Abstract
Vegetated buffer strips were evaluated for their ability to remove waterborne Cryptosporidium parvum from surface and shallow subsurface flow during simulated rainfall rates of 15 or 40 mm/h for 4 h. Log10 reductions for spiked C. parvum oocysts ranged from 1.0 to 3.1 per m of vegetated buffer, with buffers set at 5 to 20% slope, 85 to 99% fescue cover, soil textures of either silty clay (19:47:34 sand-silt-clay), loam (45:37:18), or sandy loam (70:25:5), and bulk densities of between 0.6 to 1.7 g/cm3. Vegetated buffers constructed with sandy loam or higher soil bulk densities were less effective at removing waterborne C. parvum (1- to 2-log10 reduction/m) compared to buffers constructed with silty clay or loam or at lower bulk densities (2- to 3-log10 reduction/m). The effect of slope on filtration efficiency was conditional on soil texture and soil bulk density. Based on these results, a vegetated buffer strip comprised of similar soils at a slope of ≤20% and a length of ≥3 m should function to remove ≥99.9% of C. parvum oocysts from agricultural runoff generated during events involving mild to moderate precipitation.
Cryptosporidium parvum has emerged as a widespread and persistent waterborne microbial pathogen, with specific genotypes able to be transmitted ambidirectionally between livestock and humans (e.g., amphixenotic) (6, 42, 46, 54). Although we still do not know the percentage of annual cases of human cryptosporidiosis that are attributable to livestock-derived waterborne C. parvum (39), reducing the likelihood that animal agricultural operations will contaminate surface water with infective C. parvum oocysts will help safeguard both water quality and public health (51). Several strategies exist for minimizing the likelihood that an animal agricultural operation contaminates surface water with infective C. parvum oocysts. For example, one such strategy is to reduce the incidence of C. parvum infection or the intensity of fecal shedding of C. parvum oocysts by livestock populations, thereby reducing the rate of environmental loading of C. parvum per livestock unit (26, 36). These herd-health efforts remain hampered by our poor understanding of the medical ecology of C. parvum within livestock populations (3, 4, 16, 40), how to interrupt transmission between the biological reservoir and susceptible animals (3, 40), and the lack of an affordable vaccine that has been proven to be efficacious in commercial agricultural settings (23, 47).
A second strategy is to manage the manure produced by livestock so that the survivability and off-site transport of infective C. parvum are substantially reduced (3, 20, 30, 53, 55). One strategy being advocated for minimizing the transport potential of C. parvum oocysts from animal manure to surface water is to place vegetated buffer strips between animal agricultural operations and vulnerable surface water supplies (10, 12, 15, 32, 38, 51, 59, 60). Optimal design criteria for on-farm vegetated buffer strips currently do not exist for waterborne microbial contaminants. Moreover, studies on the fate and transport of zoonotic protozoal pathogens such as C. parvum in overland and subsurface flow through such buffers are severely lacking (9, 24, 37, 38, 55; E. R. Atwill, K. W. Tate, M. R. George, and N. K. McDougald, Abstr. Am. Water Resour. Assoc. Symp. Rangeland Management Water Resour., p. 446, 1998).
Our goal for this project was to assess the ability of a meter-long vegetated buffer to remove waterborne C. parvum oocysts from overland and shallow subsurface flow as a function of soil and vegetative characteristics, slope, and rainfall intensity. Such data will help provide a basis for design criteria for vegetated buffers that remove ≥99.9% of C. parvum oocysts from agricultural runoff.
MATERIALS AND METHODS
General overall study design.
The filtration efficiency of three different types of soil (sandy loam, loam, and silty clay loam) was evaluated for three slopes (5, 10, and 20%) across two two rainfall intensities (1.5 and 4.0 cm/h), with three replicates per treatment, for a total of 54 soil box trials. Mean percent vegetative cover and mean vegetative height for each box were measured the day before the rainfall experiment. Two to four randomized boxes were irrigated simultaneously by a rainfall emitter (an experimental run), and a peristaltic pump delivered an oocyst suspension to the upper end of the boxes. Once steady-state conditions were established, the water source for the peristaltic pump was switched to an oocyst suspension for 60 min. Following oocyst application, the peristaltic pump was switched back to non-oocyst water, and the rainfall and pump then continued for an additional 180 min to quantify unbound and desorbed oocysts moving in overland and subsurface flows. Overland and subsurface flows were collected and analyzed separately.
Source and purification of C. parvum oocysts.
Naturally infected dairy calves from two local commercial dairies were the source of wild-type C. parvum oocysts. We have determined previously that these oocysts are classified as bovine genotype A by using the genotyping scheme described by Xiao et al. (58a). By using an acid-fast procedure to detect oocysts (23), samples with more than 25 oocysts per × 400 microscopic field were washed through a series of 40, 100, 200, and 270 mesh sieves. The resulting suspension was decanted off and centrifuged at 1,000 × g for 10 min. Supernatant was discarded, and the pellet washed in Tween water (0.01% Tween 80 in deionized water [vol/vol]). A discontinuous sucrose gradient was used to purify C. parvum oocysts from fecal suspensions (1). The concentration of purified oocysts was determined as the arithmetic mean of six separate counts with a phase-contrast hemacytometer. Using tap water from a local well, oocyst suspensions were diluted to 5 × 104 oocysts/ml for overland flow application. Oocysts were collected 1 to 4 days prior to each experiment in order to minimize biases associated with using aged oocysts in the project (8).
Soil types and characterization.
Soils were selected to represent a wide range of soil textures (sandy loam, loam, and silty clay loam) utilized for animal husbandry in California. The Hanford fine sandy loam, a Typic Xerorthent, was selected as the sandy soil. The Argonaut loam, a Mollic Haploxeralf, was selected as the loamy soil. The Capay silty clay, a Typic Chromoxerert, was selected as the clayey soil. Soils were air dried, gently crushed, and passed through a 2-mm-pore-diameter sieve to remove coarse fragments. The air-dried, <2-mm-particle-diameter fraction was used for the following analyses (Table 1). Soil pH was measured in water (saturated paste suspension) following a 15-min equilibration period. Particle size analysis was performed with a hydrometer in suspensions dispersed in sodium hexametaphosphate-Na carbonate (18). Organic carbon was estimated from a modified Walkley-Black analysis, assuming an organic carbon content of 58% for soil organic matter (41). Cation-exchange capacity was determined by barium acetate saturation and calcium replacement (29). The clay-size fraction (particle diameter, <2 μm) was isolated by flocculation with NaCl and sedimentation after organic matter removal with sodium hypochlorite (34) and free iron oxide removal with citrate-dithionite (28). X-ray diffraction analysis was performed with oriented clays mounted on glass slides by standard methods (58).
