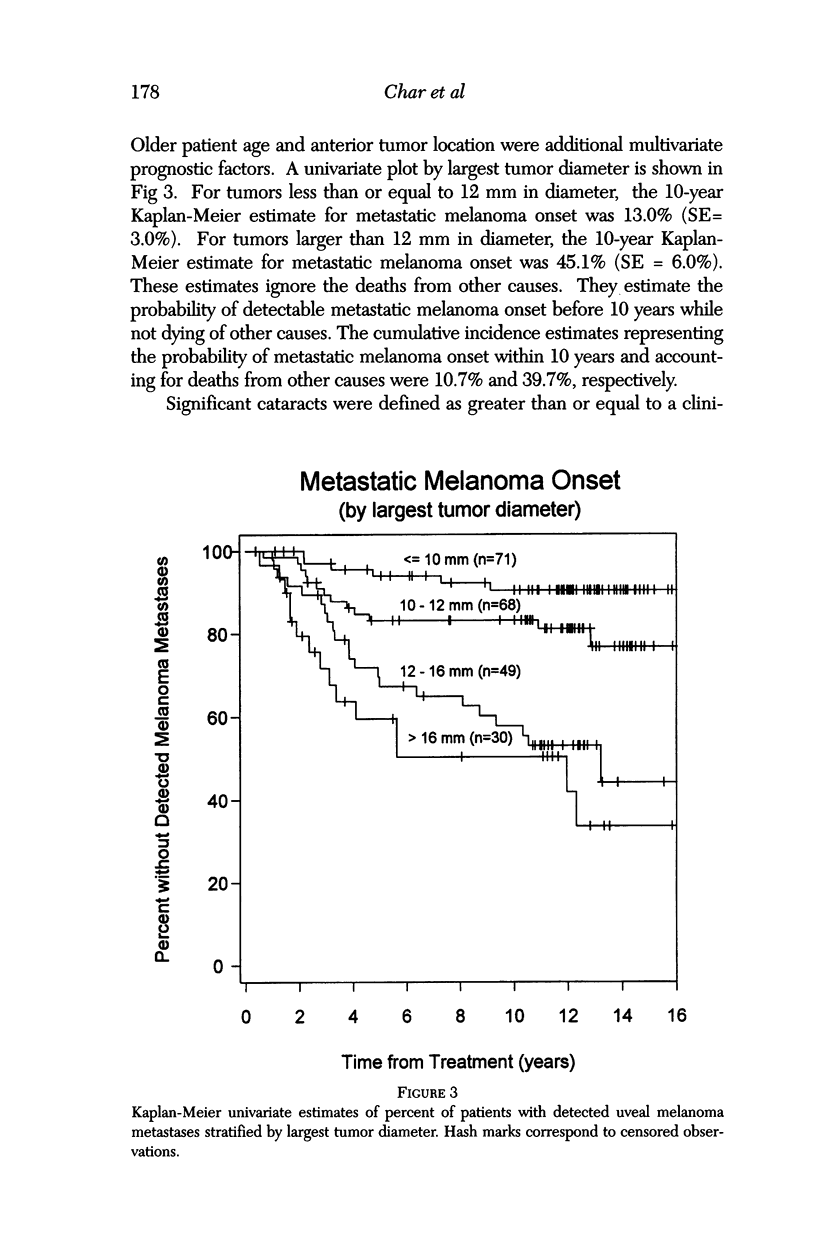
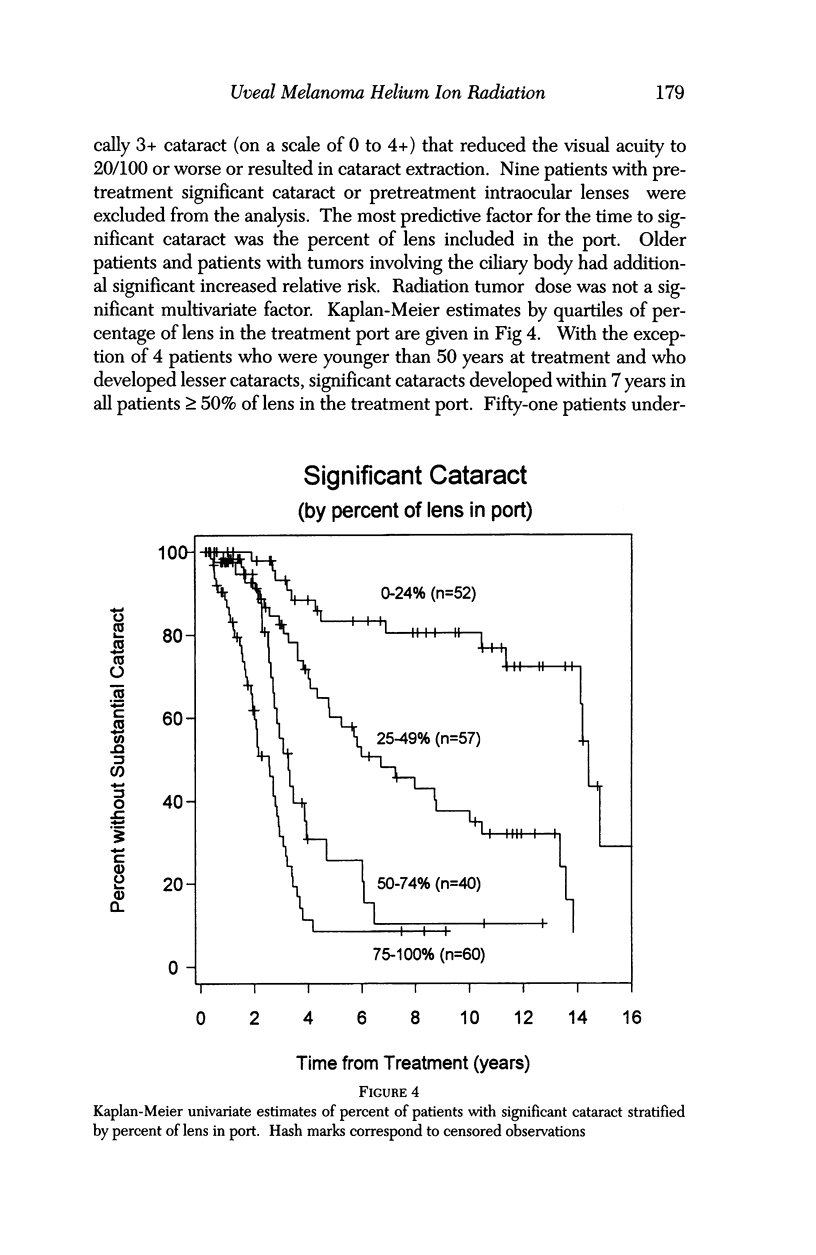
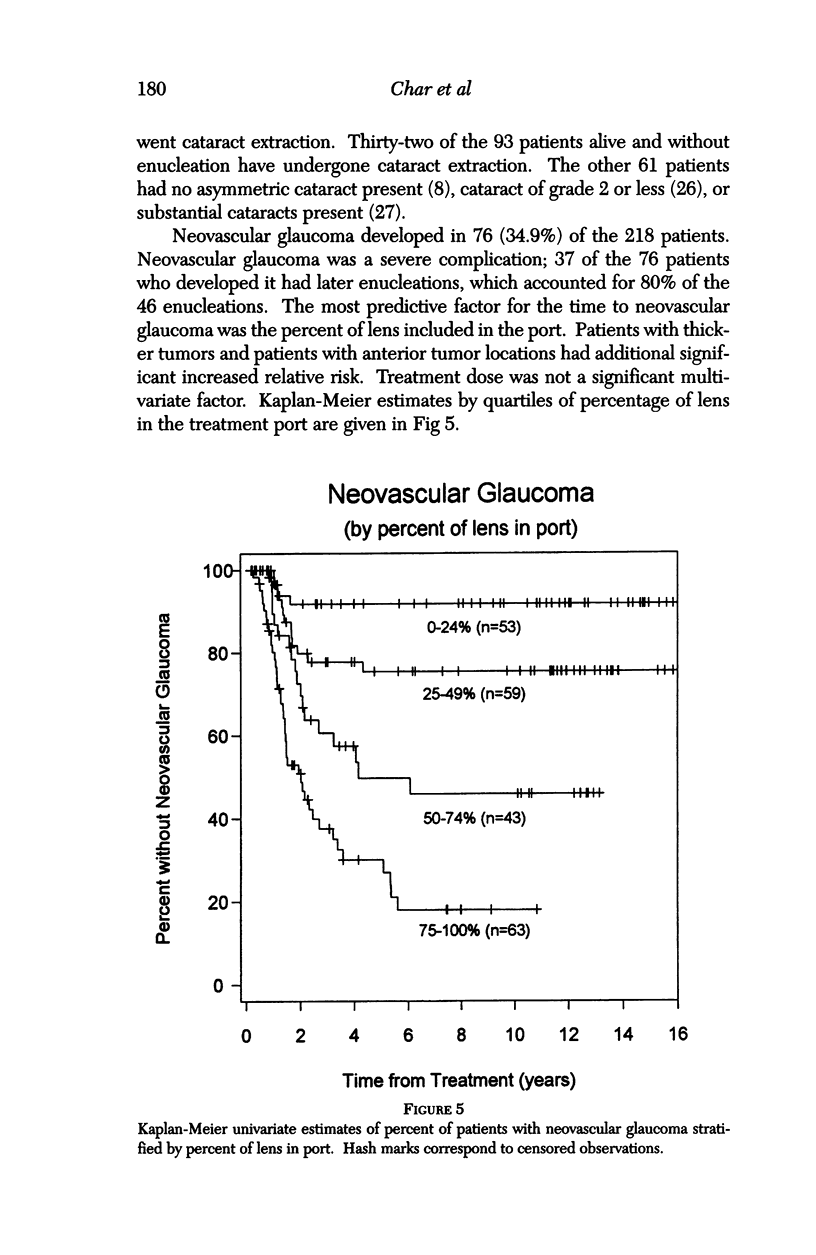
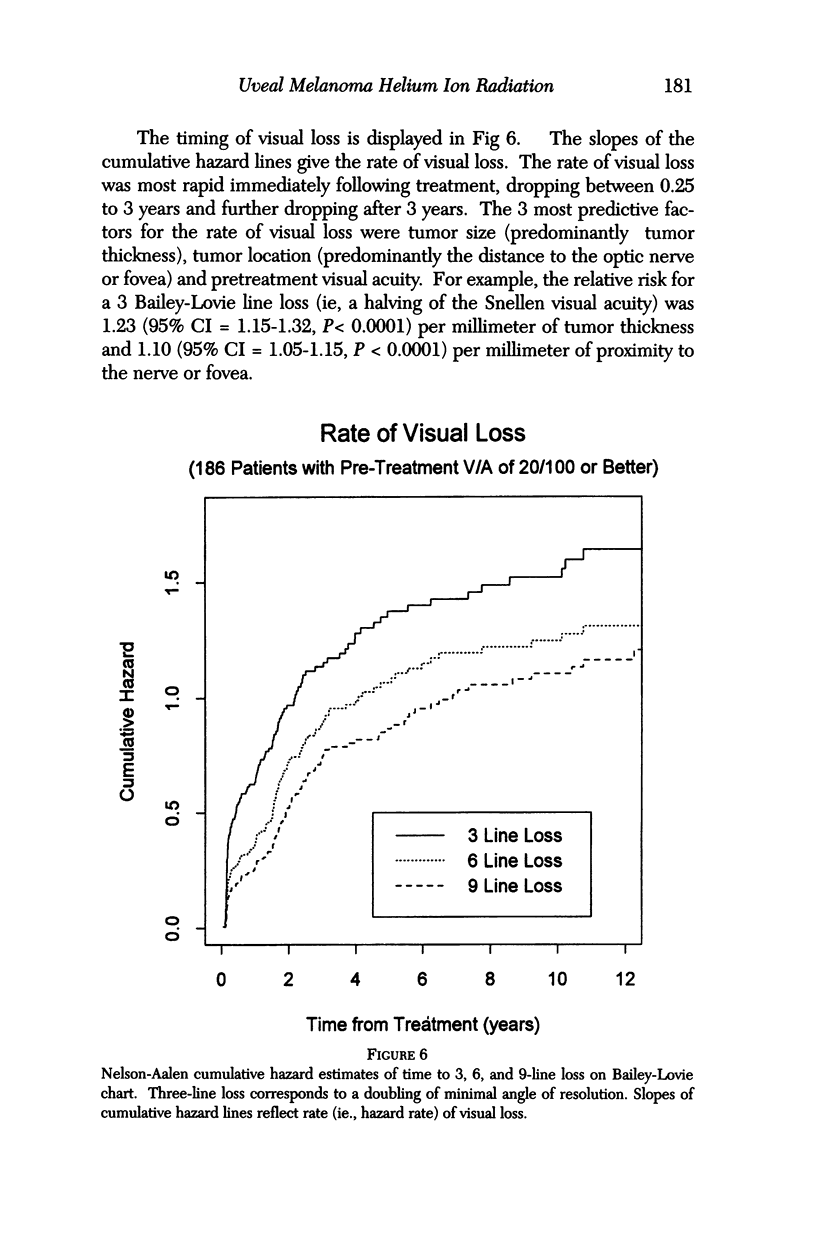
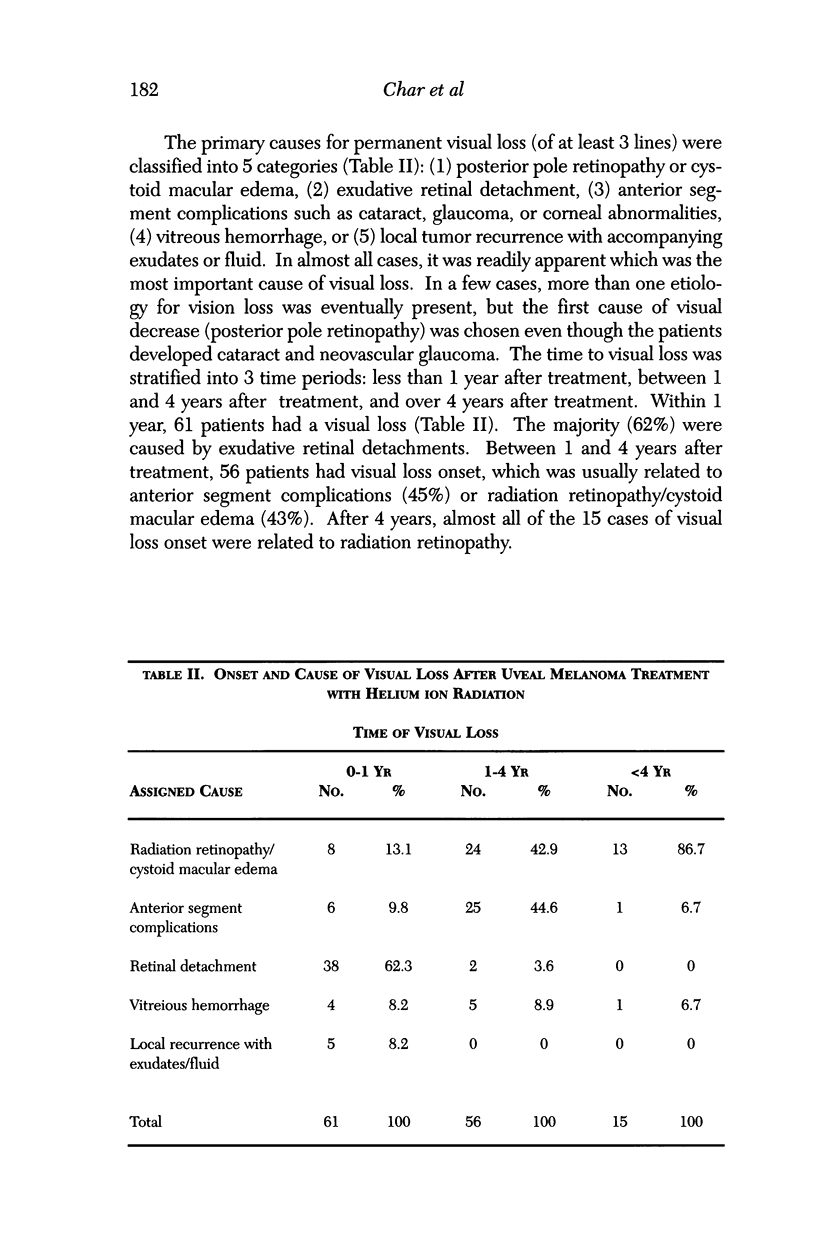
PURPOSE: To examine the results of helium ion irradiation in 218 uveal melanoma patients treated more than 10 years ago. METHODS: Retrospective review of 218 eyes treated with helium ion radiation for uveal melanoma between 1978 and 1984. Several parametric and non-parametric statistical analysis techniques were used. RESULTS: In 218 eyes treated with helium ion radiation for uveal melanoma, the mean dimension for largest basal diameter was 11.9 mm (range 5 mm to 24 mm). The mean tumor thickness was 6.7 mm (range 1.3 mm to 14.2 mm). Following helium ion radiation 208 (95.4%) of 218 eyes had local tumor control. At 10 years after radiation 46 (22.4%) of 218 eyes were enucleated; the majority (37 of 46) of enucleations were due to anterior ocular segment complications. At 10 years after radiation 102 (46.8%) of the 218 patients were dead; half had non-tumor related deaths and 51 died from metastatic melanoma. Best corrected visual acuity after radiation was > or = 20/40 in 21 of 93 eyes of patients that were alive and retained their eyes 10 or more years after treatment. In patients with tumors that were less than 6 mm in height and more than 3 mm away from the nerve or the fovea, 13 