Abstract
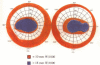
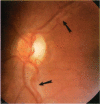
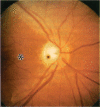
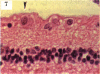
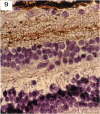
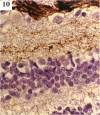
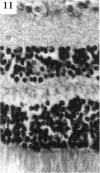
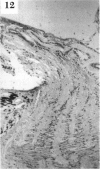
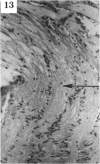
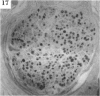
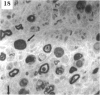
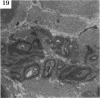
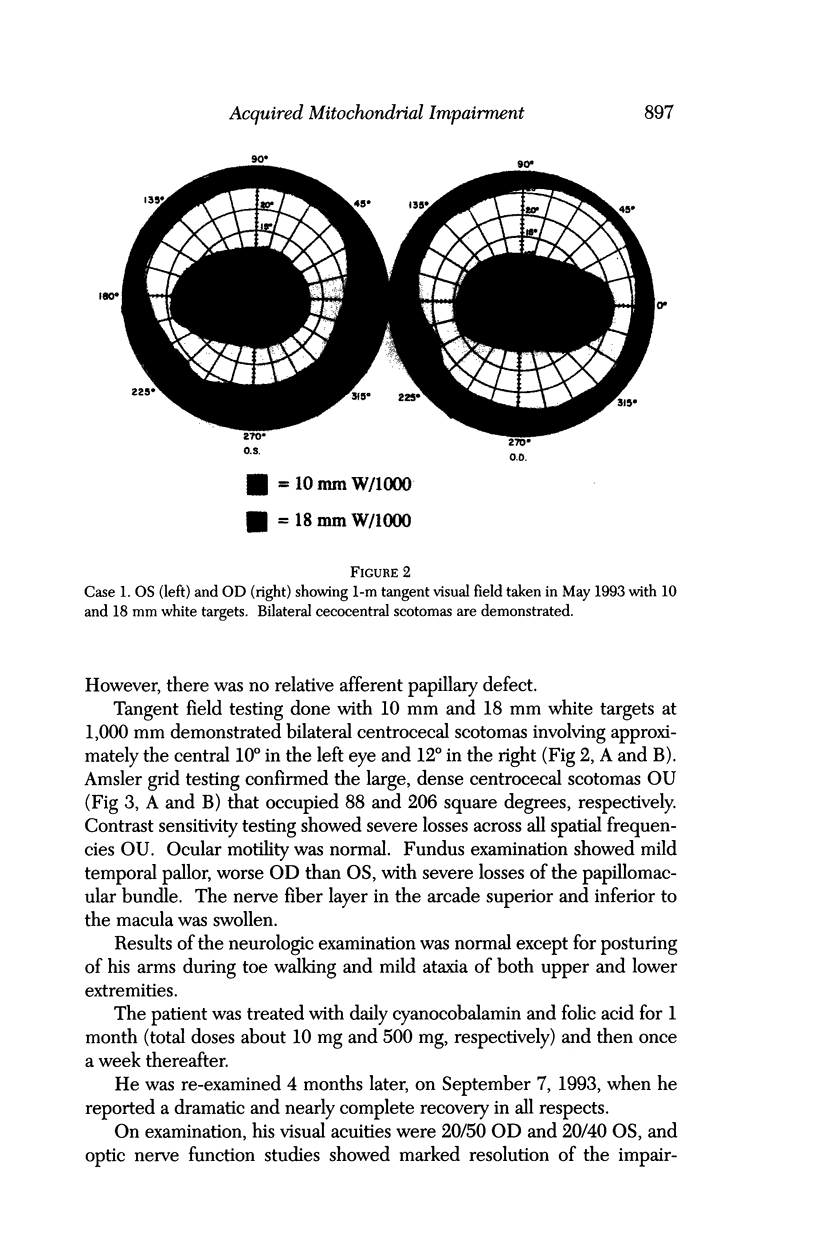
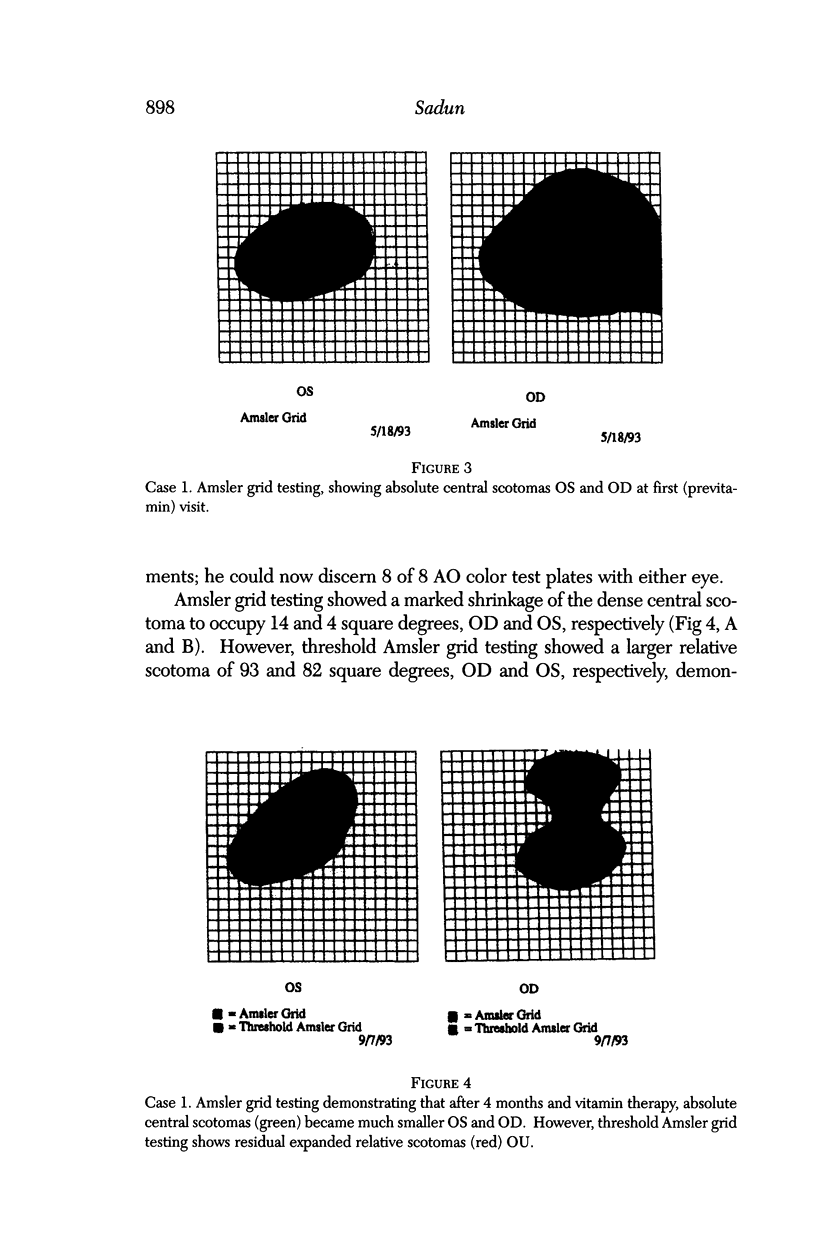
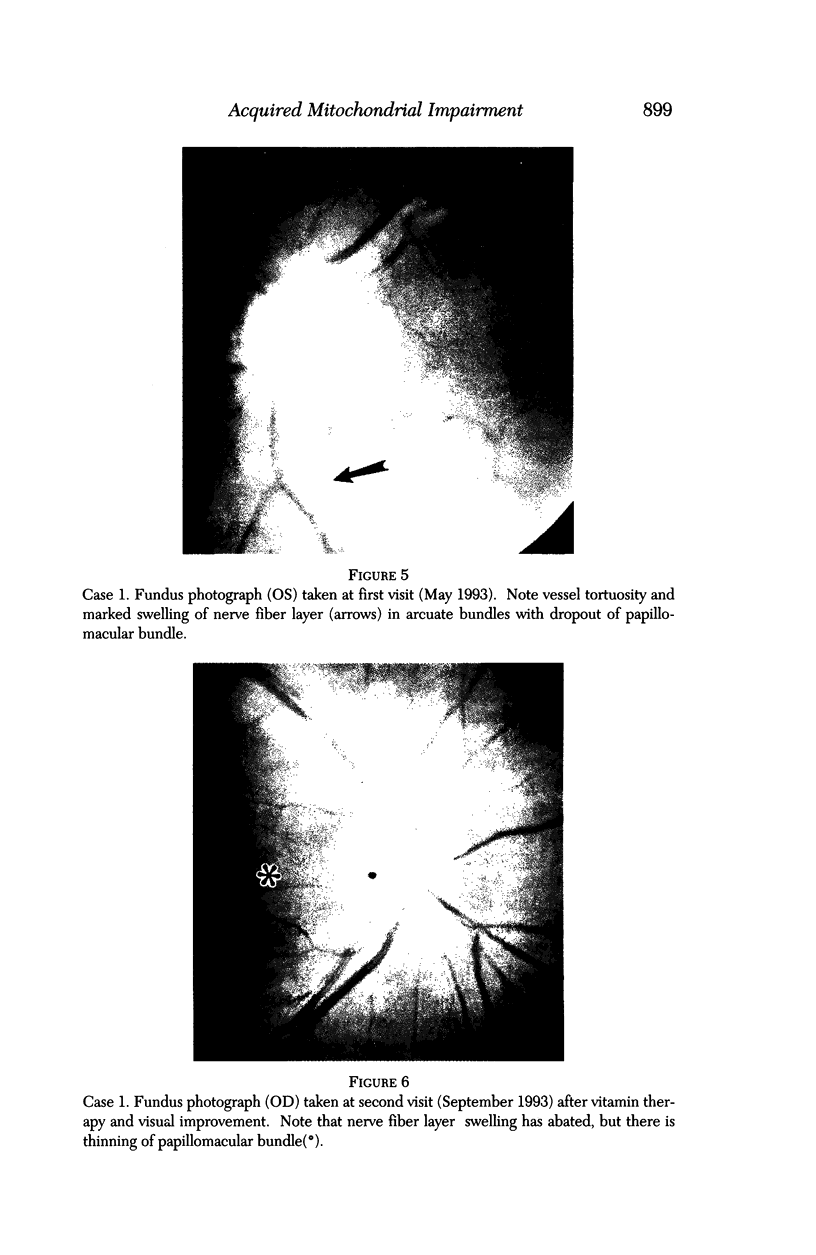
BACKGROUND: Blindness from an optic neuropathy recently occurred as an epidemic affecting 50,000 patients in Cuba (CEON) and had clinical features reminiscent of both tobacco-alcohol amblyopia (TAA) and Leber's hereditary optic neuropathy (Leber's; LHON). Selective damage to the papillomacular bundle was characteristic, and many patients also developed a peripheral neuropathy. Identified risk factors included vitamin deficiencies as well as exposure to methanol and cyanide. In all 3 syndromes, there is evidence that singular or combined insults to mitochondrial oxidative phosphorylation are associated with a clinically characteristic optic neuropathy. PURPOSE: First, to test the hypothesis that a common pathophysiologic mechanism involving impairment of mitochondria function and, consequently, axonal transport underlies both genetic optic nerve diseases such as Leber's and acquired toxic and nutritional deficiency optic neuropathies. According to this hypothesis, ATP depletion below a certain threshold leads to a blockage of orthograde axonal transport of mitochondria, which, in turn, leads to total ATP depletion and subsequent cell death. Second, to address several related questions, including (1) How does impaired energy production lead to optic neuropathy, particularly since it seems to relatively spare other metabolically active tissues, such as liver and heart? (2) Within the nervous system, why is the