TABLE 1.
Selected soil characterization data for the three topsoil horizons used in this study
| Soil type | Texture | % of soil
|
pH | % Organic carbon | % Organic matter | CEC (cmolc/kg)a | Mineralogyb | ||
|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | |||||||
| Hanford | Sandy loam | 70 | 25 | 5 | 6.7 | 1.0 | 1.7 | 10.0 | Mica, +++; kaolinite, ++ |
| Argonaut | Loam | 45 | 37 | 18 | 5.5 | 2.3 | 1.4 | 24.8 | Vermiculite, +++; chlorite, +++; kaolinite, +++; smectite, + |
| Capay | Silty clay loam | 19 | 47 | 34 | 6.0 | 0.8 | 4.0 | 28.1 | Smectite, +++; kaolinite, ++ |
CEC, cation exchange capacity (units of charge per weight of soil).
Abundance: +++, major; ++, minor; +, trace.
Soil box design and vegetation.
Twenty soil boxes were constructed of 0.75-in.-thick plywood and sealed against leaks and moisture. The dimensions were 15 cm wide by 100 cm long by 20 cm deep, allowing us to determine the filtration efficiency of a meter of vegetated buffer while allowing for reasonable root development. The box was designed to capture overland flow after it had traversed the 100-cm vegetated buffer, and subsurface flow was captured through a slot at the bottom of the seepage face. The junction between the soil surface and the inner side of the box wall was sealed with a strip of waterproof sealant to minimize seepage of surface flow along the vertical side of the box. The slope of each box was adjusted individually to achieve the desired treatment slopes of 5, 10, and 20%. Perennial fescue seed was used to vegetate the soil boxes, with approximately 50 to 70 g of seed used per box, which resulted in ≥85% vegetative cover. On the day prior to a rainfall experiment, we measured the height of the fescue for each box at points 25, 50, and 75 cm down the length of the soil box and determined a mean height from these values. Following a rainfall simulation, four soil cores of 94.3 cm3 were extracted from each box. The combined samples from each box were dried in a convection oven at 37°C for 3 days. The samples were weighed, and bulk density was calculated as: bulk density (g/cm3) = [net dry weight (g) of all 4 soil cores/(4 × 94.3 cm3)].
Rainfall emitter and storm intensity calculations.
The rainfall emitter was a drip-type box simulator (11) constructed of 0.5-in.-thick plywood and sealed with fiberglass. The dimensions were 81 cm wide, 112 cm long, and 38 cm deep. The bottom of the rainfall emitter was fitted with a uniform grid of 22- or 25-gauge needles at a density of 1 needle per 3.5 cm2, or 750 total needles. The distance between the emitter and surface of each soil box was approximately 1.8 m. This design allowed us to apply rainfall to up to four boxes simultaneously.
We selected rainfall intensities of 1.5 and 4.0 ml/cm2/h to represent a 2- to 3-year or a 20- to 30-year return interval, 4-h-duration storm, respectively. We selected these storms to be representative of those in various agricultural regions throughout the United States (25). We developed our rainfall intensity estimates as recommended by Haan et al. (21). From a range of 2- to 3-year and 20- to 30-year, 24-h rainfall amounts for agricultural areas across the United States (25), we estimated the mean rainfall intensity for each 4-h-design storm based on the Soil Conservation Service type II rainfall pattern.
Rainfall simulation experiment and collection of overland and subsurface flow.
One day before and on the morning of the experiment, each soil box was saturated with rainfall for 2 to 6 h while the rainfall emitter was calibrated for the determined storm intensity. On the day of the rainfall experiment, a four-channel peristaltic pump (Masterflex L/S; Cole-Palmer Instrument Company, Vernon Hills, Ill.) was positioned at the upper end of the boxes to deliver 10 ml of water or oocysts per min to the upper end of each of the boxes in order to simulate overland flow from upslope areas. Simultaneously, the rainfall emitter was raining at the predetermined intensity from an elevation of 1.8 m above the box. Once steady-state conditions were established, the pump was switched to an oocyst suspension (5 × 104 oocysts per ml) for 60 min, initiating the start of the experiment. Each box received 10 ml/min from the pump, or 5 × 105 oocysts/min. After 60 min, the pump was switched back to water (no oocysts), and both the simulated rain and peristaltic pump were continued for 180 min in order to quantify unbound or desorbed oocysts moving in overland or subsurface flow. All 4 h of water was collected, and the volume was recorded as 5- to 30-min composite water samples from both overland and subsurface flow ports and analyzed separately, as shown in Table 2. Approximately 3 × 107 oocysts were applied to each box.
TABLE 2.
Number of composite water samples collected per soil box during a 4-h experiment
| Flow path | No. of samples collected
|
|||||
|---|---|---|---|---|---|---|
| Preexperiment negative control | h 1 | h 2 | h 3 | h 4 | Total | |
| Overland | 1 | 12 | 6 | 4 | 2 | 26 |
| Subsurface | 1 | 4 | 4 | 3 | 2 | 14 |
Quantifying the concentration of C. parvum oocysts in overland and subsurface flow.