of 18 (72%) retained > or = 20/40. In contrast, only 11% of the patients with either thicker tumors or those close to the nerve or fovea retained that level of acuity. The actuarial enucleation rate at 5 years was 17.2% (2.7% S.E.) and at 10 years this was 22.4% (3.1% S.E). The recurrence tumor control rate at both 5 and 10 years was 5.3% (S.E 1.7%). CONCLUSIONS: Helium ion radiation of uveal melanoma is associated with good local tumor control and reasonable retention of the treated eye 10 years after treatment. In tumors that are less than 6 mm in thickness and greater than 3 mm from the optic nerve and fovea, many retain excellent vision. Approximately one-half of the deaths 10 years after treatment were due to non-tumor-related causes.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benichou J., Gail M. H. Estimates of absolute cause-specific risk in cohort studies. Biometrics. 1990 Sep;46(3):813–826. [PubMed] [Google Scholar]
- Berry G. The analysis of mortality by the subject-years method. Biometrics. 1983 Mar;39(1):173–184. [PubMed] [Google Scholar]
- Char D. H., Castro J. R., Kroll S. M., Irvine A. R., Quivey J. M., Stone R. D. Five-year follow-up of helium ion therapy for uveal melanoma. Arch Ophthalmol. 1990 Feb;108(2):209–214. doi: 10.1001/archopht.1990.01070040061031. [DOI] [PubMed] [Google Scholar]