optic nerve, and most particularly the papillomacular bundle, so highly sensitive? Although there have been previous publications on the clinical features of the Cuban epidemic of blindness, the present hypothesis and the subsequent questions have not been previously addressed. METHODS: Patients in Cuba with epidemic optic neuropathy were personally evaluated through a comprehensive neuro-ophthalmologic examination. In addition, serum, lymphocytes for DNA analysis, cerebrospinal fluid (CSF), sural nerves, and eyes with attached optic nerves were obtained from Cuban patients, as well as from Leber's patients, for study. Finally, we developed an animal model to match the low serum folic acid and high serum formate levels found in the CEON patients, by administering to rats low doses of methanol after several months of a folic acid-deficient diet. Optic nerves and other tissues obtained from these rats were analyzed and compared with those from the Cuban patients. RESULTS: Patients from the Cuban epidemic of optic neuropathy with clinical evidence of a selective loss of the papillomacular bundle did much better once their nutritional status was corrected and exposure to toxins ceased. Patients with CEON often demonstrated low levels of folic acid and high levels of formate in their blood. Histopathologic studies demonstrated losses of the longest fibers (in the sural nerve) and those of smallest caliber (papillomacular bundle) in the optic nerve, with intra-axonal accumulations just anterior to the lamina cribrosa. Our animal model duplicated the serologic changes (low folic acid, high formate) as well as these histopathologic changes. Furthermore, ultrastructural examination of rat tissues demonstrated mitochondrial changes that further matched those seen on ultrastructural examination of tissues from patients with Leber's. CONCLUSION: Mitochondria can be impaired either genetically (as in Leber's) or through acquired insults (such as nutritional or toxic factors). Either may challenge energy production in all cells of the