Abstract
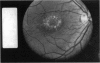
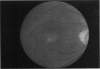
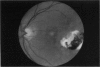
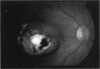
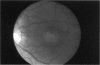
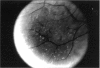
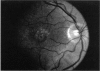
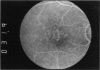
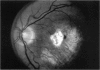
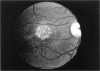
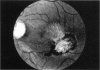
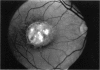
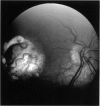
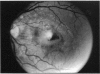
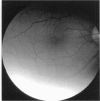
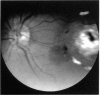
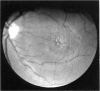
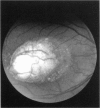
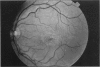
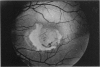
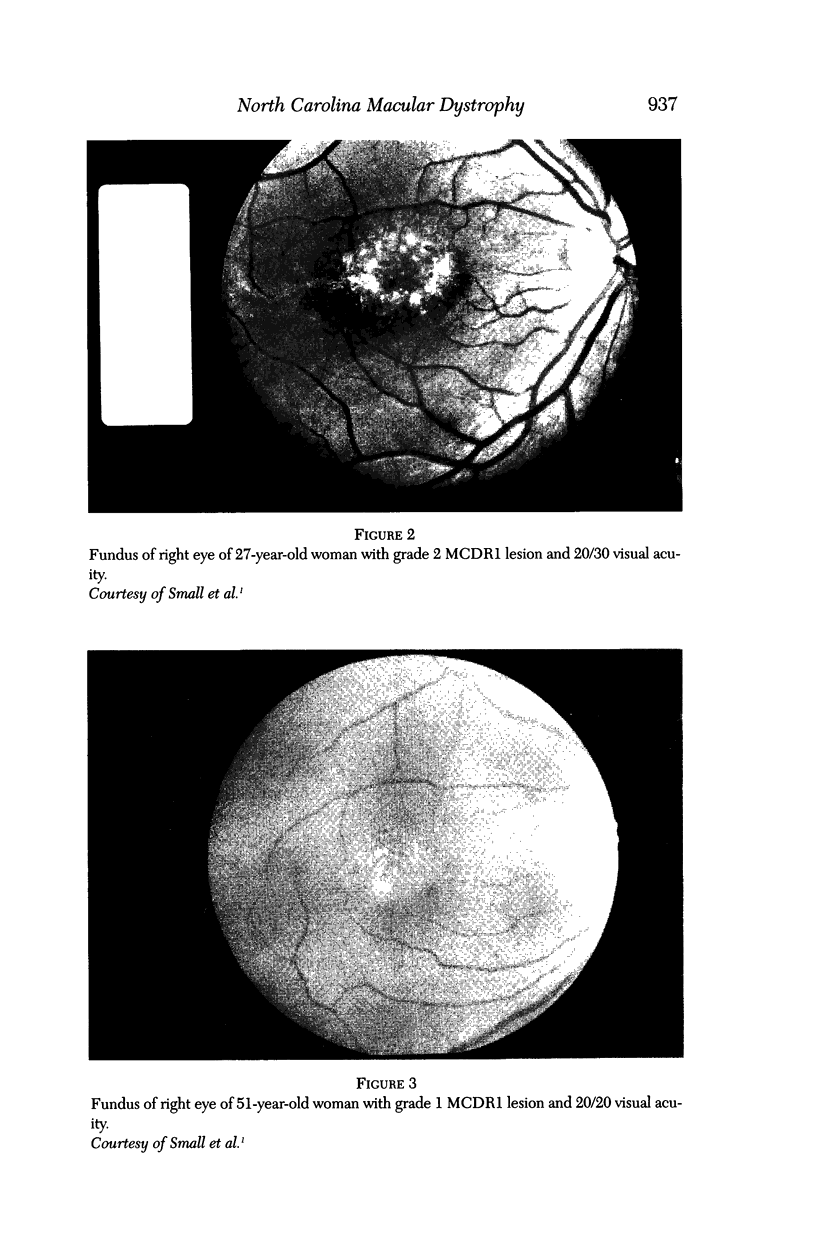