Quantitative immunofluorescent microscopy, with detailed information on the percent recovery of spiked oocysts in the source water (13), was used to enumerate C. parvum oocysts in water samples (24). One hundred microliters of a mixture containing 10% Tween 80 and 10% sodium dodecyl sulfate was added to 50-ml aliquots of overland flow and mixed for 5 min on a hand wrist shaker. Larger volumes were tested as needed. The suspension was centrifuged at 1,000 × g for 15 min, and the supernatant was removed until 1 ml of residual pellet and suspension remained. One hundred microliters of 100% formalin was added, and the suspension was transferred to a 1.5-ml microcentrifuge tube, centrifuged at 11,600 × g for 5 min, and supernatant was removed until 1 ml of residual pellet and supernatant remained. Ten microliters of suspension was enumerated for C. parvum oocysts by fluorescence microscopy with commercially prepared well slides (Meridian Diagnostics, Inc., Cincinnati, Ohio) and a fluorescein isothiocyanate-labeled anti-Cryptosporidium immunoglobulin M antibody (Waterborne, Inc., New Orleans, La.). For subsurface samples, we used a similar procedure, except that 100- to 250-ml aliquots were concentrated down to 100 to 500 μl. Positive and negative controls were included with each run.
In order to determine the percent recovery for the immunofluorescent microscopy procedure, we first conducted a sham soil box experiment with no oocysts added in order to collect clean water samples from each soil type into 50-ml centrifuge tubes. Water samples were centrifuged at 1,000 × g for 10 min, and the supernatant was removed, leaving a small sediment pellet of 10 to 50 mg in each tube. Next, a second sham soil box experiment was conducted with all of the same equipment and the usual oocyst suspension (5 × 104 oocysts per ml), except that no soil was in the box. Specifically, the soil portion of the box was bypassed to deposit the oocyst suspension (5 × 104 oocysts per ml) via synthetic tubing from the oocyst delivery point near the top of the boxes to the collecting flange at the lower end of 12 boxes. The application rate was 10 ml/min for 5 min. The source of the oocysts and the concentration of the oocyst stock solution were determined as described above. The soil box effluent (oocyst suspension) was then collected into the 50-ml tubes prepared in the first sham experiment described above, which contained the different soil sediments so as to more accurately measure the percent recovery of detection of oocysts in soil box effluent. The immunofluorescent microscopy procedure was then performed as described above.
Statistical analyses.
By using a set of composite samples as shown in Table 2, we measured the total number of C. parvum oocysts in overland and subsurface flow during 4 h of rainfall for each soil box. Our covariates (independent variables) for each treatment combination were soil type (silty clay loam, loam, and sandy loam), mean grass height (centimeters), mean soil bulk density (grams per cubic centimeter), percent slope (5, 10, and 20%), rainfall intensity (1.5 ml or 4.0 ml/h/cm2), and total volume of overland and subsurface flow during the 4-h rainstorm. Percent cover was not analyzed given the narrow range of values (85 to 99%). Mean 4-h total subsurface flow was converted to the mean infiltration rate (10 mm/h = 1 ml/cm2/h) for each soil box. Given our 4-h time frame and approximate steady-state conditions, we ignored the negligible influence of evaporation, transpiration, interception, and changes in soil water storage in our calculation of infiltration rate. The outcome (dependent) variable was the total number of C. parvum oocysts (nonfiltered or desorbed) discharging from the soil box in either overland or subsurface effluent during the 4-h rain event, adjusted for percent recovery of the assay, as determined with the following equation:
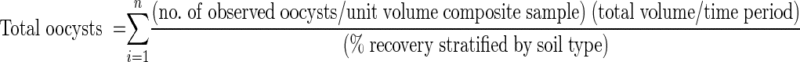 |
where n is the total number of composite samples per 4-h rain event per flow path (overland or subsurface) (Table 2), the number of observed oocysts per unit volume is the total number of C. parvum oocysts estimated via immunofluorescent microscopy for each composite sample, the total volume per time period is the volume of water collected per sampling time period (5 to 30 min) for each flow path. The percent recovery for the immunofluorescent microscopy procedure for each soil type was estimated as described below.
A linear mixed-effects regression model (48) was used to determine the association between each of our covariates (or fixed effects) and the total number of oocysts discharging off the vegetated buffer. Values for the total number of oocysts were transformed via the natural logarithm, and the experiment (15 randomized sets of two to four soil boxes per experiment) was modeled as a random effect. We used a forward-stepping algorithm to build the model, with significance for inclusion in the model set at a P value of ≤0.10, with a restricted-maximum-likelihood ratio test.
Percent recovery for the immunofluorescent microscopy procedure for each soil type was estimated by fitting a negative binomial regression model (22) to the observed number of oocysts, with soil type modeled as a categorical variable and the number of spiked oocysts (n = 500) functioning as the offset (or exposure) variable. Negative binomial regression tends to provide a better-fitting model than Poisson regression due to typical overdispersion of oocyst count data (3, 26, 56).
RESULTS
All soil boxes had from 85 to 99% vegetative cover comprised of perennial fescue, with vegetative heights ranging from 5 to 55 in. Soil properties for the silty clay loam, loam, and sandy loam are listed in Table 1. The observed infiltration rates (10 mm/h = 1 ml/cm2/h) for each soil type ranged from 0.001 to 1.8 ml/cm2/h for silty clay loam, 0.001 to 1.9 ml/cm2/h for loam, and 0.01 to 1.2 ml/cm2/h for sandy loam. Mean bulk density for each soil series ranged from 0.52 to 1.36 g/cm3 for silty clay loam, 0.88 to 1.57 g/cm3 for loam, and 1.24 to 1.84 g/cm3 for sandy loam. Differences in bulk density within each soil series were due to the inherent inability to replicate soil compaction within the range realized for these soils under field conditions.
Compared to the initial application of approximately 3 × 107 oocysts, the total number of oocysts that were collected in the effluent (nonfiltered or desorbed oocysts) ranged from 3.5 × 103 to 1.9 × 107 oocysts (0.01 to 65% of initial oocyst load). The majority of oocysts in the effluent were transported in overland flow (i.e., median percentages of oocysts that were transported in overland compared to subsurface flow were 99.8 and 0.2%, respectively). The ranges of flow rates were 1,600 to 6,700 ml/h for overland flow and 0.4 to 2,800 ml/h for subsurface flow. The median percentages of total effluent that were collected from overland or subsurface flow were 96 and 4%, respectively.