body. While this challenge may be met through certain compensatory mechanisms (such as in the size, shape, or number of the mitochondria), there exists in neurons a threshold which, once passed, leads to catastrophic changes. This threshold may be that point at which mitochondrial derangement leads to such ATP depletion that axonal transport is compromised, and decreased mitochondrial transport results in even further ATP depletion. Neurons are singularly dependent on the axonal transport of mitochondria. (
Full text
PDF

















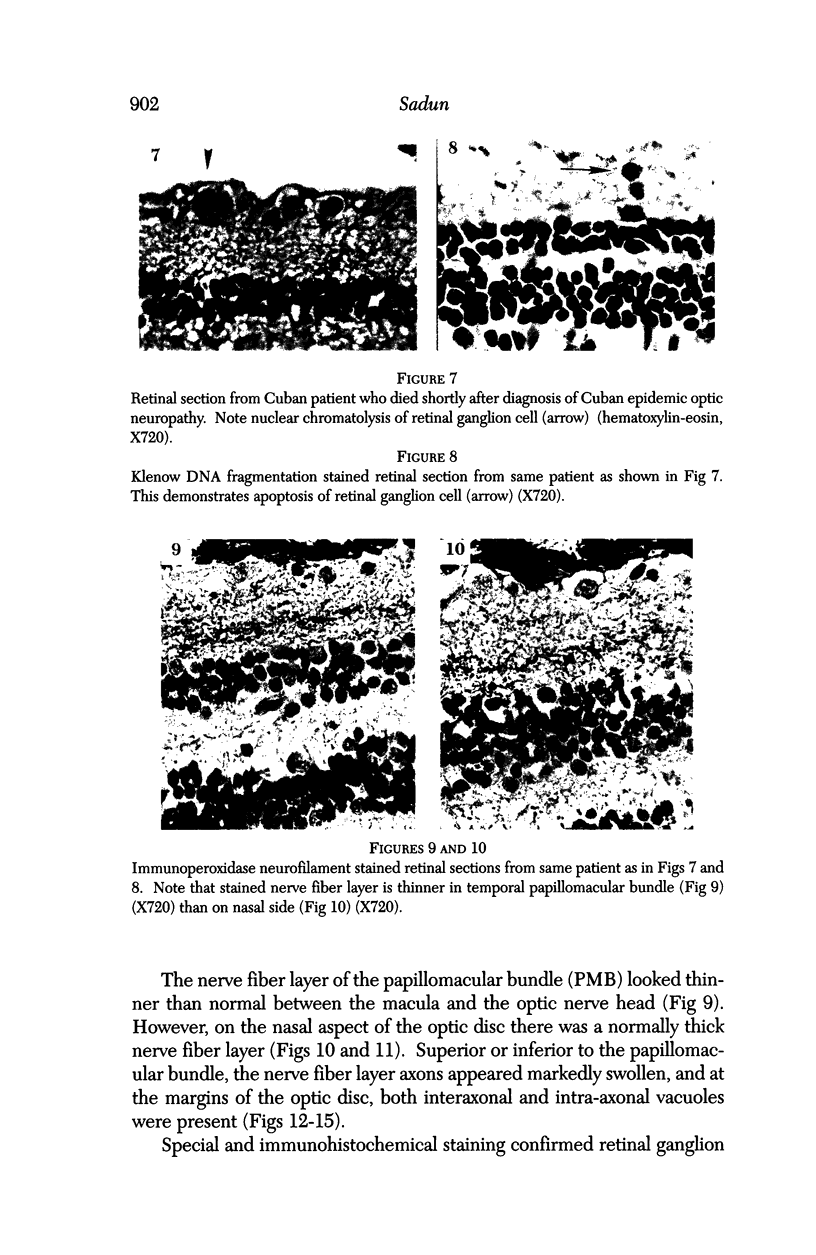
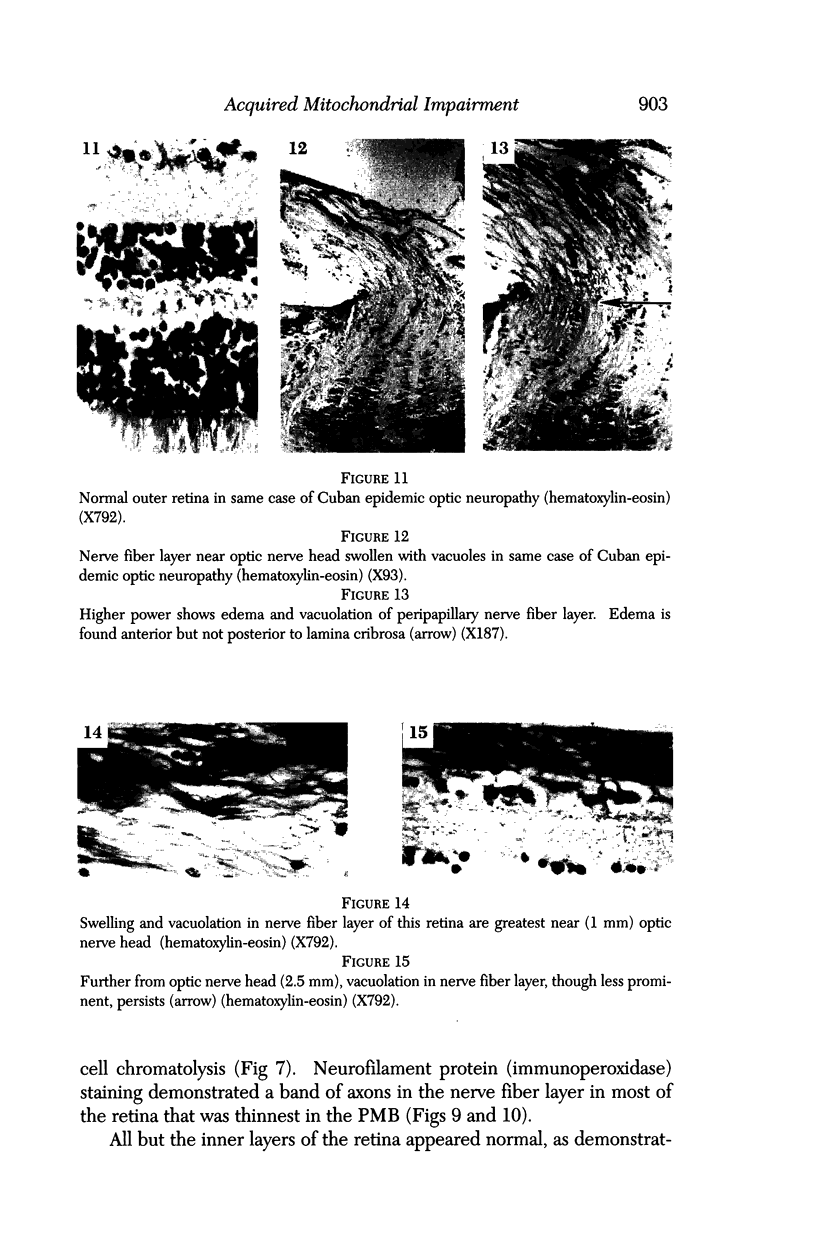

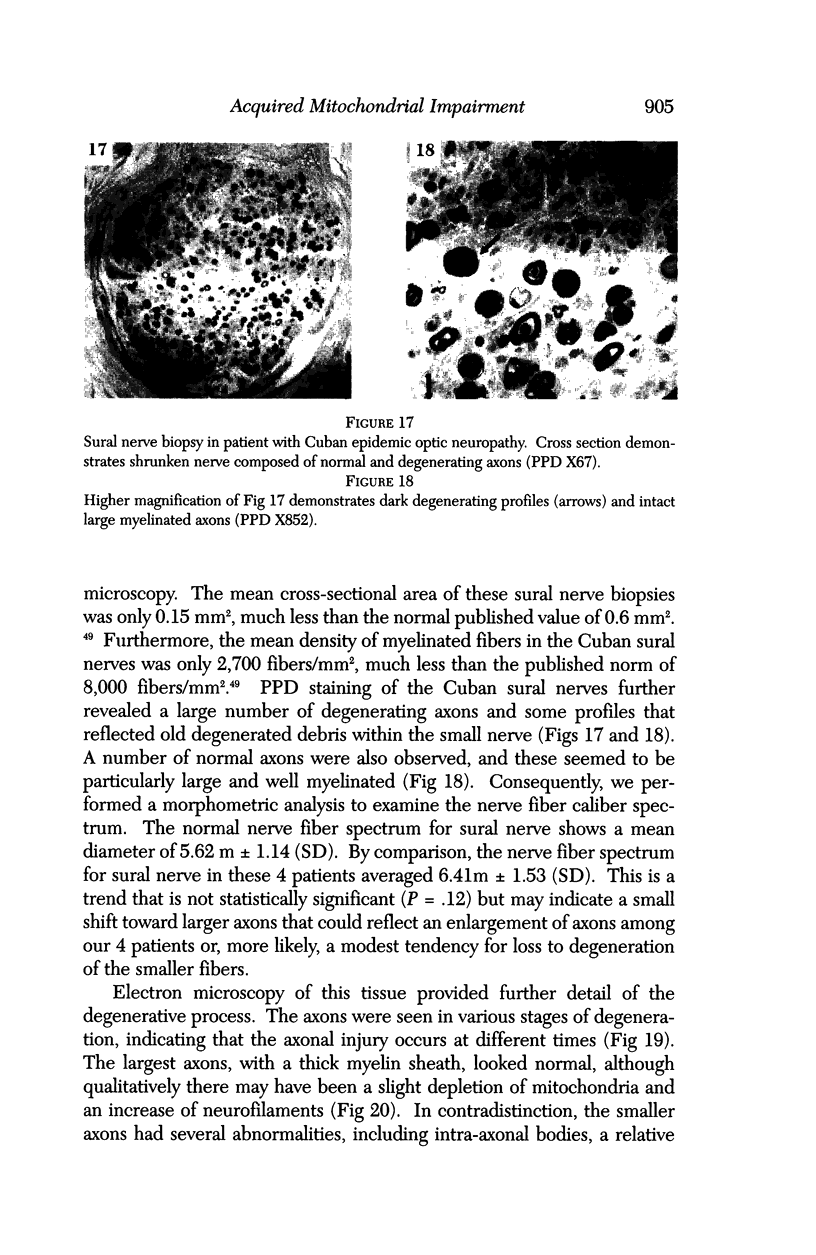
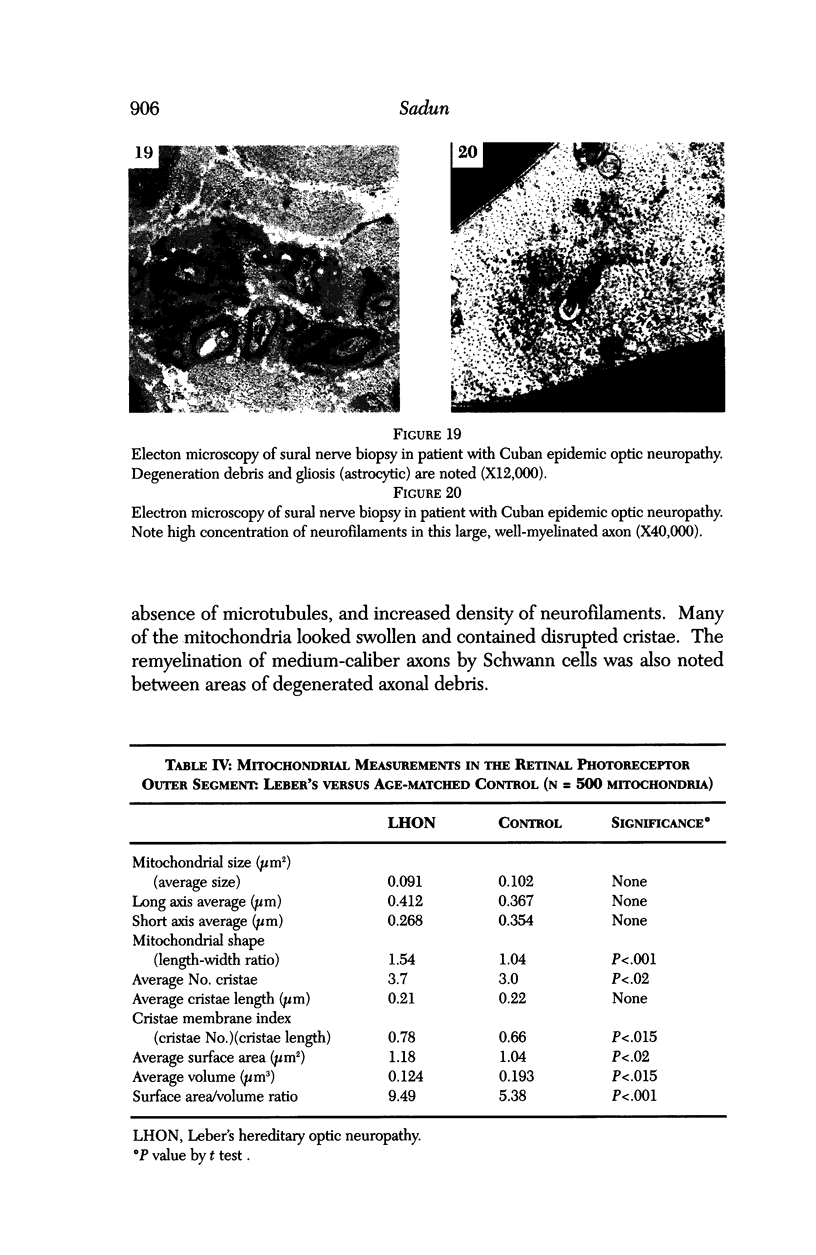



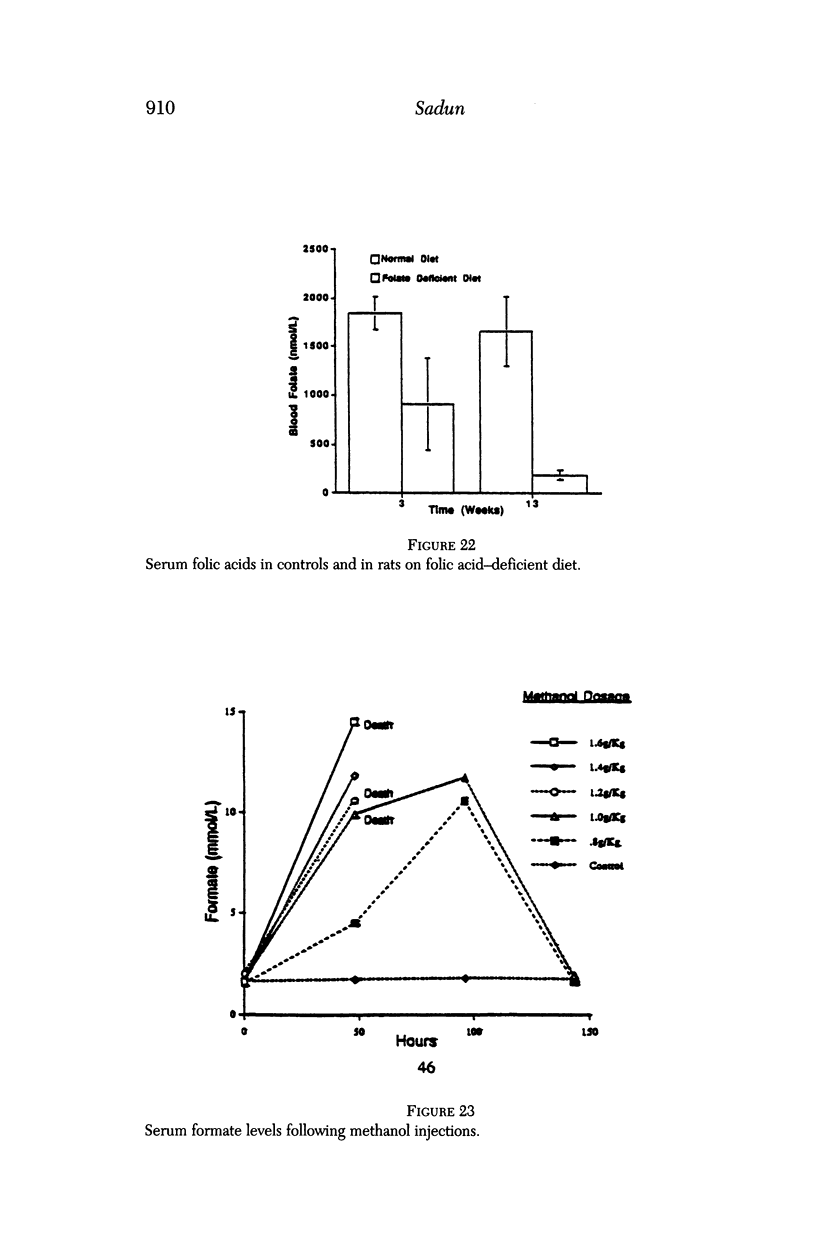

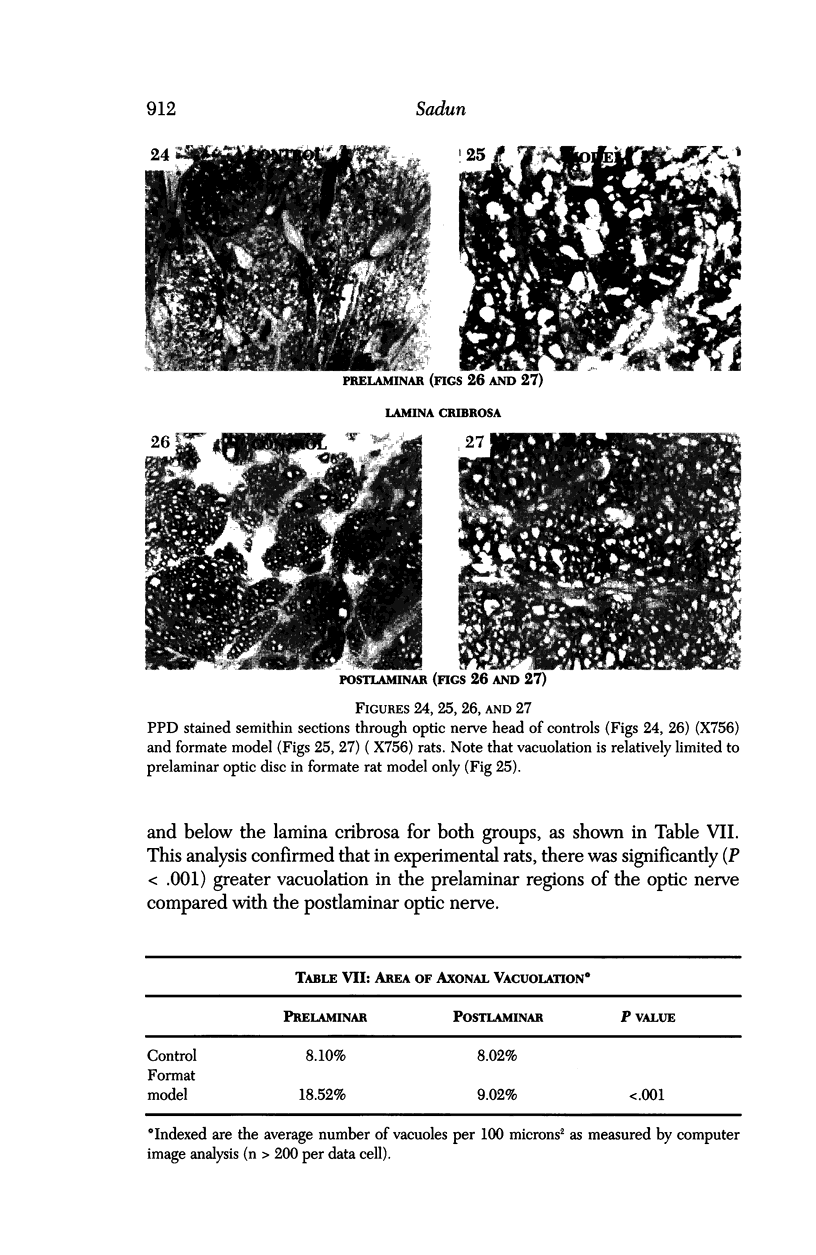
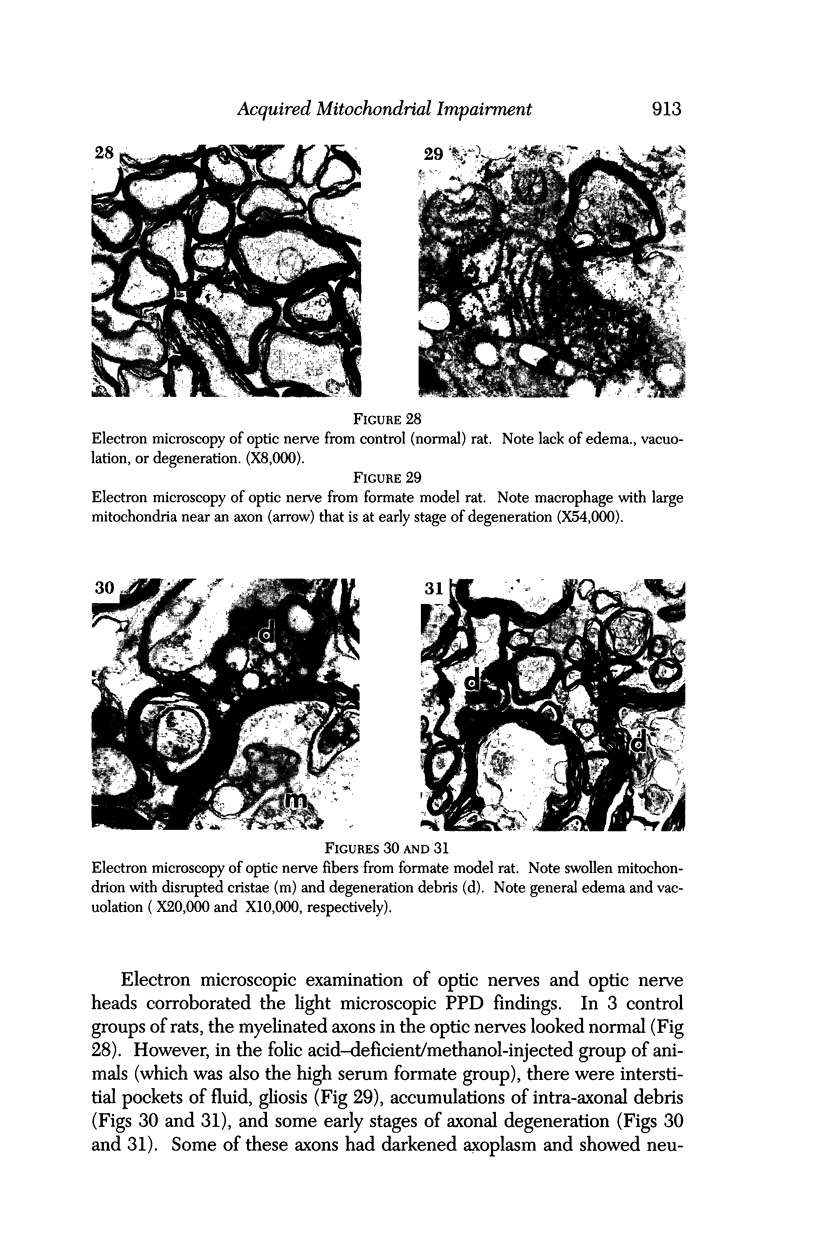





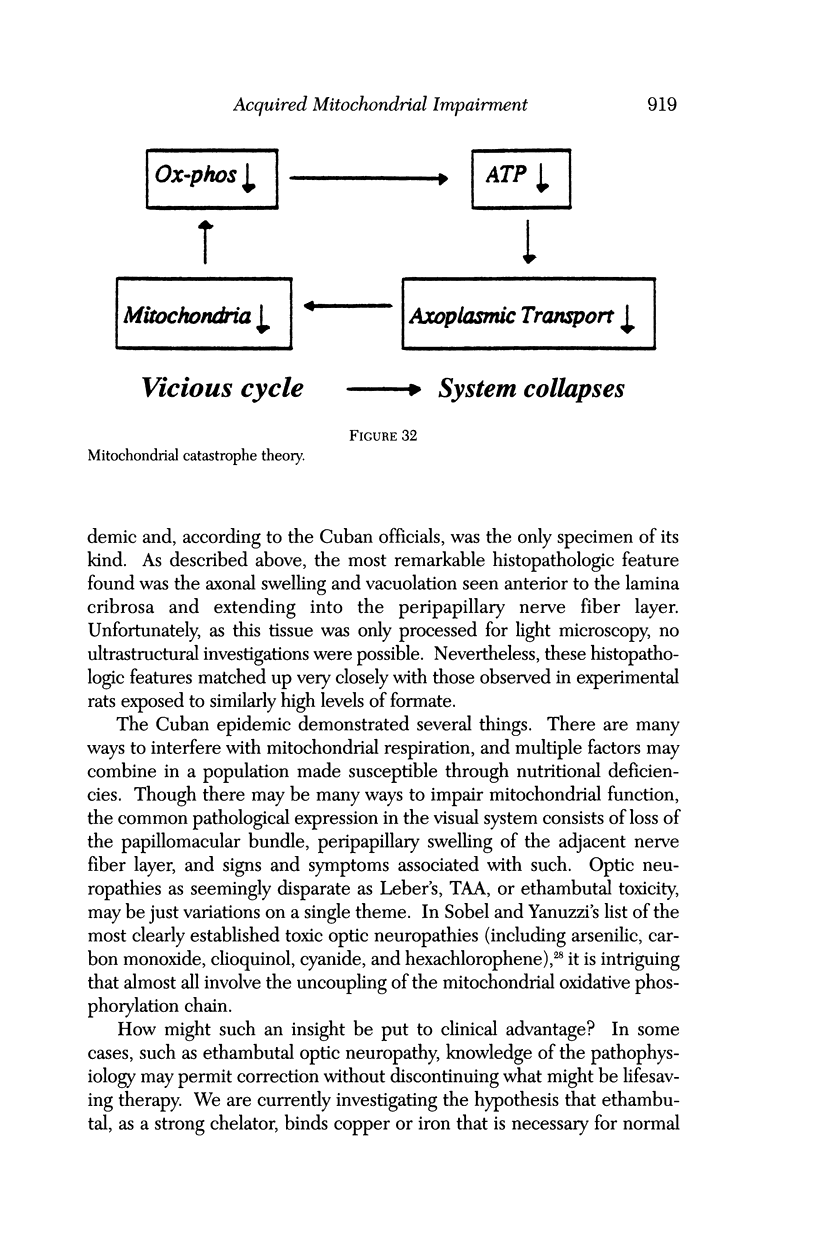




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Li Y. Y., Heher E. C., Kimble C. R. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci. 1992 Mar;12(3):840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENTON C. D., Jr, CALHOUN F. P., Jr The ocular effects of methyl alcohol poisoning: report of a catastrophe involving three hundred and twenty persons. Trans Am Acad Ophthalmol Otolaryngol. 1952 Nov-Dec;56(6):875–885. [PubMed] [Google Scholar]
- Beal M. F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992 Feb;31(2):119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- Borrajero I., Pérez J. L., Domínguez C., Chong A., Coro R. M., Rodríguez H., Gómez N., Román G. C., Navarro-Román L. Epidemic neuropathy in Cuba: morphological characterization of peripheral nerve lesions in sural nerve biopsies. J Neurol Sci. 1994 Dec 1;127(1):68–76. doi: 10.1016/0022-510x(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J. Axonal transport: a cell-biological method for studying proteins that associate with the cytoskeleton. Methods Cell Biol. 1982;25(Pt B):365–398. doi: 10.1016/s0091-679x(08)61434-x. [DOI] [PubMed] [Google Scholar]
