Abstract
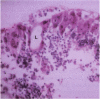
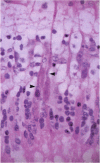
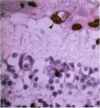
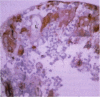
PURPOSE: Although cytomegalovirus (CMV) retinitis is known to occur in association with retinal microangiopathy in individuals with marked immunodeficiency, glial cells are believed to be the initial target cells in the development of retinitis. Moreover, it has been hypothesized that CMV gains access to the retinal glia because of altered vascular permeability. In an attempt to address the hypothesis, we studied 30 autopsy eyes of AIDS patients with systemic CMV infection, with or without clinically apparent CMV retinitis. METHODS: The autopsy eyes were processed in three ways. First, dual immunohistochemical studies were done by using anti-CMV antibodies for immediate early, early, and late antigens. The retinal cell types infected with the virus were then determined by using anti-GFAP, anti-VonWillebrand's factor, neuronal specific enolase, and leukocyte marker CD68. Second, selected eyes were processed for in situ hybridization with DNA probe specific to CMV. Third, an eye with clinically apparent CMV retinitis was submitted for electron microscopic examination. RESULTS: At the site of retinal necrosis in those eyes with a clinical diagnosis of CMV retinitis, the immunohistochemical, in situ hybridization, and ultrastructural examinations revealed that CMV was present primarily in the Müller cells and in perivascular glial cells. Adjacent to these infected cells, focal areas of positive staining for CMV antigen were seen in the glial cells, neuronal cells, and retinal pigment epithelial cells. At these sites most of the retinal capillaries were devoid of endothelial cells. Few vessels located at the advancing margin of retinal necrosis showed the presence of viral proteins in the endothelial cells. CONCLUSIONS: The present results indicate that retinal vascular endothelial cells could be the initial target in the development of viral retinitis, with subsequent spread of the infection to perivascular glia, Müller cells, and other retinal cells, including the retinal pigment epithelium.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burd E. M., Pulido J. S., Puro D. G., O'Brien W. J. Replication of human cytomegalovirus in human retinal glial cells. Invest Ophthalmol Vis Sci. 1996 Sep;37(10):1957–1966. [PubMed] [Google Scholar]
- Collier A. C., Meyers J. D., Corey L., Murphy V. L., Roberts P. L., Handsfield H. H. Cytomegalovirus infection in homosexual men. Relationship to sexual practices, antibody to human immunodeficiency virus, and cell-mediated immunity. Am J Med. 1987 Mar 23;82(3 Spec No):593–601. doi: 10.1016/0002-9343(87)90105-7. [DOI] [PubMed] [Google Scholar]
- Coskuncan N. M., Jabs D. A., Dunn J. P., Haller J. A., Green W. R., Vogelsang G. B., Santos G. W. The eye in bone marrow transplantation. VI. Retinal complications. Arch Ophthalmol. 1994 Mar;112(3):372–379. doi: 10.1001/archopht.1994.01090150102031. [DOI] [PubMed] [Google Scholar]
- De Venecia G., Zu Rhein G. M., Pratt M. V., Kisken W. Cytomegalic inclusion retinitis in an adult. Arch Ophthalmol. 1971 Jul;86(1):44–57. doi: 10.1001/archopht.1971.01000010046010. [DOI] [PubMed] [Google Scholar]
- Galea P., Vermot-Desroches C., Le Contel C., Wijdenes J., Chermann J. C. Circulating cell adhesion molecules in HIV1-infected patients as indicator markers for AIDS progression. Res Immunol. 1997 Feb;148(2):109–117. doi: 10.1016/s0923-2494(97)82482-0. [DOI] [PubMed] [Google Scholar]
