Abstract
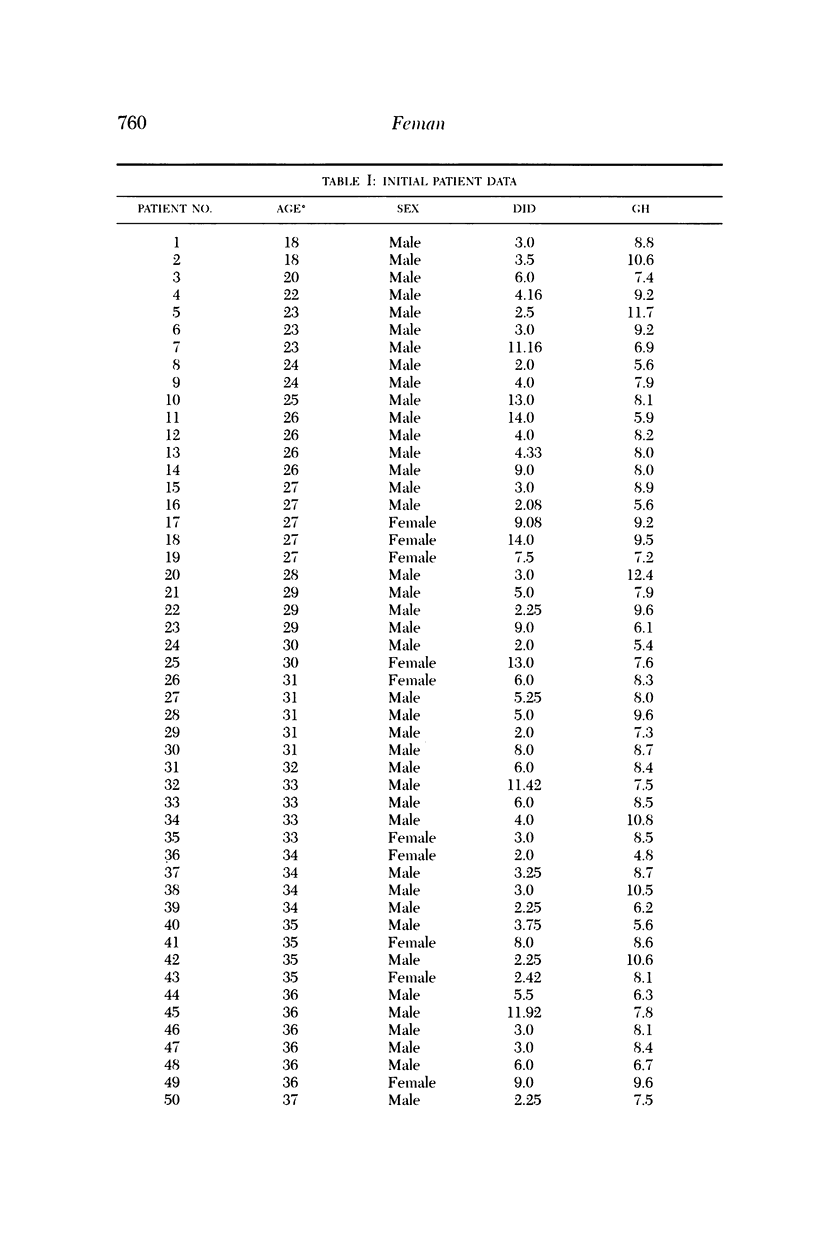
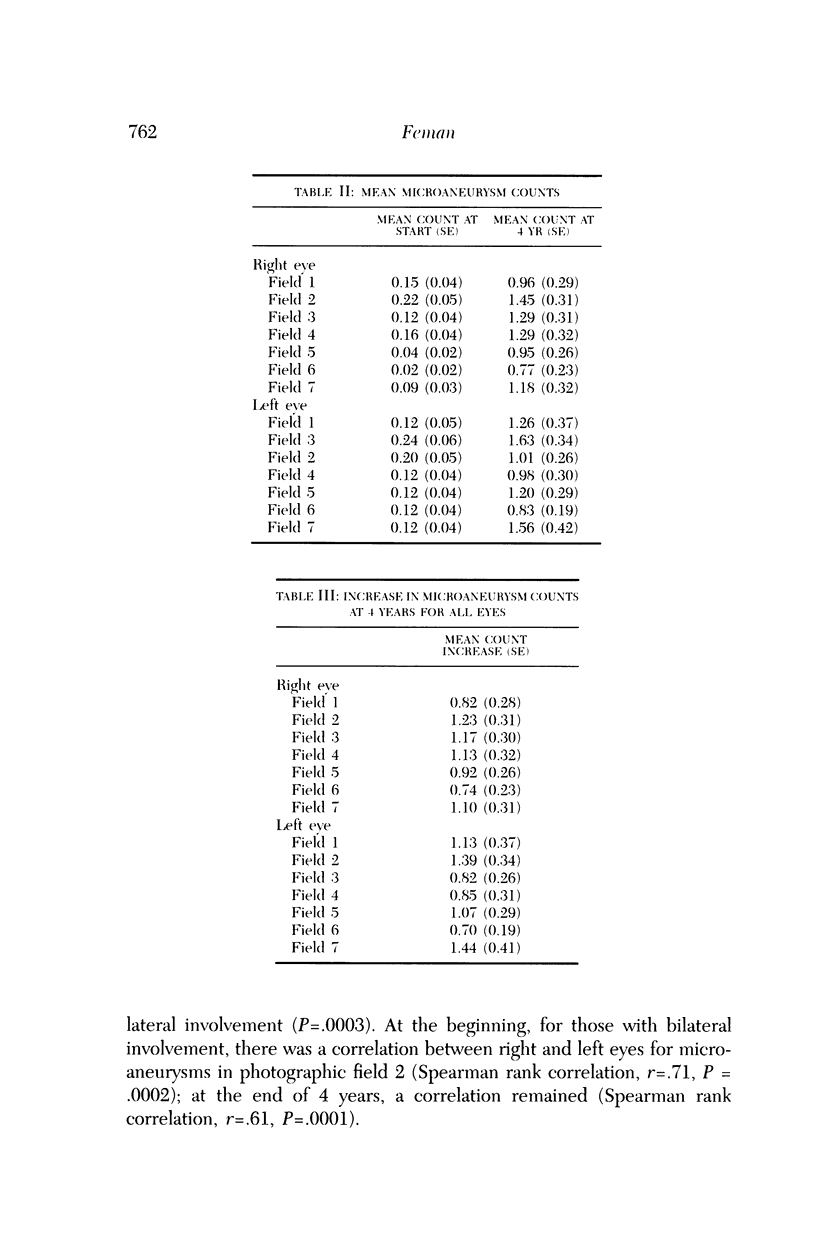
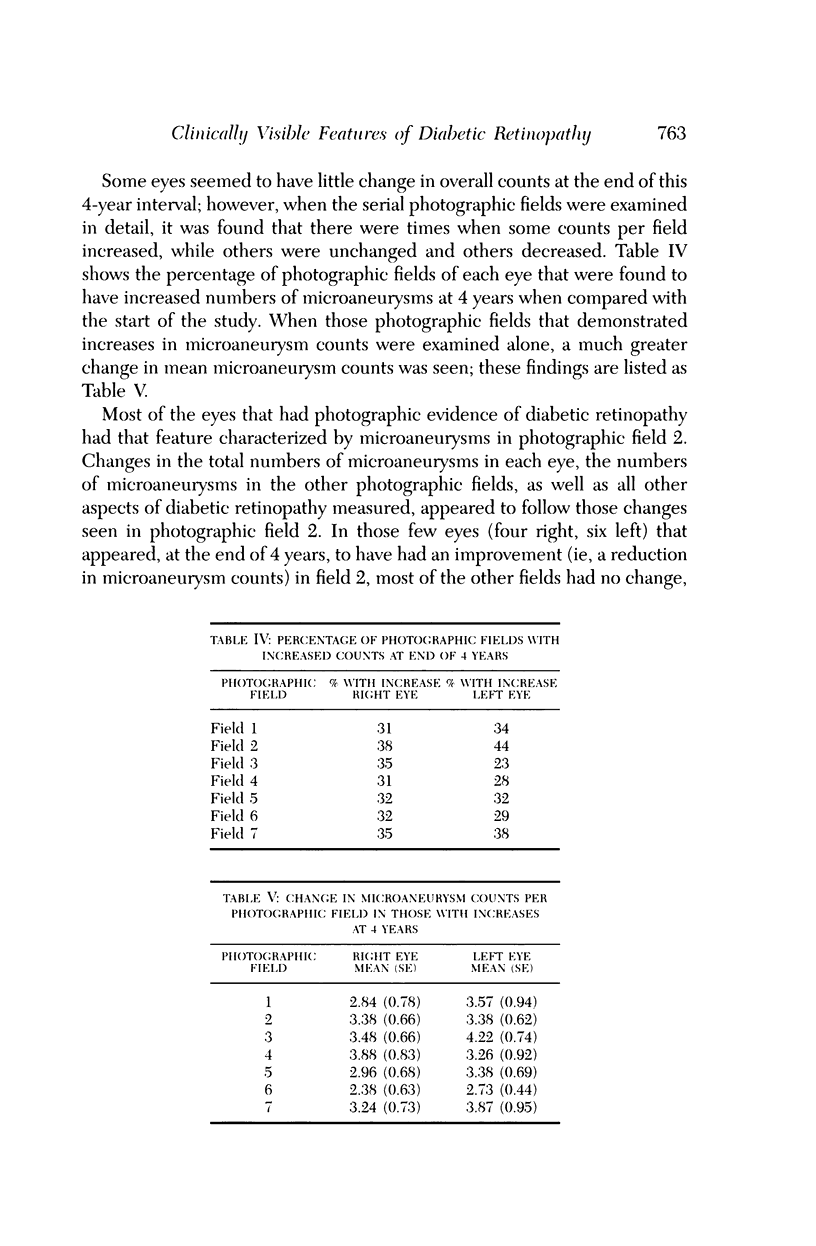
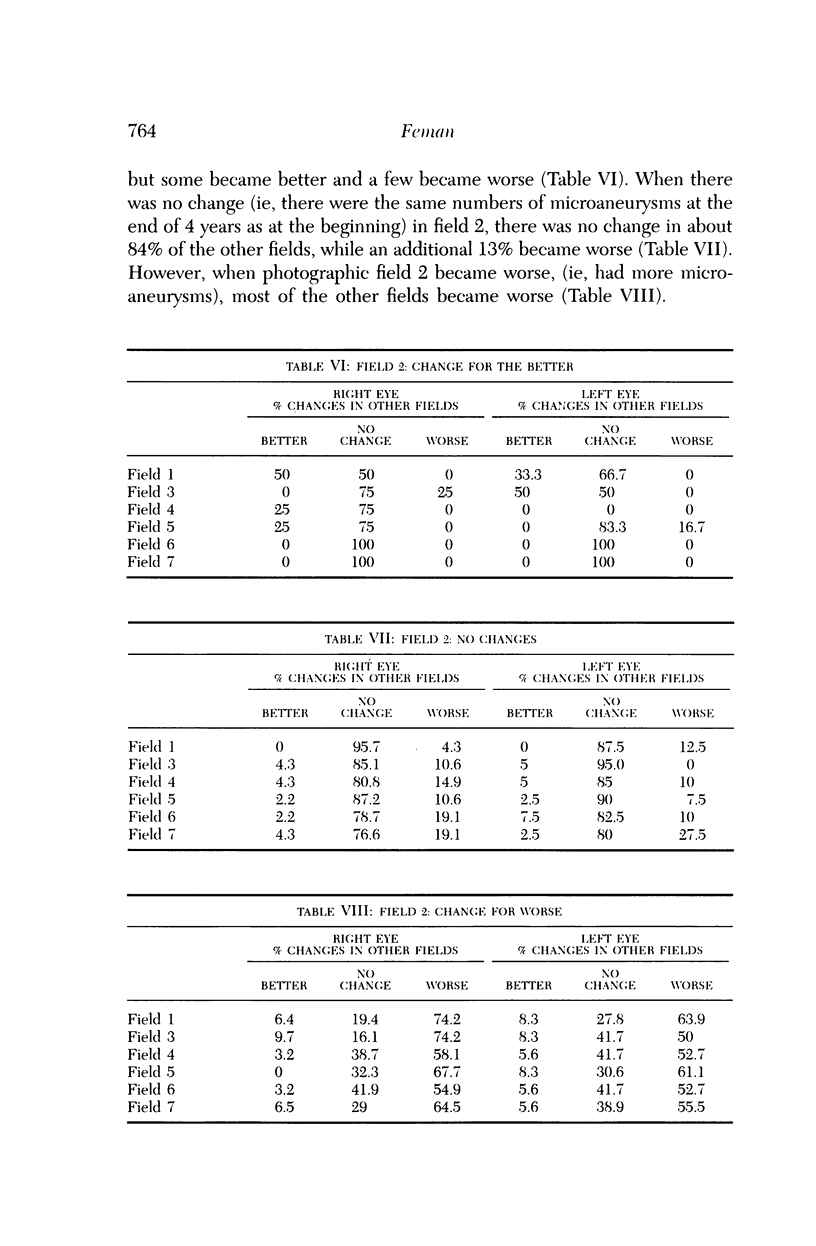
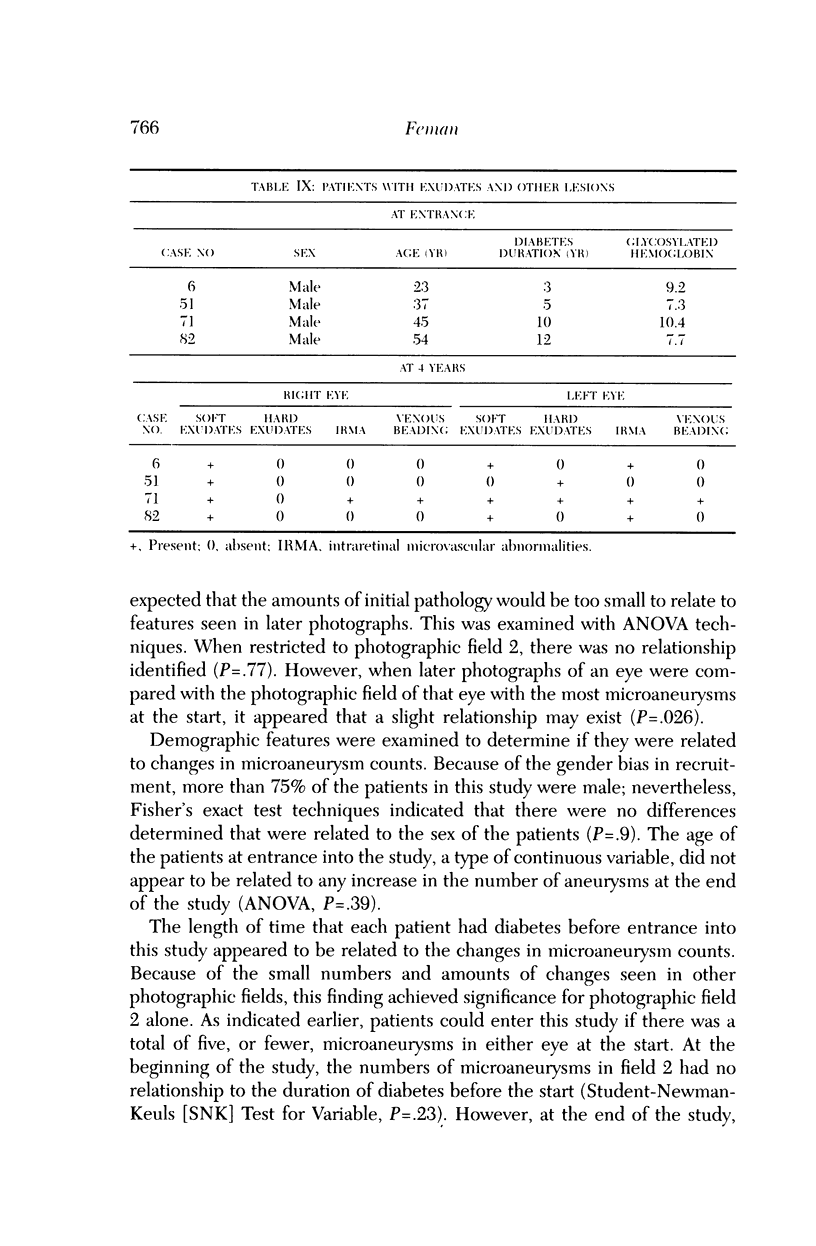
Microaneurysms are the first features of human diabetic retinopathy that can be detected with common clinical techniques. These are found, most often, in photographic field 2 (that is, an area occupying 30 degrees of the ocular fundus centered on the middle of the macula). After the first microaneurysms develop, there will be a tendency for more to appear; however, over time many of the original microaneurysms will become no longer visible with clinical techniques, while other, newer, microaneurysms mature. After the onset of microaneurysms, several years may pass before any other diabetic retinopathic lesions develop. Lesions other than microaneurysms were uncommon in this study; the following is a list in decreasing frequency: retinal hemorrhages, soft exudates, IRMA, hard exudates, and venous beading. During the 4 years of this study, there were no other diabetic retinopathic lesions detected. The duration of insulin-dependent diabetes mellitus was related to the rate of change in microaneurysm counts. The age and sex of patients did not affect this rate of change. The accuracy of metabolic control, as determined by glycosylated hemoglobin levels, may influence this rate of change; however, this was detected only at the extremes of measurement in this study. The equipment available to most ophthalmologists can detect the earliest clinical aspects of diabetic retinopathy. These features can be quantified in a reproducible manner with standardized photographic techniques to permit satisfactory data analysis.