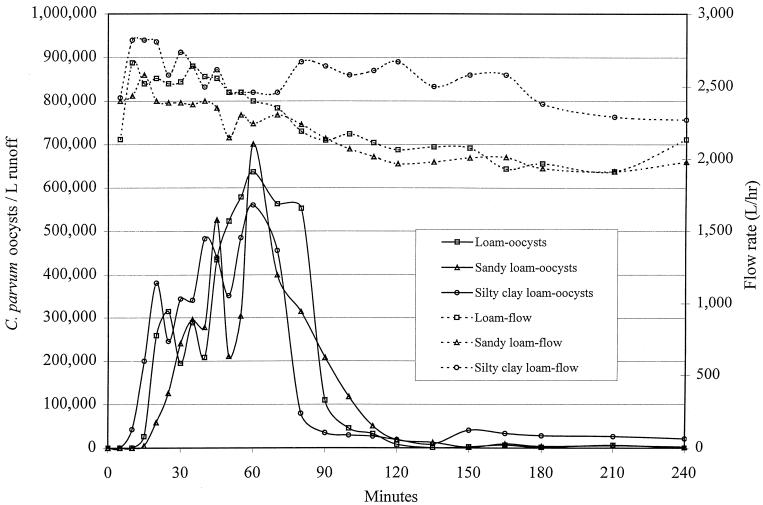
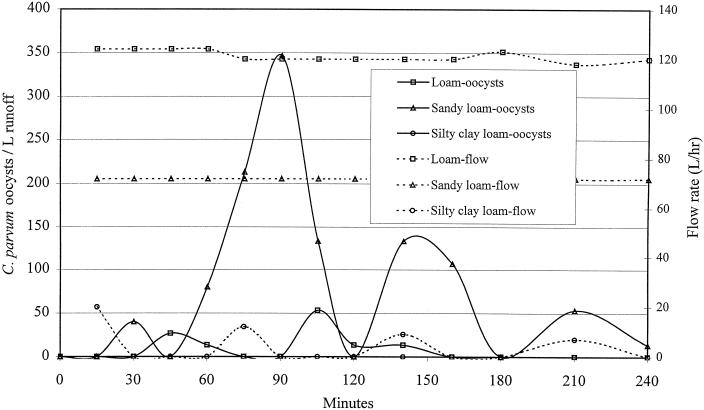
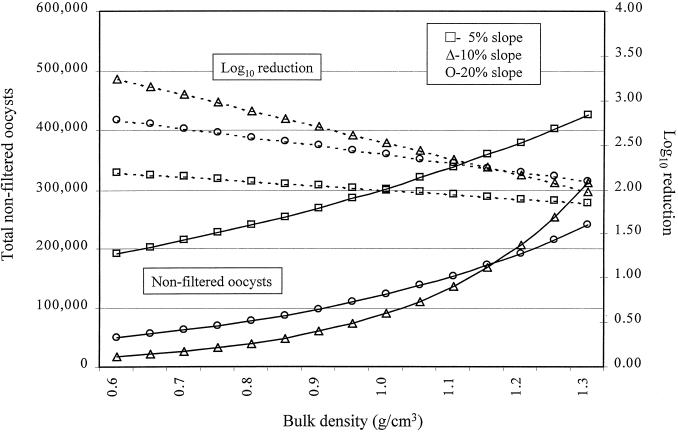
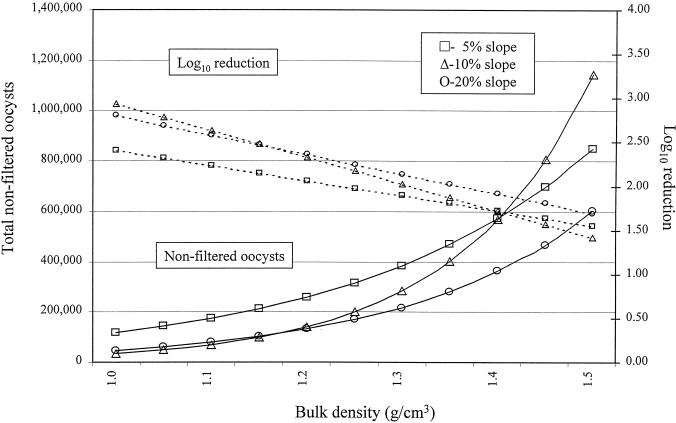
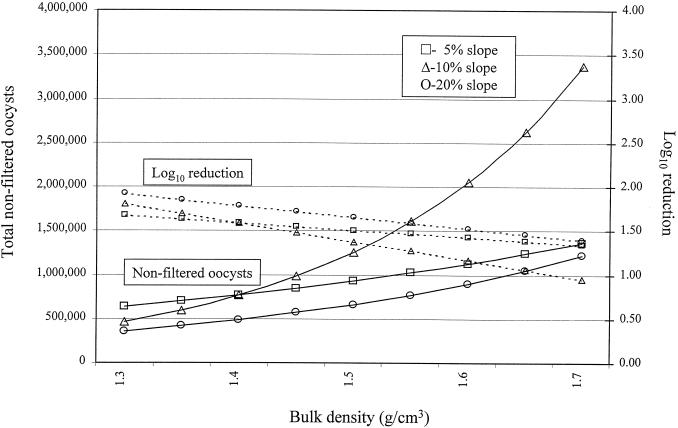
A linear mixed-effects model was fitted to the data and used to evaluate the association between biotic and abiotic factors and the natural logarithm of the total number of nonfiltered and desorbed oocysts in the effluent of overland or subsurface flow. Examples of the raw data for the breakthrough curve for C. parvum are shown in Fig. 1 and 2. Prior to statistical modeling, values for total enumerated oocysts were adjusted for the percent recovery of our assay (Table 3). Factors significantly associated with total oocysts in the effluent of overland flow (proportional to the inverse of filtration efficiency) were soil type and percent slope of the vegetated buffer, but this association was strongly influenced by the bulk density (Table 4 and Fig. 3, 4, and 5). For example, at a 5% slope, for every increase of bulk density of 0.1 g/cm3, the total numbers of oocysts in the effluent of overland flow (oocysts avoiding filtration or detaching during transport across the buffer) were increased by approximately 12% [e(1.14 × 0.1) − 1 = 0.12], 49% {e[(1.14 + 2.83) × 0.1] − 1 = 0.49}, and 21% {e[(1.14 + 0.77) × 0.1] − 1 = 0.2112} for silty clay loam, loam, and sandy loam, respectively. Alternatively, at a 5% slope and for every increase of 0.1 g/cm3 in bulk density, the log10 reductions were reduced by approximately 2.5, 8.0, and 5.0% for silty clay loam, loam, and sandy loam, respectively. In other words, increasing the soil's bulk density substantially reduced the ability of the buffer to remove waterborne oocysts in overland flow (Fig. 3 to 5). In general, buffers constructed of sandy loam compared to the other two soils were least effective at removing oocysts in overland flow. For buffers constructed of silty clay loam and loam, positioning the soil box at a higher slope was in general more effective at removing oocysts than buffers at a 5% slope, but this trend did not hold at higher bulk densities nor for the sandy loam (Fig. 3 to 5). Given that soil type, slope, and the interactions with bulk density were in the regression model, infiltration rate, percent vegetative cover, vegetative height, rate of total overland flow, and precipitation rate were not associated with filtration efficiency.