- Char D. H., Castro J. R., Quivey J. M., Phillips T. L., Irvine A. R., Stone R. D., Kroll S. Uveal melanoma radiation. 125I brachytherapy versus helium ion irradiation. Ophthalmology. 1989 Dec;96(12):1708–1715. [PubMed] [Google Scholar]
- Char D. H., Kroll S. M., Miller T., Castro J., Quivey J. Irradiated uveal melanomas: cytopathologic correlation with prognosis. Am J Ophthalmol. 1996 Oct;122(4):509–513. doi: 10.1016/s0002-9394(14)72110-5. [DOI] [PubMed] [Google Scholar]
- Char D. H. Metastatic choroidal melanoma. Am J Ophthalmol. 1978 Jul;86(1):76–80. doi: 10.1016/0002-9394(78)90018-1. [DOI] [PubMed] [Google Scholar]
- Char D. H., Quivey J. M., Castro J. R., Kroll S., Phillips T. Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology. 1993 Oct;100(10):1547–1554. doi: 10.1016/s0161-6420(93)31446-6. [DOI] [PubMed] [Google Scholar]
- Char D. H., Saunders W., Castro J. R., Quivey J. M., Irvine A. R., Stone R. D., Crawford J. B., Barricks M., Lonn L. I., Hilton G. F. Helium ion therapy for choroidal melanoma. Ophthalmology. 1983 Oct;90(10):1219–1225. doi: 10.1016/s0161-6420(83)34405-5. [DOI] [PubMed] [Google Scholar]
- Char D. H., Stone R. D., Irvine A. R., Crawford J. B., Hilton G. F., Lonn L. I., Schwartz A. Diagnostic modalities in choroidal melanoma. Am J Ophthalmol. 1980 Feb;89(2):223–230. doi: 10.1016/0002-9394(80)90115-4. [DOI] [PubMed] [Google Scholar]