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi Y., Kador P. F., Kuwabara T., Kinoshita J. H. Aldose reductase localization in human retinal mural cells. Invest Ophthalmol Vis Sci. 1983 Nov;24(11):1516–1519. [PubMed] [Google Scholar]
- Ashton N. Pathophysiology of retinal cotton-wool spots. Br Med Bull. 1970 May;26(2):143–150. doi: 10.1093/oxfordjournals.bmb.a070766. [DOI] [PubMed] [Google Scholar]
- COGAN D. G., KUWABARA T. CAPILLARY SHUNTS IN THE PATHOGENESIS OF DIABETIC RETINOPATHY. Diabetes. 1963 Jul-Aug;12:293–300. doi: 10.2337/diab.12.4.293. [DOI] [PubMed] [Google Scholar]
- COGAN D. G., KUWABARA T. CAPILLARY SHUNTS IN THE PATHOGENESIS OF DIABETIC RETINOPATHY. Diabetes. 1963 Jul-Aug;12:293–300. doi: 10.2337/diab.12.4.293. [DOI] [PubMed] [Google Scholar]
- COGAN D. G., TOUSSAINT D., KUWABARA T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961 Sep;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- Caird F. I., Burditt A. F., Draper G. J. Diabetic retinopathy. A further study of prognosis for vision. Diabetes. 1968 Mar;17(3):121–123. doi: 10.2337/diab.17.3.121. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Kao C. S., Chang C. J., Wu T. J., Fu C. C., Chen C. J., Tai T. Y. Prevalence and risk factors of diabetic retinopathy among noninsulin-dependent diabetic subjects. Am J Ophthalmol. 1992 Dec 15;114(6):723–730. doi: 10.1016/s0002-9394(14)74051-6. [DOI] [PubMed] [Google Scholar]
- Constable I. J., Knuiman M. W., Welborn T. A., Cooper R. L., Stanton K. M., McCann V. J., Grose G. C. Assessing the risk of diabetic retinopathy. Am J Ophthalmol. 1984 Jan;97(1):53–61. doi: 10.1016/0002-9394(84)90446-x. [DOI] [PubMed] [Google Scholar]
- De Venecia G., Davis M., Engerman R. Clinicopathologic correlations in diabetic retinopathy. I. Histology and fluorescein angiography of microaneurysms. Arch Ophthalmol. 1976 Oct;94(10):1766–1773. doi: 10.1001/archopht.1976.03910040540013. [DOI] [PubMed] [Google Scholar]
- Doft B. H., Kingsley L. A., Orchard T. J., Kuller L., Drash A., Becker D. The association between long-term diabetic control and early retinopathy. Ophthalmology. 1984 Jul;91(7):763–769. doi: 10.1016/s0161-6420(84)34235-x. [DOI] [PubMed] [Google Scholar]
- Engerman R. L., Kern T. S. Experimental galactosemia produces diabetic-like retinopathy. Diabetes. 1984 Jan;33(1):97–100. doi: 10.2337/diab.33.1.97. [DOI] [PubMed] [Google Scholar]
- FRIEDENWALD J. S. Diabetic retinopathy. Am J Ophthalmol. 1950 Aug;33(8):1187–1199. doi: 10.1016/0002-9394(50)90988-3. [DOI] [PubMed] [Google Scholar]
- Frank R. N., Hoffman W. H., Podgor M. J., Joondeph H. C., Lewis R. A., Margherio R. R., Nachazel D. P., Jr, Weiss H., Christopherson K. W., Cronin M. A. Retinopathy in juvenile-onset type I diabetes of short duration. Diabetes. 1982 Oct;31(10):874–882. doi: 10.2337/diab.31.10.874. [DOI] [PubMed] [Google Scholar]
- Herman I. M., D'Amore P. A. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985 Jul;101(1):43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerneld B., Algvere P. Relationship of duration and onset of diabetes to prevalence of diabetic retinopathy. Am J Ophthalmol. 1986 Oct 15;102(4):431–437. doi: 10.1016/0002-9394(86)90069-3. [DOI] [PubMed] [Google Scholar]
- Kinoshita J. H. Cataracts in galactosemia. The Jonas S. Friedenwald Memorial Lecture. Invest Ophthalmol. 1965 Oct;4(5):786–799. [PubMed] [Google Scholar]