FIG. 1.
Raw data for discharge of C. parvum oocysts from overland flow. The slope was 5%, and the 4-h precipitation rate was 1.5 ml/cm2/h.
FIG. 2.
Raw data for discharge of C. parvum oocysts from subsurface flow. The slope was 5%, and the 4-h precipitation rate was 1.5 ml/cm2/h.
TABLE 3.
Recovery for the oocyst delivery and collection system in combination with the direct immunofluorescent assay
| Soila | Expected no. of oocystsb | Mean no. of oocysts detected (range) | Mean % recovery (range) |
|---|---|---|---|
| Silty clay loam | 500 | 317 (280-346) | 63 (56-69) |
| Loam | 500 | 376 (258-496) | 75 (52-99) |
| Sandy loam | 500 | 405 (344-500) | 81 (69-100) |
There were four replicates per soil type for silty clay loam and sandy loam and eight replicates for loam.
The expected number of 500 oocysts was generated by using a 0.01-ml volume from a stock solution containing 5 × 104 oocysts per ml.
TABLE 4.
Final linear mixed-effects regression modelsa for abiotic factors associated with vegetated buffer strip filtration of waterborne C. parvum oocysts in overland flow
| Abiotic factor(s) | Log mean no. of oocysts (SD)b | Regression coefficient for log mean no. of oocysts (95% CI)b | P valuec |
|---|---|---|---|
| Soil series | |||
| Silty clay loamd | 12.77 (2.26) | 0.0 | |
| Loam | 12.84 (2.08) | −3.78 (−6.90, 0.66) | 0.02 |
| Sandy loam | 14.25 (1.83) | −0.60 (−2.81, 1.61) | 0.56 |
| Slope | |||
| 5% | 13.92 (1.95) | 0.0 | |
| 10% | 13.87 (1.79) | −4.24 (−7.02, −1.45) | 0.004 |
| 20% | 12.04 (2.23) | −2.04 (−3.22, −0.85) | 0.001 |
| Bulk densitye | 1.14 (−1.59, 3.86) | 0.40 | |
| Soil-bulk density interaction | |||
| Silty clay loam | 0.0 | ||
| Loam | 2.83 (0.24, 5.42) | 0.03 | |
| Sandy loam | 0.77 (−0.72, 2.27) | 0.30 | |
| Slope-bulk density interaction | |||
| 5% slope | 0.0 | ||
| 10% slope | 3.02 (0.90, 5.15) | 0.007 | |
| 20% slope | 1.13 (0.20, 2.06) | 0.02 | |
| Intercept | 11.48 (7.76, 15.2) | 0.001 |
Abiotic factors (e.g., soil type and slope) were modeled as fixed effects. The experiment (15 randomized sets of two to four soil boxes per experiment) was modeled as a random effect. The outcome variable was the natural logarithm of the total number of oocysts in overland flow effluent during the 4-h precipitation event, with ∼3 × 107 oocysts applied to the head of each soil box.
Total number of oocysts collected from the effluent, transformed via the natural logarithm.
Significance was determined at a P value of ≤0.10 with a restricted-maximum-likelihood ratio test.
Referent category
Bulk density: grams per cubic centimeter of oven-dried soil.
FIG. 3.
Modeled filtration efficiency of a 1-m vegetated buffer as a function of bulk density and percent slope with silty clay loam. The initial load was 3 × 107 oocysts.
FIG. 4.
Modeled filtration efficiency of a 1-m vegetated buffer as a function of bulk density and percent slope with loam. The initial load was 3 × 107 oocysts.
FIG. 5.
Modeled filtration efficiency of a 1-m vegetated buffer as a function of bulk density and percent slope with sandy loam. The initial load was 3 × 107 oocysts.
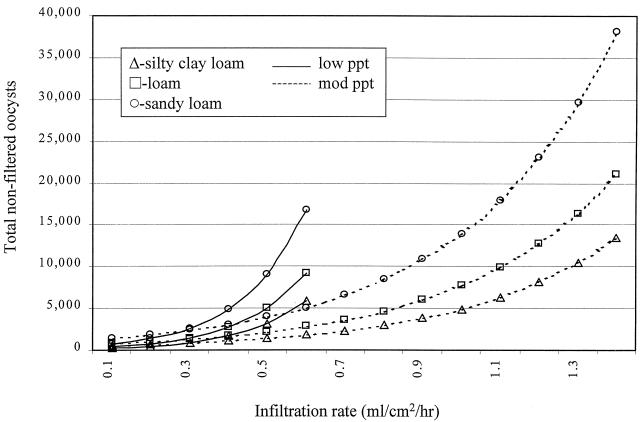
Factors significantly associated with total oocysts in the effluent of subsurface flow were soil type and infiltration rate, but this association was strongly influenced by the rainfall intensity (Table 5 and Fig. 6). For every increase of 0.1 ml/cm2/h (1 mm/h) in the infiltration rate, the total number of oocysts in the effluent of subsurface flow was increased by 86% [e(6.21 × 0.1) − 1 = 0.86] and 29% {e[(6.21-3.67) × 0.1] − 1 = 0.29} for lower (−1.5 ml/cm2/h) and higher (−4.0 ml/cm2/h) rainfall intensities, respectively. Buffers constructed of sandy loam resulted in 81% more oocysts in subsurface effluent [e(0.60) − 1 = 0.81] relative to buffers constructed of silty clay loam. All other biotic and abiotic factors were not significant.