- Coleman D. J., Silverman R. H., Ursea R., Rondeau M. J., Lizzi F. L. Ultrasonically induced hyperthermia for adjunctive treatment of intraocular malignant melanoma. Retina. 1997;17(2):109–117. doi: 10.1097/00006982-199703000-00005. [DOI] [PubMed] [Google Scholar]
- Davidorf F. H., Makley T. A., Lang J. R. Radiotherapy of malignant melanoma of the choroid. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976 Sep-Oct;81(5):849–861. [PubMed] [Google Scholar]
- Duker J. S., Augsburger J. J., Shields J. A. Noncontiguous local recurrence of posterior uveal melanoma after cobalt 60 episcleral plaque therapy. Arch Ophthalmol. 1989 Jul;107(7):1019–1022. doi: 10.1001/archopht.1989.01070020081035. [DOI] [PubMed] [Google Scholar]
- Egan K. M., Gragoudas E. S., Seddon J. M., Glynn R. J., Munzenreider J. E., Goitein M., Verhey L., Urie M., Koehler A. The risk of enucleation after proton beam irradiation of uveal melanoma. Ophthalmology. 1989 Sep;96(9):1377–1383. doi: 10.1016/s0161-6420(89)32738-2. [DOI] [PubMed] [Google Scholar]
- Finger P. T., Buffa A., Mishra S., Berson A., Bosworth J. L., Vikram B. Palladium 103 plaque radiotherapy for uveal melanoma. Clinical experience. Ophthalmology. 1994 Feb;101(2):256–263. doi: 10.1016/s0161-6420(94)31338-8. [DOI] [PubMed] [Google Scholar]
- Gragoudas E. S., Egan K. M., Seddon J. M., Walsh S. M., Munzenrider J. E. Intraocular recurrence of uveal melanoma after proton beam irradiation. Ophthalmology. 1992 May;99(5):760–766. doi: 10.1016/s0161-6420(92)31900-1. [DOI] [PubMed] [Google Scholar]