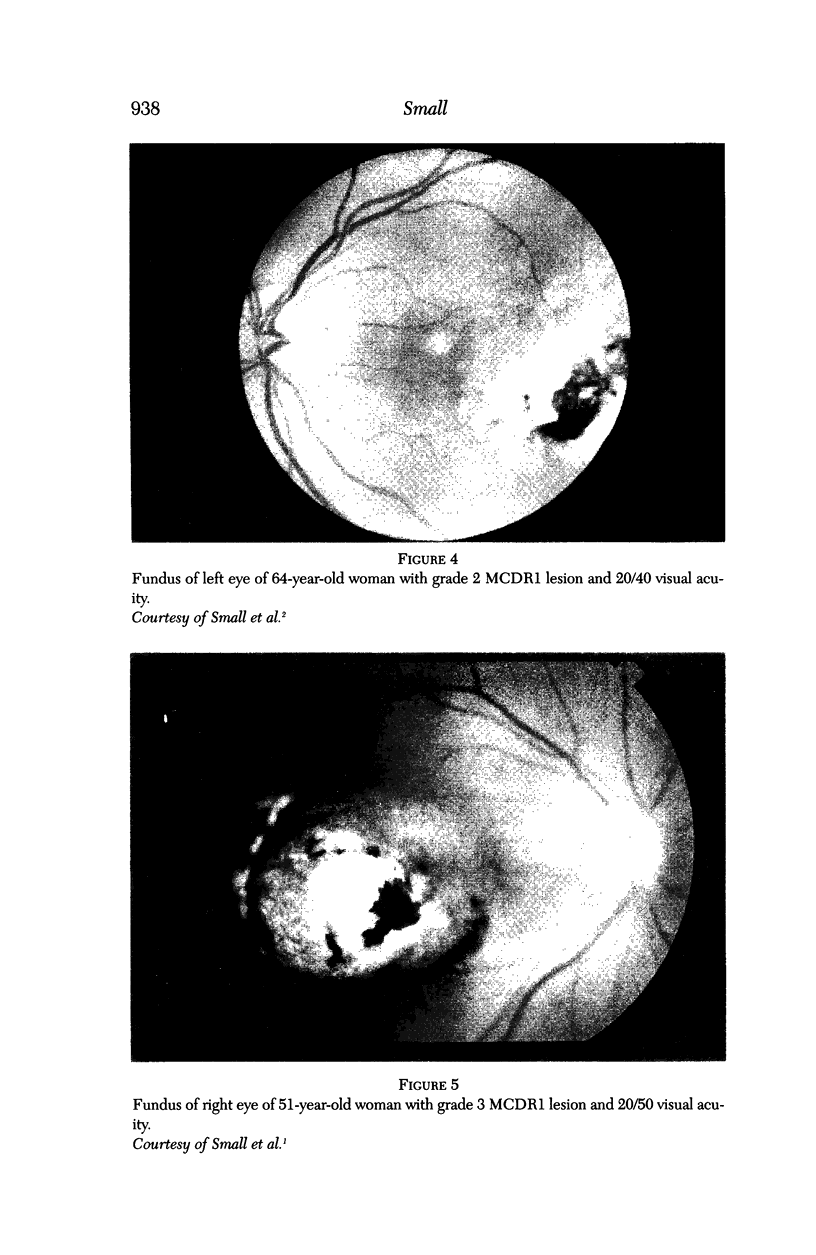
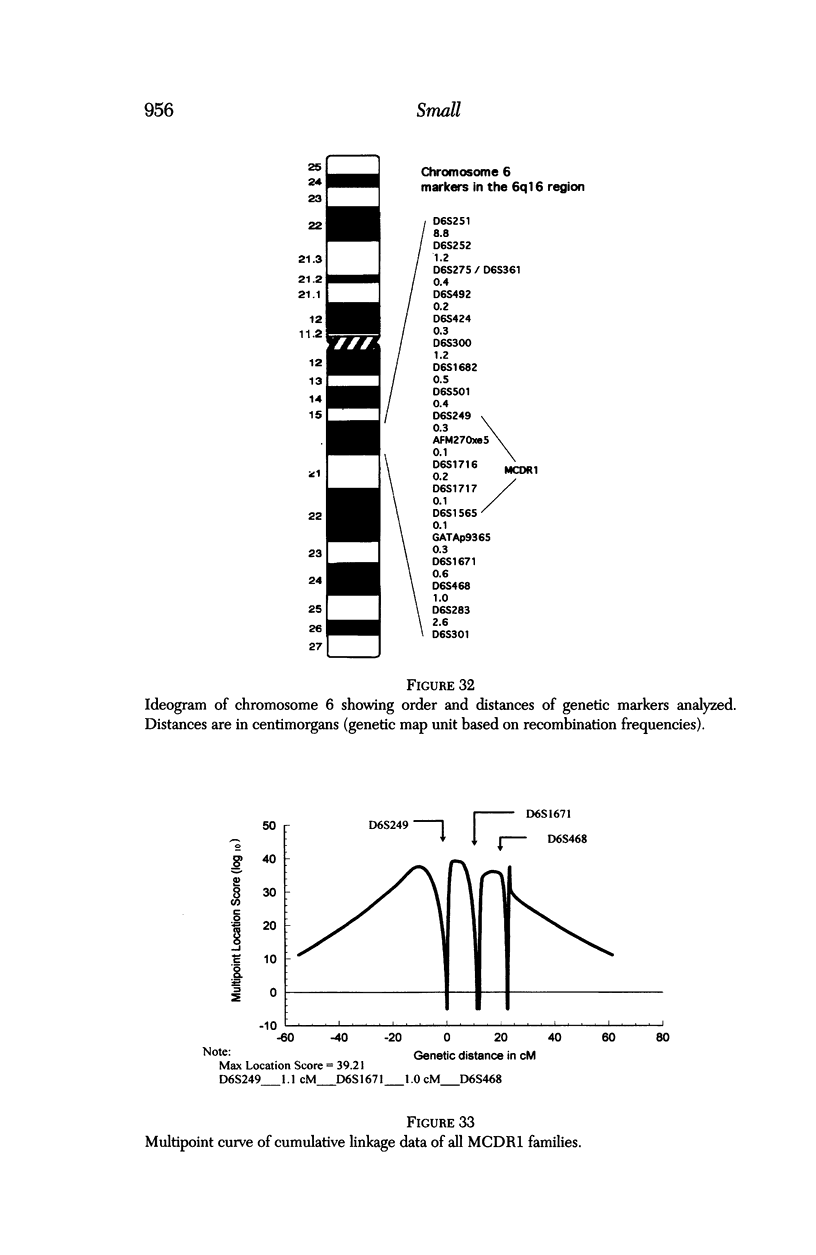
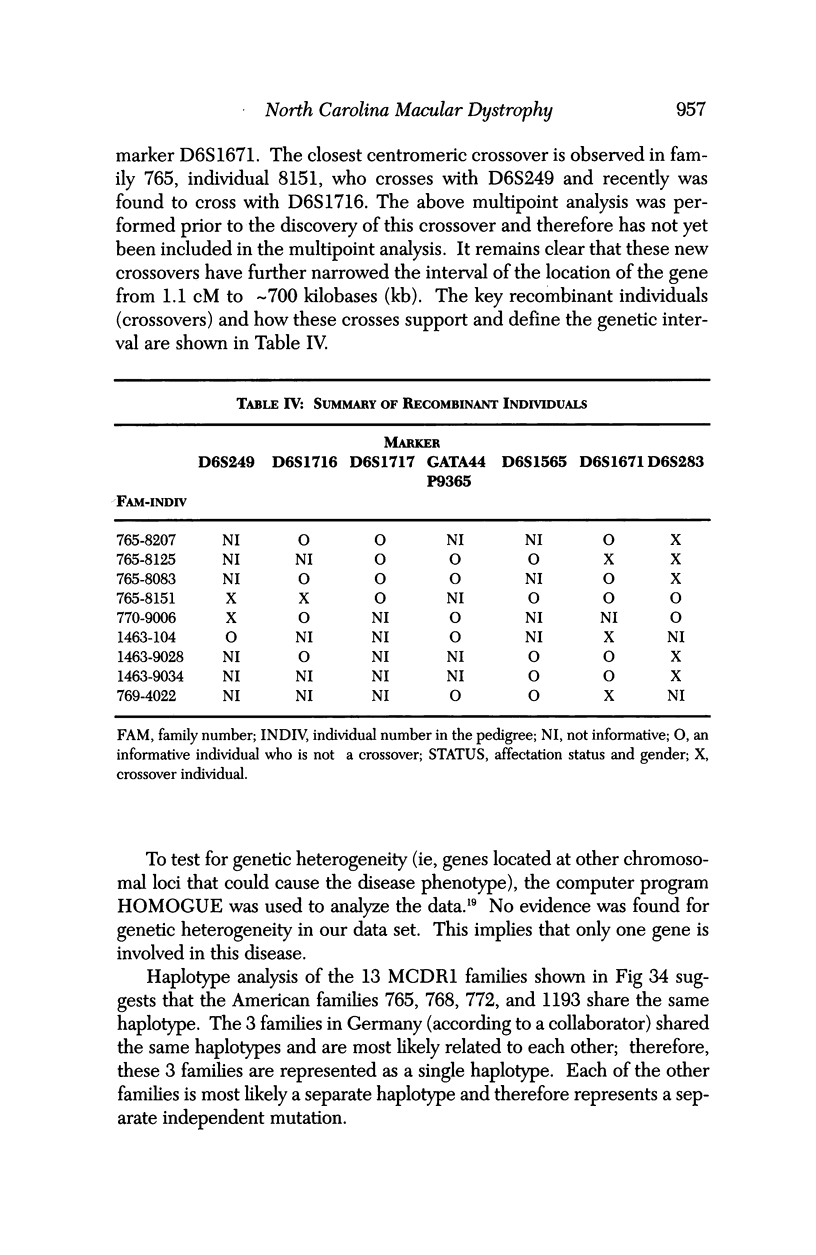
PURPOSE: To study the North Carolina macular dystrophy phenotype (MCDR1) in multiple families of different ethnic backgrounds, to determine the genetic relationships of these families, and to determine the minimal candidate region of the MCDR1 gene. METHODS: Thirteen families with the North Carolina MCDR1 were ascertained. These families were of various ethnic and geographic origins, such as Caucasian, Mayan Indian, African American, French, British, German, and American. Extensive genealogical investigations were performed for all families. A total of 232 members of these families underwent comprehensive ophthalmic examinations, including blood collection for genotyping. Of these, 117 were found to be affected