- Brady S. Motor-neuron diseases. Interfering with the runners. Nature. 1995 May 4;375(6526):12–13. doi: 10.1038/375012a0. [DOI] [PubMed] [Google Scholar]
- Brontë-Stewart J., Pettigrew A. R., Foulds W. S. Toxic optic neuropathy and its experimental production. Trans Ophthalmol Soc U K. 1976 Sep;96(3):355–358. [PubMed] [Google Scholar]
- Carroll F. D. Jamaican optic neuropathy in immigrants to the United States. Am J Ophthalmol. 1971 Jan;71(1 Pt 2):261–265. doi: 10.1016/0002-9394(71)90398-9. [DOI] [PubMed] [Google Scholar]
- Cohen E. D., Miller R. F. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis Neurosci. 1994 Mar-Apr;11(2):317–332. doi: 10.1017/s0952523800001668. [DOI] [PubMed] [Google Scholar]
- Collard J. F., Côté F., Julien J. P. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995 May 4;375(6526):61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- DeVita E. G., Miao M., Sadun A. A. Optic neuropathy in ethambutol-treated renal tuberculosis. J Clin Neuroophthalmol. 1987 Jun;7(2):77–86. [PubMed] [Google Scholar]
- Deacon R., Perry J., Lumb M., Chanarin I. Formate metabolism in the cobalamin-inactivated rat. Br J Haematol. 1990 Mar;74(3):354–359. doi: 10.1111/j.1365-2141.1990.tb02595.x. [DOI] [PubMed] [Google Scholar]
- Dethlefs R., Naraqi S. Ocular manifestations and complications of acute methyl alcohol intoxication. Med J Aust. 1978 Nov 4;2(10):483–485. doi: 10.5694/j.1326-5377.1978.tb131655.x. [DOI] [PubMed] [Google Scholar]
- Eells J. T., Black K. A., Tedford C. E., Tephly T. R. Methanol toxicity in the monkey: effects of nitrous oxide and methionine. J Pharmacol Exp Ther. 1983 Nov;227(2):349–353. [PubMed] [Google Scholar]
- Eells J. T., Makar A. B., Noker P. E., Tephly T. R. Methanol poisoning and formate oxidation in nitrous oxide-treated rats. J Pharmacol Exp Ther. 1981 Apr;217(1):57–61. [PubMed] [Google Scholar]
- Eells J. T. Methanol-induced visual toxicity in the rat. J Pharmacol Exp Ther. 1991 Apr;257(1):56–63. [PubMed] [Google Scholar]
- Golnik K. C., Schaible E. R. Folate-responsive optic neuropathy. J Neuroophthalmol. 1994 Sep;14(3):163–169. [PubMed] [Google Scholar]