- Gragoudas E. S., Egan K. M., Walsh S. M., Regan S., Munzenrider J. E., Taratuta V. Lens changes after proton beam irradiation for uveal melanoma. Am J Ophthalmol. 1995 Feb;119(2):157–164. doi: 10.1016/s0002-9394(14)73868-1. [DOI] [PubMed] [Google Scholar]
- Gragoudas E. S., Seddon J. M., Egan K., Glynn R., Munzenrider J., Austin-Seymour M., Goitein M., Verhey L., Urie M., Koehler A. Long-term results of proton beam irradiated uveal melanomas. Ophthalmology. 1987 Apr;94(4):349–353. doi: 10.1016/s0161-6420(87)33456-6. [DOI] [PubMed] [Google Scholar]
- Guthoff R., Haase J., von Domarus D., Draeger J., Lauritzen K. Das Regressionsverhalten des Aderhautmelanoms nach Strahlentherapie--ein neuer prognostischer Parameter? Klin Monbl Augenheilkd. 1990 Jan;196(1):6–10. doi: 10.1055/s-2008-1046119. [DOI] [PubMed] [Google Scholar]
- Guyer D. R., Mukai S., Egan K. M., Seddon J. M., Walsh S. M., Gragoudas E. S. Radiation maculopathy after proton beam irradiation for choroidal melanoma. Ophthalmology. 1992 Aug;99(8):1278–1285. doi: 10.1016/s0161-6420(92)31832-9. [DOI] [PubMed] [Google Scholar]
- Karlsson U. L., Augsburger J. J., Shields J. A., Markoe A. M., Brady L. W., Woodleigh R. Recurrence of posterior uveal melanoma after 60Co episcleral plaque therapy. Ophthalmology. 1989 Mar;96(3):382–388. doi: 10.1016/s0161-6420(89)32882-x. [DOI] [PubMed] [Google Scholar]