with the disorder. Genetic linkage simulation studies were performed using the computer program SIMLINK. Two-point linkage analysis, haplotype analysis, and multipoint linkage analyses were performed using the computer programs M-LINK, VITESSE, and FASTLINK. RESULTS: The clinical features were consistent with the diagnosis of North Carolina macular dystrophy in all families studied. Multipoint linkage analysis and haplotype analysis indicate that the MCDR1 gene is in the 1.1-centimorgan (cM) interval between the genetic markers D6D249 and D6S1671, with a maximum LOD score of 40.03. There was no evidence of genetic heterogeneity. Families 765, 768, 772, 1193, and 1292 shared the same chromosomal haplotype in this region, suggesting they are the result of the same ancestral mutation. The remaining families each likely represent independent origins of the mutation in the MCDR1 gene. North Carolina macular dystrophy is present worldwide and does not emanate from a single founder from North Carolina.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dausset J., Cann H., Cohen D., Lathrop M., Lalouel J. M., White R. Centre d'etude du polymorphisme humain (CEPH): collaborative genetic mapping of the human genome. Genomics. 1990 Mar;6(3):575–577. doi: 10.1016/0888-7543(90)90491-c. [DOI] [PubMed] [Google Scholar]
- Fetkenhour C. L., Gurney N., Dobbie J. G., Choromokos E. Central areolar pigment epithelial dystrophy. Am J Ophthalmol. 1976 Jun;81(6):745–753. doi: 10.1016/0002-9394(76)90357-3. [DOI] [PubMed] [Google Scholar]
- Frank H. R., Landers M. B., 3rd, Williams R. J., Sidbury J. B. A new dominant progressive foveal dystrophy. Am J Ophthalmol. 1974 Dec;78(6):903–916. doi: 10.1016/0002-9394(74)90800-9. [DOI] [PubMed] [Google Scholar]