TABLE 5.
Final linear mixed-effects regression modelsa for abiotic factors associated with vegetated buffer strip filtration of waterborne C. parvum in subsurface flow
| Abiotic factor(s) | Log mean no. of oocysts (SD)b | Regression coefficient for log mean no. of oocysts (95% CI)b | P valuec |
|---|---|---|---|
| Soil series | |||
| Silty clay loamd | 7.00 (3.57) | 0.0 | |
| Loam | 6.56 (4.02) | −0.44 (−1.28, 0.39) | 0.29 |
| Sandy loam | 7.25 (3.50) | 0.60 (0.11, 1.08) | 0.02 |
| Rain series | |||
| 2- to 3-yr return intervald | 5.70 (3.68) | 0.0 | |
| 20- to 30-yr return interval | 8.30 (3.12) | 1.02 (−0.45, 2.49) | 0.16 |
| Infiltration ratee | 2.25 (1.10, 3.40) | 0.001 | |
| Infiltration rate-rain series interaction | |||
| 2- to 3-yr return intervald | 0.0 | ||
| 20- to 30-yr return interval | −3.67 (−6.65, −0.72) | 0.02 | |
| Intercept | 5.41 (4.02, 6.80) | 0.001 |
Abiotic factors (e.g., soil type and slope) were modeled as fixed effects. The experiment (15 randomized sets of two to four soil boxes per experiment) was modeled as a random effect. The outcome variable was the natural logarithm of the total number of oocysts in subsurface flow effluent during the 4-h precipitation event, with ∼3 × 107 oocysts applied to the head of each soil box.
Total no. of oocysts collected in the effluent, transformed via the natural logarithm.
Significance was determined at a P value of ≤0.10 with a restricted-maximum-likelihood ratio test.
Referent category.
Infiltration rate: number of milliliters of water passing through the subsurface per square centimeter of soil surface per hour, equivalent to 10 mm/h.
FIG. 6.
Modeled number of total oocysts in subsurface flow effluent as a function of infiltration rate, as predicted by the linear mixed-effects regression model. The initial load was 3 × 107 oocysts, stratified by soil type and precipitation rate (1.5 or 4.0 ml/cm2/h).
Significant random effects were present from experiment to experiment in our study, whereby two to four soil boxes were positioned underneath the rainfall simulator per experiment. Approximately 67 and 45% of the total variation for log total oocysts in the effluent of overland and subsurface flows, respectively, were produced by interexperiment variation. Such variation is not uncommon for these types of experiments; for example, enumeration of C. parvum oocysts via microscopy from replicate samples can induce substantial intersample variation in oocyst counts (3, 26, 56). Both the coefficients and their associated standard errors for our regression model were adjusted for these random effects (48).
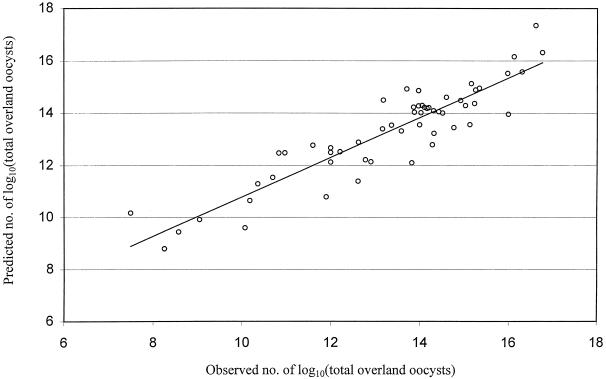
There was reasonable agreement between the observed number of total nonfiltered and desorbed oocysts in overland flow and the values predicted by the linear mixed-effects model (Fig. 7). Therefore, coefficients from the linear mixed-effects models were used to calculate the mean filtration efficiency (log10 reduction) associated with the use of vegetated buffers. These predictions were calculated as a function of soil type, slope of buffer, and interactions with bulk density, given that these factors were significant in the regression model (Fig. 3 to 5). Hence, the mean log10 reduction of waterborne C. parvum oocysts (bovine genotype A) associated with a 100-cm-long vegetated buffer under these experimental conditions ranged from 1.0 to 3.1 (Table 6). Oocyst counts in subsurface flow were excluded from these calculations, because in almost all cases, total oocyst counts in subsurface flow did not exceed 10,000 oocysts (0.03% of total), resulting in a negligible effect on the calculated log10 reduction of waterborne C. parvum oocysts.
FIG. 7.
Observed number of total C. parvum oocysts in overland flow effluent compared to predictions generated by the linear mixed-effects regression model.
TABLE 6.
Predictions of log10 reduction of waterborne C. parvum oocysts in overland flow attributable to 1 meter of vegetated buffer strip filtration
| Soil type and slope of vegetated buffer strip | Log10 reduction of C. parvum oocysts/m of buffer strip at bulk density (g/cm3) ofa:
|
|||
|---|---|---|---|---|
| 0.66 | 1.0 | 1.33 | 1.66 | |
| Silty clay loam | ||||
| 5% slope | 2.2 | 2.0 | 1.8 | |
| 10% slope | 3.1 | 2.5 | 1.9 | |
| 20% slope | 2.7 | 2.4 | 2.1 | |
| Loam | ||||
| 5% slope | 2.4 | 1.8 | ||
| 10% slope | 2.9 | 1.9 | ||
| 20% slope | 2.8 | 2.1 | ||
| Sandy loam | ||||
| 5% slope | 1.7 | 1.4 | ||
| 10% slope | 1.7 | 1.0 | ||
| 20% slope | 1.9 | 1.4 | ||
Predictions were calculated as log10 [(mean number of total oocysts discharged from buffer in overland flow effluent, estimated from the linear mixed-effects regression model)/(3 × 107 spiked oocysts)].