- Kleineidam M., Augsburger J. J., Hernandez C., Glennon P., Brady L. W. Cataractogenesis after Cobalt-60 eye plaque radiotherapy. Int J Radiat Oncol Biol Phys. 1993 Jul 15;26(4):625–630. doi: 10.1016/0360-3016(93)90279-5. [DOI] [PubMed] [Google Scholar]
- Mameghan H., Fisher R., Mameghan J., Brook S. Results of external beam radiotherapy in 448 patients with clinically localized adenocarcinoma of the prostate. Aust N Z J Surg. 1994 Jun;64(6):389–394. doi: 10.1111/j.1445-2197.1994.tb02236.x. [DOI] [PubMed] [Google Scholar]
- Meecham W. J., Char D. H., Kroll S., Castro J. R., Blakely E. A. Anterior segment complications after helium ion radiation therapy for uveal melanoma. Radiation cataract. Arch Ophthalmol. 1994 Feb;112(2):197–203. doi: 10.1001/archopht.1994.01090140073026. [DOI] [PubMed] [Google Scholar]
- Moreno J. G., Ahlering T. E. Late local complications after definitive radiotherapy for prostatic adenocarcinoma. J Urol. 1992 Mar;147(3 Pt 2):926–928. doi: 10.1016/s0022-5347(17)37424-4. [DOI] [PubMed] [Google Scholar]
- Packer S., Rotman M. Radiotherapy of choroidal melanoma with iodine-125. Ophthalmology. 1980 Jun;87(6):582–590. doi: 10.1016/s0161-6420(80)35194-4. [DOI] [PubMed] [Google Scholar]