- Glasgow B. J., Weisberger A. K. A quantitative and cartographic study of retinal microvasculopathy in acquired immunodeficiency syndrome. Am J Ophthalmol. 1994 Jul 15;118(1):46–56. doi: 10.1016/s0002-9394(14)72841-7. [DOI] [PubMed] [Google Scholar]
- Henderly D. E., Atalla L. R., Freeman W. R., Rao N. A. Demonstration of cytomegalovirus retinitis by in situ DNA hybridization. Retina. 1988;8(3):177–181. doi: 10.1097/00006982-198808030-00005. [DOI] [PubMed] [Google Scholar]
- Holland G. N., Pepose J. S., Pettit T. H., Gottlieb M. S., Yee R. D., Foos R. Y. Acquired immune deficiency syndrome. Ocular manifestations. Ophthalmology. 1983 Aug;90(8):859–873. doi: 10.1016/s0161-6420(83)80009-8. [DOI] [PubMed] [Google Scholar]
- Hoover D. R., Peng Y., Saah A., Semba R., Detels R. R., Rinaldo C. R., Jr, Phair J. P. Occurrence of cytomegalovirus retinitis after human immunodeficiency virus immunosuppression. Arch Ophthalmol. 1996 Jul;114(7):821–827. doi: 10.1001/archopht.1996.01100140035004. [DOI] [PubMed] [Google Scholar]
- Jabs D. A., Bartlett J. G. AIDS and ophthalmology: a period of transition. Am J Ophthalmol. 1997 Aug;124(2):227–233. doi: 10.1016/s0002-9394(14)70789-5. [DOI] [PubMed] [Google Scholar]
- Jabs D. A., Green W. R., Fox R., Polk B. F., Bartlett J. G. Ocular manifestations of acquired immune deficiency syndrome. Ophthalmology. 1989 Jul;96(7):1092–1099. doi: 10.1016/s0161-6420(89)32794-1. [DOI] [PubMed] [Google Scholar]
- Jabs D. A. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc. 1995;93:623–683. [PMC free article] [PubMed] [Google Scholar]
- Kuppermann B. D., Petty J. G., Richman D. D., Mathews W. C., Fullerton S. C., Rickman L. S., Freeman W. R. Correlation between CD4+ counts and prevalence of cytomegalovirus retinitis and human immunodeficiency virus-related noninfectious retinal vasculopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993 May 15;115(5):575–582. doi: 10.1016/s0002-9394(14)71453-9. [DOI] [PubMed] [Google Scholar]
- Lange M., Klein E. B., Kornfield H., Cooper L. Z., Grieco M. H. Cytomegalovirus isolation from healthy homosexual men. JAMA. 1984 Oct 12;252(14):1908–1910. [PubMed] [Google Scholar]
- Pepose J. S., Holland G. N., Nestor M. S., Cochran A. J., Foos R. Y. Acquired immune deficiency syndrome. Pathogenic mechanisms of ocular disease. Ophthalmology. 1985 Apr;92(4):472–484. doi: 10.1016/s0161-6420(85)34008-3. [DOI] [PubMed] [Google Scholar]
- Pereira L., Maidji E., Tugizov S., Jones T. Deletion mutants in human cytomegalovirus glycoprotein US9 are impaired in cell-cell transmission and in altering tight junctions of polarized human retinal pigment epithelial cells. Scand J Infect Dis Suppl. 1995;99:82–87. [PubMed] [Google Scholar]
- Plachter B., Sinzger C., Jahn G. Cell types involved in replication and distribution of human cytomegalovirus. Adv Virus Res. 1996;46:195–261. doi: 10.1016/s0065-3527(08)60073-1. [DOI] [PubMed] [Google Scholar]
- Plachter B., Sinzger C., Jahn G. Cell types involved in replication and distribution of human cytomegalovirus. Adv Virus Res. 1996;46:195–261. doi: 10.1016/s0065-3527(08)60073-1. [DOI] [PubMed] [Google Scholar]