- Hermsen V. M., Judisch G. F. Central areolar pigment epithelial dystrophy. Ophthalmologica. 1984;189(1-2):69–72. doi: 10.1159/000309388. [DOI] [PubMed] [Google Scholar]
- Klein R., Bresnick G. An inherited central retinal pigment epithelial dystrophy. Birth Defects Orig Artic Ser. 1982;18(6):281–296. [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefler W. H., Wadsworth J. A., Sidbury J. B., Jr Hereditary macular degeneration and amino-aciduria. Am J Ophthalmol. 1971 Jan;71(1 Pt 2):224–230. doi: 10.1016/0002-9394(71)90394-1. [DOI] [PubMed] [Google Scholar]
- Leveille A. S., Morse P. H., Kiernan J. P. Autosomal dominant central pigment epithelial and choroidal degeneration. Ophthalmology. 1982 Dec;89(12):1407–1413. doi: 10.1016/s0161-6420(82)34621-7. [DOI] [PubMed] [Google Scholar]
- O'Connell J. R., Weeks D. E. The VITESSE algorithm for rapid exact multilocus linkage analysis via genotype set-recoding and fuzzy inheritance. Nat Genet. 1995 Dec;11(4):402–408. doi: 10.1038/ng1295-402. [DOI] [PubMed] [Google Scholar]
- Ott J. Computer-simulation methods in human linkage analysis. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4175–4178. doi: 10.1073/pnas.86.11.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauleikhoff D., Sauer C. G., Müller C. R., Radermacher M., Merz A., Weber B. H. Clinical and genetic evidence for autosomal dominant North Carolina macular dystrophy in a German family. Am J Ophthalmol. 1997 Sep;124(3):412–415. doi: 10.1016/s0002-9394(14)70842-6. [DOI] [PubMed] [Google Scholar]
- Sandberg M. A., Jacobson S. G., Berson E. L. Foveal cone electroretinograms in retinitis pigmentosa and juvenile maular degeneration. Am J Ophthalmol. 1979 Oct;88(4):702–707. doi: 10.1016/0002-9394(79)90669-x. [DOI] [PubMed] [Google Scholar]