- Jacobs J. M., Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain. 1985 Dec;108(Pt 4):897–924. doi: 10.1093/brain/108.4.897. [DOI] [PubMed] [Google Scholar]
- Jacobsen D., McMartin K. E. Methanol and ethylene glycol poisonings. Mechanism of toxicity, clinical course, diagnosis and treatment. Med Toxicol. 1986 Sep-Oct;1(5):309–334. doi: 10.1007/BF03259846. [DOI] [PubMed] [Google Scholar]
- Jestico J. V., O'Brien M. D., Teoh R., Toseland P. A., Wong H. C. Whole blood cyanide levels in patients with tobacco amblyopia. J Neurol Neurosurg Psychiatry. 1984 Jun;47(6):573–578. doi: 10.1136/jnnp.47.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns D. R., Sadun A. A. Cuban epidemic optic neuropathy. Mitochondrial DNA analysis. J Neuroophthalmol. 1994 Sep;14(3):130–134. [PubMed] [Google Scholar]
- Johns D. R., Smith K. H., Miller N. R., Sulewski M. E., Bias W. B. Identical twins who are discordant for Leber's hereditary optic neuropathy. Arch Ophthalmol. 1993 Nov;111(11):1491–1494. doi: 10.1001/archopht.1993.01090110057023. [DOI] [PubMed] [Google Scholar]
- Johns D. R. The molecular genetics of Leber's hereditary optic neuropathy. Arch Ophthalmol. 1990 Oct;108(10):1405–1407. doi: 10.1001/archopht.1990.01070120053027. [DOI] [PubMed] [Google Scholar]
- Johnson B. M., Sadun A. A. Ultrastructural and paraphenylene studies of degeneration in the primate visual system: degenerative remnants persist for much longer than expected. J Electron Microsc Tech. 1988 Feb;8(2):179–183. doi: 10.1002/jemt.1060080204. [DOI] [PubMed] [Google Scholar]
- Kluck R. M., Bossy-Wetzel E., Green D. R., Newmeyer D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997 Feb 21;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Kozak S. F., Inderlied C. B., Hsu H. Y., Heller K. B., Sadun A. A. The role of copper on ethambutol's antimicrobial action and implications for ethambutol-induced optic neuropathy. Diagn Microbiol Infect Dis. 1998 Feb;30(2):83–87. doi: 10.1016/s0732-8893(97)00217-4. [DOI] [PubMed] [Google Scholar]