- Rao N. A., Font R. L. Toxoplasmic retinochoroiditis: electron-microscopic and immunofluorescence studies of formalin-fixed tissue. Arch Ophthalmol. 1977 Feb;95(2):273–277. doi: 10.1001/archopht.1977.04450020074012. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Morris S., Zipeto D., Fessel J., Wolitz R., Dowling A., Merigan T. C. Quantitation of human cytomegalovirus DNA from peripheral blood cells of human immunodeficiency virus-infected patients could predict cytomegalovirus retinitis. J Infect Dis. 1995 Jan;171(1):177–182. doi: 10.1093/infdis/171.1.177. [DOI] [PubMed] [Google Scholar]
- Rummelt V., Rummelt C., Jahn G., Wenkel H., Sinzger C., Mayer U. M., Naumann G. O. Triple retinal infection with human immunodeficiency virus type 1, cytomegalovirus, and herpes simplex virus type 1. Light and electron microscopy, immunohistochemistry, and in situ hybridization. Ophthalmology. 1994 Feb;101(2):270–279. doi: 10.1016/s0161-6420(94)31336-4. [DOI] [PubMed] [Google Scholar]
- Schmitt-Gräff A., Neuen-Jacob E., Rettig B., Sundmacher R. Evidence for cytomegalovirus and human immunodeficiency virus infection of the retina in AIDS. Virchows Arch A Pathol Anat Histopathol. 1990;416(3):249–253. doi: 10.1007/BF01678984. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Freeman W. R., Wiley C. A., McCutchan J. A. Immune predispositions for cytomegalovirus retinitis in AIDS. The HNRC Group. J Clin Invest. 1995 Apr;95(4):1741–1746. doi: 10.1172/JCI117851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman J. S., Orellana J., Friedman A. H., Teich S. A. Acquired immunodeficiency syndrome (AIDS). Surv Ophthalmol. 1987 May-Jun;31(6):384–410. doi: 10.1016/0039-6257(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Seilhean D., Dzia-Lepfoundzou A., Sazdovitch V., Cannella B., Raine C. S., Katlama C., Bricaire F., Duyckaerts C., Hauw J. J. Astrocytic adhesion molecules are increased in HIV-1-associated cognitive/motor complex. Neuropathol Appl Neurobiol. 1997 Apr;23(2):83–92. [PubMed] [Google Scholar]
- Seilhean D., Kobayashi K., He Y., Uchihara T., Rosenblum O., Katlama C., Bricaire F., Duyckaerts C., Hauw J. J. Tumor necrosis factor-alpha, microglia and astrocytes in AIDS dementia complex. Acta Neuropathol. 1997 May;93(5):508–517. doi: 10.1007/s004010050646. [DOI] [PubMed] [Google Scholar]
- Shepp D. H., Match M. E., Ashraf A. B., Lipson S. M., Millan C., Pergolizzi R. Cytomegalovirus glycoprotein B groups associated with retinitis in AIDS. J Infect Dis. 1996 Jul;174(1):184–187. doi: 10.1093/infdis/174.1.184. [DOI] [PubMed] [Google Scholar]
- Shinkai M., Bozzette S. A., Powderly W., Frame P., Spector S. A. Utility of urine and leukocyte cultures and plasma DNA polymerase chain reaction for identification of AIDS patients at risk for developing human cytomegalovirus disease. J Infect Dis. 1997 Feb;175(2):302–308. doi: 10.1093/infdis/175.2.302. [DOI] [PubMed] [Google Scholar]
- Shinkai M., Bozzette S. A., Powderly W., Frame P., Spector S. A. Utility of urine and leukocyte cultures and plasma DNA polymerase chain reaction for identification of AIDS patients at risk for developing human cytomegalovirus disease. J Infect Dis. 1997 Feb;175(2):302–308. doi: 10.1093/infdis/175.2.302. [DOI] [PubMed] [Google Scholar]
- Waldman W. J., Knight D. A., Huang E. H. An in vitro model of T cell activation by autologous cytomegalovirus (CMV)-infected human adult endothelial cells: contribution of CMV-enhanced endothelial ICAM-1. J Immunol. 1998 Apr 1;160(7):3143–3151. [PubMed] [Google Scholar]
- Yoser S. L., Forster D. J., Rao N. A. Systemic viral infections and their retinal and choroidal manifestations. Surv Ophthalmol. 1993 Mar-Apr;37(5):313–352. doi: 10.1016/0039-6257(93)90064-e. [DOI] [PubMed] [Google Scholar]