DISCUSSION
Vegetated buffer strips are being advocated by agencies such as the U.S. Department of Agriculture as a practical method to reduce waterborne transport of C. parvum and other zoonotic microbial pathogens from animal agricultural operations to nearby surface water supplies (51). The assumption is that by placing a vegetated buffer of adequate width between livestock populations and adjacent surface water supplies, microorganisms that are shed in the feces or urine of livestock will be removed (strained or adsorbed) as they are transported via advective and dispersive processes through the vegetated buffer (10, 12, 15, 32, 59, 60). In testing this assumption, we determined that a 100-cm-long vegetated buffer with 85 to 99% perennial fescue cover and under conditions involving a 5 to 20% slope, silty clay loam, loam, or sandy loam and precipitation rates of 1.5 or 4.0 ml/cm2/h, produced a 1.0- to 3.1-log10 mean reduction in waterborne C. parvum oocysts (bovine genotype A) (Table 6).
To put these estimated values for the filtration efficiency of a vegetated buffer into a public health perspective, a variety of domestic and wild mammalian species, including humans, have been shown to shed in excess of 106 C. parvum oocysts per individual per day during the course of infection (5, 31, 57). In comparison, the 50% infective dose (ID50) for livestock-derived C. parvum for healthy human adult volunteers ranges from approximately 10 to 1,000 (42). This suggests that a length of several meters of vegetated buffer may be needed to generate adequate water quality protection from relatively few infected individuals that are located in close proximity to surface water supplies. Appropriate modifications would also need to be made to buffer length for such factors as higher animal densities or higher rates of endemic cryptosporidiosis for specific mammalian populations (2, 4, 5, 40). Alternatively, the risk of waterborne transmission of C. parvum from entire livestock production systems (feedlot, cow to calf, dairy, etc.) at large distances from source water supplies, such as ≥30 m, should be quite minimal if the filtration efficiency of the intervening buffer has been properly maintained (e.g., adequate soil porosity and vegetative cover) and if the preferential flow paths and large macropores have been kept to a minimum (e.g., few rills, gullies, and complexes of small mammal burrows).
We calculated previously that at a 95% confidence level, the mean number of C. parvum oocysts being shed in the feces of California adult beef cows was around 1.7 × 105 oocysts per animal per day (26). With these estimates, a California herd comprised of 500 adult beef cows (no calves or yearlings) would on average produce an estimated maximum 8.5 × 107 oocysts/day. Given our range of a 1.0- to 3.1-log10 reduction produced by a meter of vegetated buffer, 3 to 7 m of equivalent buffer would be needed to reduce the translocation potential of freshly deposited C. parvum oocysts to below 1 oocyst for events involving low to moderate precipitation (≤4 cm/h). Such predictions are still crude approximations, due to the absence from our calculations of additional parameters that markedly influence microbial water quality risk from entire livestock operations (20, 26, 27, 55). Most notably, obtaining valid and precise methods for characterizing microbial risk from oocysts in aged fecal material remains problematic due to the observation that the translocation potential of C. parvum oocysts in aged fecal material (2 to 7 days old during summer) appears to be 3 log10 less than that of fresh fecal material, indicating that the majority of bovine-derived oocysts are trapped in aged fecal material (unpublished observation). In addition, exposure of oocysts to a variety of common environmental stressors can result in rapid loss of viability or infectivity (50). Furthermore, much of the fecal material produced by cattle is deposited in hydrologically remote areas, even when cattle use surface water supplies (e.g., streams) as their source of drinking water (17, 33). Although these additional processes collectively function to decrease the risk to water quality for a given C. parvum loading rate and buffer width, this reduction in risk to water quality is offset somewhat by the relatively large amount of aged compared to fresh fecal material located on the soil surface of livestock production systems (27).
The conceptual model motivating these experiments on buffer strip filtration is that C. parvum oocysts (or any gastrointestinal microbe) are deposited in fecal material, whereby a precipitation event of sufficient intensity erodes the upper layer of the fecal pat and releases oocysts onto the wetted soil surface. When rainfall intensity exceeds the infiltration capacity of the site, these waterborne oocysts are entrained into the prevailing surface flow (e.g., Hortonian overland-sheet flow, preferential flow in rills, and exfiltration in saturated variable source areas), wherein they are transported both downslope and infiltrate into the subsurface, the distance and comparative rates being the result of a variety of hydrogeological and other local factors governing overland flow over an infiltrating surface (55). Given that vegetative buffer strips are intended to intercept and remove these waterborne contaminants before they reach a specified downslope site, processes that enhance infiltration of contaminated surface runoff into the subsurface should improve buffer strip filtration. Therefore, for a given soil texture, we hypothesize that the ability of a vegetated buffer strip to remove waterborne zoonotic microorganisms from agricultural runoff is in part a function of the bulk density or porosity and pore size distribution of the underlying soil. For a specific soil, as bulk density is decreased, total porosity is increased. In general, this will function to increase effective porosity, leading to an increase in intrinsic permeability, increasing hydraulic conductivity, and thereby causing the infiltration rate (i) of the vegetated buffer to increase in some monotonic fashion. Under natural conditions, variations of bulk density in surface soils are caused by variation in plant and root density, microbial activity, and density and activity of soil macroorganisms (e.g., insects and worms), as well as by the amount of cultivation. In the soil box experiments, variations in bulk density are due to nonhomogeneous packing of the artificially filled boxes. Like macrobiotic activities in natural soils, artificial packing results in reduced bulk density due primarily to an increase in the macropore and mesopore space, not due to an increase in the micropore space. On the other hand, bulk density differences between different soil textures (i.e., sand, silt, and clay) can be largely attributed to an increase in micropore space, which does not increase the intrinsic permeability or infiltration capacity of the soil.
Under constant rainfall intensity (r) where r > i and once field saturated steady-state conditions exist (as in our experiment), buffers with lower bulk densities due to increased macro- and mesopore space should exhibit increased saturated hydraulic conductivity, leading to an increase in the proportion of total flow infiltrating into the subsurface. This enhanced partitioning of total flow into the subsurface should improve the ability of a buffer to remove via straining and adsorption waterborne zoonotic microorganisms that are being transported via advective and dispersive processes. Derivations of this general hypothesis have been presented previously for a variety of surface-subsurface interfacial systems, including, for example, the removal of silt particles from sheet flow on rangeland (45).