- Kroemer G. Mitochondrial implication in apoptosis. Towards an endosymbiont hypothesis of apoptosis evolution. Cell Death Differ. 1997 Aug;4(6):443–456. doi: 10.1038/sj.cdd.4400266. [DOI] [PubMed] [Google Scholar]
- Lee E. W., Garner C. D., Terzo T. S. Animal model for the study of methanol toxicity: comparison of folate-reduced rat responses with published monkey data. J Toxicol Environ Health. 1994 Jan;41(1):71–82. doi: 10.1080/15287399409531827. [DOI] [PubMed] [Google Scholar]
- Lincoff N. S., Odel J. G., Hirano M. 'Outbreak' of optic and peripheral neuropathy in Cuba? JAMA. 1993 Jul 28;270(4):511–518. [PubMed] [Google Scholar]
- Makar A. B., Tephly T. R. Improved estimation of formate in body fluids and tissues. Clin Chem. 1982 Feb;28(2):385–385. [PubMed] [Google Scholar]
- Martin-Amat G., Tephly T. R., McMartin K. E., Makar A. B., Hayreh M. S., Hayreh S. S., Baumbach G., Cancilla P. Methyl alcohol poisoning. II. Development of a model for ocular toxicity in methyl alcohol poisoning using the rhesus monkey. Arch Ophthalmol. 1977 Oct;95(10):1847–1850. doi: 10.1001/archopht.1977.04450100149021. [DOI] [PubMed] [Google Scholar]
- McMartin K. E., Ambre J. J., Tephly T. R. Methanol poisoning in human subjects. Role for formic acid accumulation in the metabolic acidosis. Am J Med. 1980 Mar;68(3):414–418. doi: 10.1016/0002-9343(80)90113-8. [DOI] [PubMed] [Google Scholar]
- McMartin K. E., Martin-Amat G., Makar A. B., Tephly T. R. Methanol poisoning. V. Role of formate metabolism in the monkey. J Pharmacol Exp Ther. 1977 Jun;201(3):564–572. [PubMed] [Google Scholar]
- Murray T. G., Burton T. C., Rajani C., Lewandowski M. F., Burke J. M., Eells J. T. Methanol poisoning. A rodent model with structural and functional evidence for retinal involvement. Arch Ophthalmol. 1991 Jul;109(7):1012–1016. doi: 10.1001/archopht.1991.01080070124049. [DOI] [PubMed] [Google Scholar]