- Quivey J. M., Augsburger J., Snelling L., Brady L. W. 125I plaque therapy for uveal melanoma. Analysis of the impact of time and dose factors on local control. Cancer. 1996 Jun 1;77(11):2356–2362. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2356::AID-CNCR26>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Quivey J. M., Char D. H., Phillips T. L., Weaver K. A., Castro J. R., Kroll S. M. High intensity 125-iodine (125I) plaque treatment of uveal melanoma. Int J Radiat Oncol Biol Phys. 1993 Jul 15;26(4):613–618. doi: 10.1016/0360-3016(93)90277-3. [DOI] [PubMed] [Google Scholar]
- Stallard H. B. Malignant melanoblastoma of the choroid. Bibl Ophthalmol. 1968;75:16–38. [PubMed] [Google Scholar]
- Summanen P., Immonen I., Heikkonen J., Tommila P., Laatikainen L., Tarkkanen A. Survival of patients and metastatic and local recurrent tumor growth in malignant melanoma of the uvea after ruthenium plaque radiotherapy. Ophthalmic Surg. 1993 Feb;24(2):82–90. [PubMed] [Google Scholar]
- Vrabec T. R., Augsburger J. J., Gamel J. W., Brady L. W., Hernandez C., Woodleigh R. Impact of local tumor relapse on patient survival after cobalt 60 plaque radiotherapy. Ophthalmology. 1991 Jun;98(6):984–988. doi: 10.1016/s0161-6420(91)32193-6. [DOI] [PubMed] [Google Scholar]
- Zografos L., Bercher L., Egger E., Chamot L., Gailloud C., Uffer S., Perret C., Markovits C. Le traitement des tumeurs oculaires par faisceau de protons accélérés. 7 ans d'expérience. Klin Monbl Augenheilkd. 1992 May;200(5):431–435. doi: 10.1055/s-2008-1045785. [DOI] [PubMed] [Google Scholar]