- Small K. W., Hermsen V., Gurney N., Fetkenhour C. L., Folk J. C. North Carolina macular dystrophy and central areolar pigment epithelial dystrophy. One family, one disease. Arch Ophthalmol. 1992 Apr;110(4):515–518. doi: 10.1001/archopht.1992.01080160093040. [DOI] [PubMed] [Google Scholar]
- Small K. W., Killian J., McLean W. C. North Carolina's dominant progressive foveal dystrophy: how progressive is it? Br J Ophthalmol. 1991 Jul;75(7):401–406. doi: 10.1136/bjo.75.7.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small K. W. North Carolina macular dystrophy, revisited. Ophthalmology. 1989 Dec;96(12):1747–1754. doi: 10.1016/s0161-6420(89)32655-8. [DOI] [PubMed] [Google Scholar]
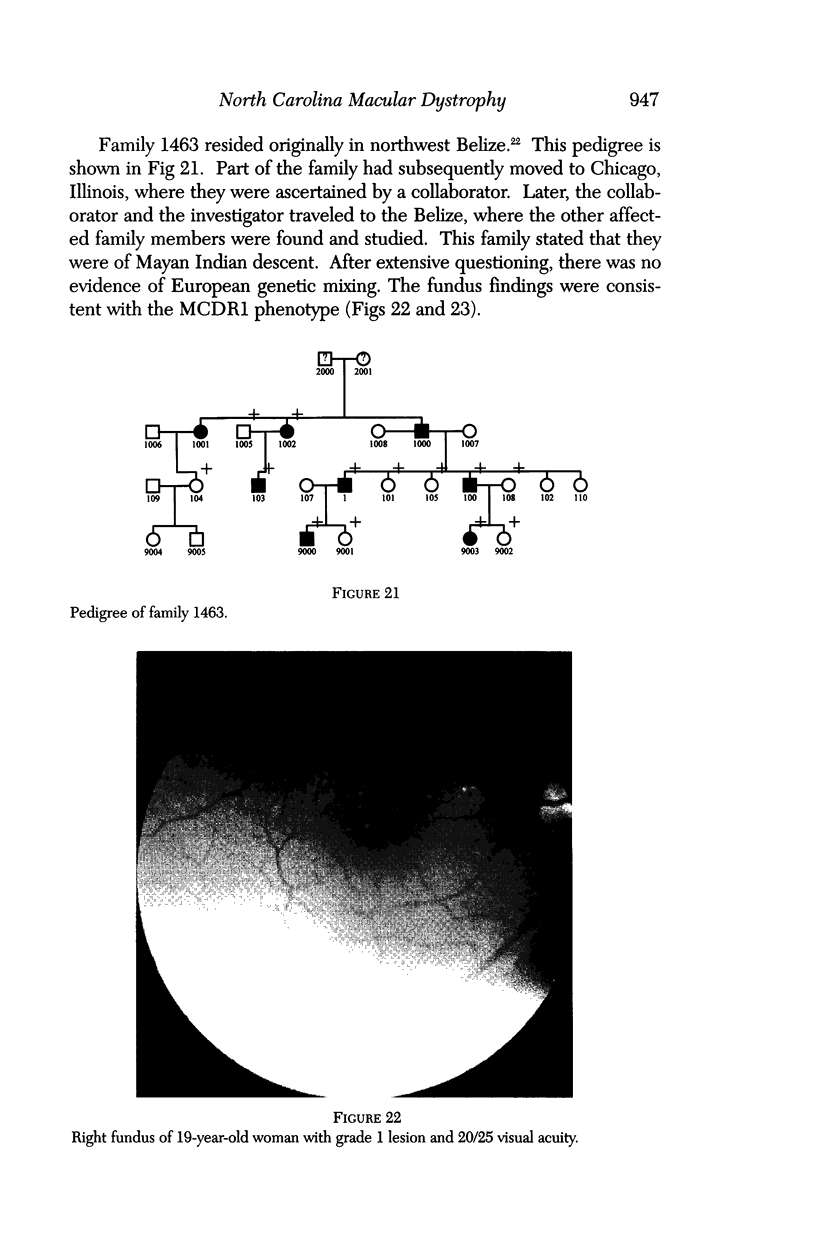