- Newman N. J., Torroni A., Brown M. D., Lott M. T., Fernandez M. M., Wallace D. C. Epidemic neuropathy in Cuba not associated with mitochondrial DNA mutations found in Leber's hereditary optic neuropathy patients. Cuba Neuropathy Field Investigation Team. Am J Ophthalmol. 1994 Aug 15;118(2):158–168. doi: 10.1016/s0002-9394(14)72895-8. [DOI] [PubMed] [Google Scholar]
- Newman N. J., Wallace D. C. Mitochondria and Leber's hereditary optic neuropathy. Am J Ophthalmol. 1990 Jun 15;109(6):726–730. doi: 10.1016/s0002-9394(14)72445-6. [DOI] [PubMed] [Google Scholar]
- Nicholls P. Formate as an inhibitor of cytochrome c oxidase. Biochem Biophys Res Commun. 1975 Nov 17;67(2):610–616. doi: 10.1016/0006-291x(75)90856-6. [DOI] [PubMed] [Google Scholar]
- Nicholls P. The effect of formate on cytochrome aa3 and on electron transport in the intact respiratory chain. Biochim Biophys Acta. 1976 Apr 9;430(1):13–29. doi: 10.1016/0005-2728(76)90218-8. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen E., Hoyt W. F., Nummelin K., Schatz H. Fundus findings in Leber's hereditary optic neuroretinopathy. III. Fluorescein angiographic studies. Arch Ophthalmol. 1984 Jul;102(7):981–989. doi: 10.1001/archopht.1984.01040030783017. [DOI] [PubMed] [Google Scholar]
- POTTS A. M. The visual toxicity of methanol. VI. The clinical aspects of experimental methanol poisoning treated with base. Am J Ophthalmol. 1955 Feb;39(2 Pt 2):86–92. [PubMed] [Google Scholar]
- Radius R. L., Anderson D. R. Fast axonal transport in early experimental disc edema. Invest Ophthalmol Vis Sci. 1980 Feb;19(2):158–168. [PubMed] [Google Scholar]
- Rizzo J. F., 3rd Adenosine triphosphate deficiency: a genre of optic neuropathy. Neurology. 1995 Jan;45(1):11–16. doi: 10.1212/wnl.45.1.11. [DOI] [PubMed] [Google Scholar]
- Sadun A. A., Martone J. F. Cuba: response of medical science to a crisis of optic and peripheral neuropathy. Int Ophthalmol. 1994;18(6):373–378. doi: 10.1007/BF00930318. [DOI] [PubMed] [Google Scholar]
- Sadun A. A., Martone J. F., Muci-Mendoza R., Reyes L., DuBois L., Silva J. C., Roman G., Caballero B. Epidemic optic neuropathy in Cuba. Eye findings. Arch Ophthalmol. 1994 May;112(5):691–699. doi: 10.1001/archopht.1994.01090170139037. [DOI] [PubMed] [Google Scholar]