- Klein B. E., Davis M. D., Segal P., Long J. A., Harris W. A., Haug G. A., Magli Y. L., Syrjala S. Diabetic retinopathy. Assessment of severity and progression. Ophthalmology. 1984 Jan;91(1):10–17. doi: 10.1016/s0161-6420(84)34374-3. [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B. E., Moss S. E., Shrago E. S., Spennetta T. L. Glycosylated hemoglobin in a population-based study of diabetes. Am J Epidemiol. 1987 Sep;126(3):415–428. doi: 10.1093/oxfordjournals.aje.a114673. [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B. E., Moss S. E. Visual impairment in diabetes. Ophthalmology. 1984 Jan;91(1):1–9. [PubMed] [Google Scholar]
- Klein R., Meuer S. M., Moss S. E., Klein B. E. The relationship of retinal microaneurysm counts to the 4-year progression of diabetic retinopathy. Arch Ophthalmol. 1989 Dec;107(12):1780–1785. doi: 10.1001/archopht.1989.01070020862028. [DOI] [PubMed] [Google Scholar]
- Kohner E. M., Dollery C. T., Bulpitt C. J. Cotton-wool spots in diabetic retinopathy. Diabetes. 1969 Oct;18(10):691–704. doi: 10.2337/diab.18.10.691. [DOI] [PubMed] [Google Scholar]
- Muraoka K., Shimizu K. Intraretinal neovascularization in diabetic retinopathy. Ophthalmology. 1984 Dec;91(12):1440–1446. doi: 10.1016/s0161-6420(84)34125-2. [DOI] [PubMed] [Google Scholar]
- Palmberg P., Smith M., Waltman S., Krupin T., Singer P., Burgess D., Wendtlant T., Achtenberg J., Cryer P., Santiago J. The natural history of retinopathy in insulin-dependent juvenile-onset diabetes. Ophthalmology. 1981 Jul;88(7):613–618. doi: 10.1016/s0161-6420(81)34975-6. [DOI] [PubMed] [Google Scholar]
- Puklin J. E., Tamborlane W. V., Felig P., Genel M., Sherwin R. S. Influence of long-term insulin infusion pump treatment of type I diabetes on diabetic retinopathy. Ophthalmology. 1982 Jul;89(7):735–747. doi: 10.1016/s0161-6420(82)34730-2. [DOI] [PubMed] [Google Scholar]
- Robison W. G., Jr, Kador P. F., Kinoshita J. H. Retinal capillaries: basement membrane thickening by galactosemia prevented with aldose reductase inhibitor. Science. 1983 Sep 16;221(4616):1177–1179. doi: 10.1126/science.6612330. [DOI] [PubMed] [Google Scholar]
- Robison W. G., Jr, Nagata M., Laver N., Hohman T. C., Kinoshita J. H. Diabetic-like retinopathy in rats prevented with an aldose reductase inhibitor. Invest Ophthalmol Vis Sci. 1989 Nov;30(11):2285–2292. [PubMed] [Google Scholar]
- Sharma N. K., Archer D. B., Hadden D. R., Merrett J. D., Maguire C. J. Morbidity and mortality in patients with diabetic retinopathy. Trans Ophthalmol Soc U K. 1980 Apr;100(Pt 1):83–89. [PubMed] [Google Scholar]
- Speiser P., Gittelsohn A. M., Patz A. Studies on diabetic retinopathy. 3. Influence of diabetes on intramural pericytes. Arch Ophthalmol. 1968 Sep;80(3):332–337. doi: 10.1001/archopht.1968.00980050334007. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Wyman M., Ferris F., 3rd, Kador P. F. Diabeteslike preproliferative retinal changes in galactose-fed dogs. Arch Ophthalmol. 1992 Sep;110(9):1295–1302. doi: 10.1001/archopht.1992.01080210113037. [DOI] [PubMed] [Google Scholar]
- WOLTER J. R. Pathology of a cotton-wool spot. Am J Ophthalmol. 1959 Oct;48:473–485. doi: 10.1016/0002-9394(59)90883-9. [DOI] [PubMed] [Google Scholar]
- YAMASHITA T., BECKER B. The basement membrane in the human diabetic eye. Diabetes. 1961 May-Jun;10:167–174. doi: 10.2337/diab.10.3.167. [DOI] [PubMed] [Google Scholar]
- Yanoff M. Ocular pathology of diabetes mellitus. Am J Ophthalmol. 1969 Jan;67(1):21–38. doi: 10.1016/0002-9394(69)90004-x. [DOI] [PubMed] [Google Scholar]