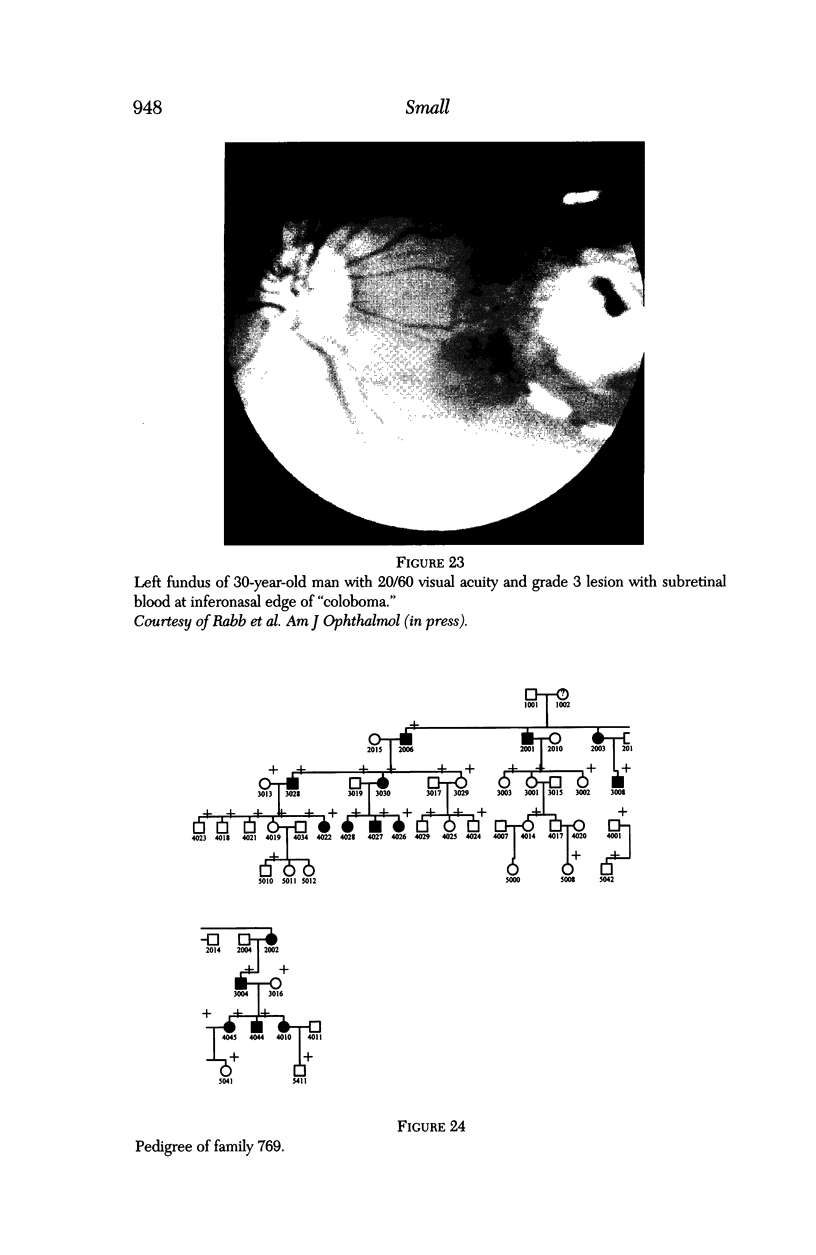
- Small K. W., Weber J. L., Hung W. Y., Vance J., Roses A., Pericak-Vance M. North Carolina macular dystrophy: exclusion map using RFLPs and microsatellites. Genomics. 1991 Nov;11(3):763–766. doi: 10.1016/0888-7543(91)90087-u. [DOI] [PubMed] [Google Scholar]
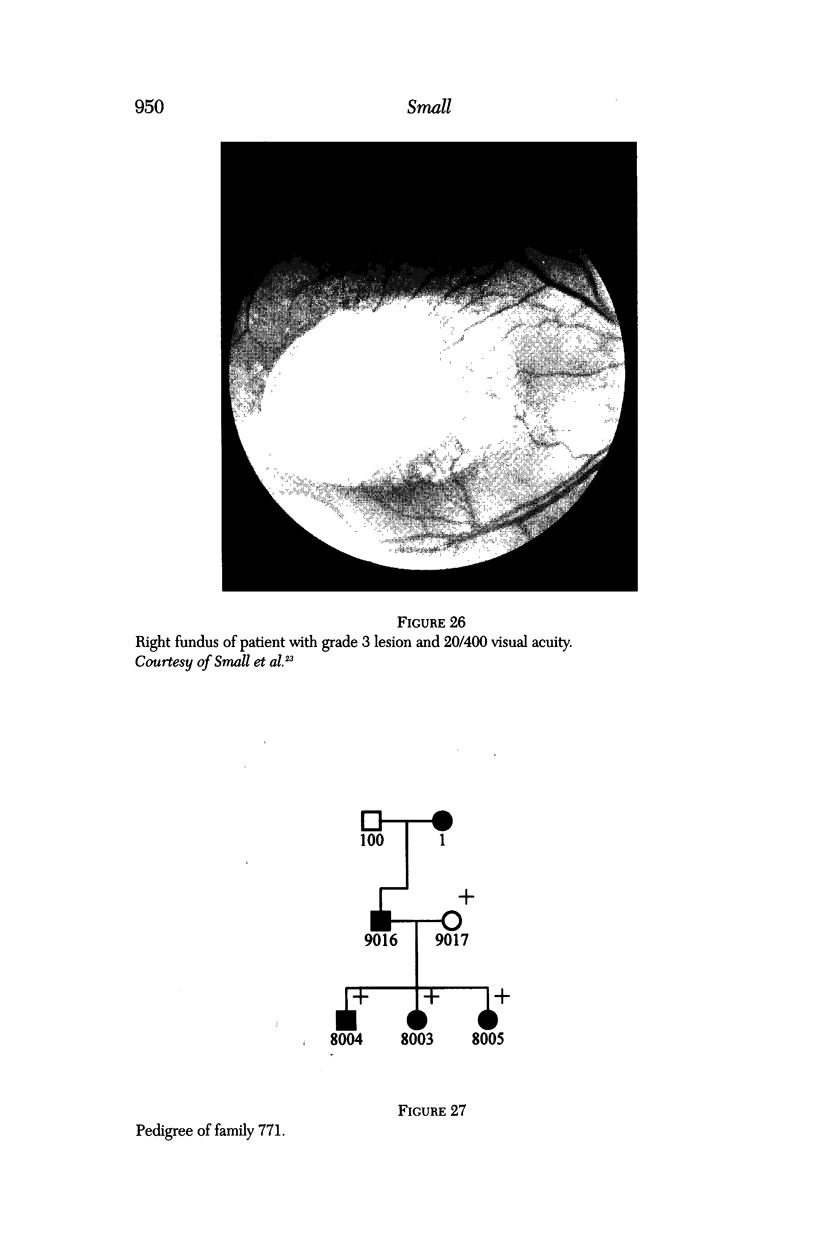
- Small K. W., Weber J. L., Roses A., Lennon F., Vance J. M., Pericak-Vance M. A. North Carolina macular dystrophy is assigned to chromosome 6. Genomics. 1992 Jul;13(3):681–685. doi: 10.1016/0888-7543(92)90141-e. [DOI] [PubMed] [Google Scholar]
- Small K. W., Weber J., Roses A., Pericak-Vance P. North Carolina macular dystrophy (MCDR1). A review and refined mapping to 6q14-q16.2. Ophthalmic Paediatr Genet. 1993 Dec;14(4):143–150. doi: 10.3109/13816819309042913. [DOI] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]