- Sadun A. A., Martone J. F., Reyes L., Du Bois L., Roman G. C., Caballero B. Optic and peripheral neuropathy in Cuba. JAMA. 1994 Mar 2;271(9):663–664. [PubMed] [Google Scholar]
- Sadun A. A., Smith L. E., Kenyon K. R. Paraphenylenediamine: a new method for tracing human visual pathways. J Neuropathol Exp Neurol. 1983 Mar;42(2):200–206. [PubMed] [Google Scholar]
- Schumer R. A., Podos S. M. The nerve of glaucoma! Arch Ophthalmol. 1994 Jan;112(1):37–44. doi: 10.1001/archopht.1994.01090130047015. [DOI] [PubMed] [Google Scholar]
- Sejersted O. M., Jacobsen D., Ovrebø S., Jansen H. Formate concentrations in plasma from patients poisoned with methanol. Acta Med Scand. 1983;213(2):105–110. doi: 10.1111/j.0954-6820.1983.tb03699.x. [DOI] [PubMed] [Google Scholar]
- Torroni A., Brown M. D., Lott M. T., Newman N. J., Wallace D. C. African, Native American, and European mitochondrial DNAs in Cubans from Pinar del Rio Province and implications for the recent epidemic neuropathy in Cuba. Cuba Neuropathy Field Investigation Team. Hum Mutat. 1995;5(4):310–317. doi: 10.1002/humu.1380050407. [DOI] [PubMed] [Google Scholar]
- Tso M. O., Hayreh S. S. Optic disc edema in raised intracranial pressure. III. A pathologic study of experimental papilledema. Arch Ophthalmol. 1977 Aug;95(8):1448–1457. doi: 10.1001/archopht.1977.04450080158022. [DOI] [PubMed] [Google Scholar]
- Wall M., Sadun A. A. Threshold Amsler grid testing. Cross-polarizing lenses enhance yield. Arch Ophthalmol. 1986 Apr;104(4):520–523. doi: 10.1001/archopht.1986.01050160076015. [DOI] [PubMed] [Google Scholar]
- Yang J., Liu X., Bhalla K., Kim C. N., Ibrado A. M., Cai J., Peng T. I., Jones D. P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997 Feb 21;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Zeevalk G. D., Nicklas W. J. Mechanisms underlying initiation of excitotoxicity associated with metabolic inhibition. J Pharmacol Exp Ther. 1991 May;257(2):870–878. [PubMed] [Google Scholar]