For our project, this hypothesis was generally correct for each of our three soils. As the bulk density was decreased, likely due to the increased density of micro-, macro-, and mesopores, greater amounts of the oocysts were removed from the surface effluent exiting the buffer (Fig. 3 to 5). At the same time, despite the fact that an increase in the infiltration rate was associated with increased numbers of oocysts in the subsurface effluent (Fig. 6), but this increase in oocysts in the subsurface outflow was insignificant compared to the decrease in unfiltered oocyst in the surface outflow. These observations suggest that management practices that decrease the bulk density and increase the infiltration capacity of agricultural soils could benefit water quality with respect to microbial contamination. Alternatively, land use practices that compact the soil or reduce its porosity may have a negative impact on microbial water quality by reducing the ability of vegetated buffers, grazed pastures, paddocks, and grazed rangeland to remove microbial contaminants from overland or shallow subsurface flow. For example, Blackburn (7) and Gifford and Hawkins (19) summarized a body of work illustrating that improperly managed livestock production practices can lead to soil compaction that reduces soil porosity and infiltration capacity. Reduced infiltration capacity was associated with increased surface runoff, soil erosion, and transport of nonpoint source pollutants. Soil bulk density is a common measure of soil compaction, and it has been long established that soil bulk density is negatively correlated with soil porosity and infiltration capacity, while being positively correlated with surface runoff (35, 43, 44, 49).
With respect to our set of three soils (silty clay loam, loam, and sandy loam), buffers constructed of sandy loam were least effective at removing oocysts from both overland and subsurface flow. Relative to the silty clay loam and loam, sandy loam had substantially greater amounts of sand (70%) than the other two soils (≤45%) and was dominated by macropores (pore diameters >0.08 mm) compared to the other two soils that were dominated by mesopores (0.03 to 0.08 mm) or micropores (<0.03 mm). Moreover, the silty clay loam and loam featured significant macropore space due to artificial packing, shrinking and swelling, and macrobiotic activity. At the same time, the sandy loam exhibited the highest bulk density. The sandy loam was the soil that was most closely packed to resemble natural conditions and had probably the fewest preferential and macropore flow paths due to shrinking and swelling, macrobiotic activity, or packing nonuniformity. We found previously by using 10-cm columns with saturated, sandy sediments that increasing the effective grain diameter from 180 to 1,400 μm was associated with a 2-log10 increase in the number of C. parvum oocysts eluting from the column (24). An additional soil difference was that sandy loam had a higher pH, 6.7, compared to pHs of 6.0 and 5.5 for silty clay loam and loam, respectively. Drozd and Schwartzbrod (14) estimate the zeta potential on the surface of fresh C. parvum oocysts in river water to be −23.7, −21.4, and −19.5 for the pH levels observed for these three soils (silty clay loam, loam, and sandy loam), respectively. Hence, the higher pH associated with the sandy loam will generate a more negatively charged soil surface in combination with a more negatively charged oocyst wall, creating less favorable conditions for oocyst attachment to the soil surface compared to those in the other soils. Similarly, elevated pH has been shown to inhibit attachment of negatively charged bacteria onto mineral surfaces under saturated conditions (52).
In general, oocyst removal efficiencies were greater for boxes with higher slopes (10 and 20%) than for boxes with a 5% slope, with this trend particularly strong at lower bulk densities. This effect of slope on oocyst removal could not be explained by a different infiltration rate (and hence, overland flow rate), due to the observation that the infiltration rate under steady-state conditions did not significantly change with slope within the conditions of this experiment. If we assume relatively uniform soil moisture and given that the overland flow rates were similar across the three slopes used in these experiments, removal efficiency should increase monotonically (albeit not necessarily linearly) with increasing slope due to a decreased thickness of the Hortonian sheet flow and a subsequent increased likelihood of oocyst filtration. The decreased thickness of the Hortonian sheet flow results when the overland flow rate (liters/time = Q) is held constant, but sheet-flow velocity (Q/area) is increased due to the higher slope. Interestingly, we did not observe a monotonic increase in filtration efficiency as slope was increased. To explain this nonmonotonic behavior, we speculate that the soil moisture near the soil surface was relatively uniform only for boxes set at a 5% slope. At slopes of 10% and particularly 20%, the soil moisture near the soil surface was lower for upslope sections compared to downslope sections within the soil box, due to the boundary conditions imposed by the soil box on the subsurface drainage flow. Thus, infiltration rates were nonuniform along the soil box surface for boxes with higher slopes, resulting in the observed nonmonotonic behavior of filtration efficiency as a function of slope. Resolving this interplay between slope, thickness of Hortonian sheet flow, and heterogeneities of the infiltration rate on filtration efficiency of a vegetative buffer will require a more controlled study than was performed herein.
Based on the data presented above and within the limitations of our experimental design, vegetated buffer strips of similar soils with bulk densities between 0.6 to 1.7 g/cm3, ≤20% slope, and widths of at least 3 m should generally function to remove 99.9% of C. parvum oocysts from overland flow generated during events involving low to moderate precipitation (≤4 cm/h). These minimal design criteria for a 3-log10 reduction are recommended under the assumption that vegetated buffers will be actively maintained such that overland and subsurface preferential flow paths are kept to a minimum. Communities with high levels of water quality risk intolerance, substantial amounts of recreational contact with surface water, or watersheds with high rates of C. parvum loading may want to strengthen these criteria in order to further minimize overland flow of C. parvum.
Acknowledgments
This project was funded by a grant from the International Life Sciences Institute North America, Technical Committee on Food Microbiology.
REFERENCES
- 1.Arrowood, M. J., and C. R. Sterling. 1987. Isolation of Cryptosporidium parvum and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J. Parasitol. 73:314-319. [PubMed] [Google Scholar]
- 2.Atwill, E. R., R. A. Sweitzer, M. Das Graças C. Pereira, I. A. Gardner, D. Van Vuren, and W. M. Boyce. 1997. Prevalence of and associated risk factors for shedding Cryptosporidium parvum and Giardia within feral pig populations in California. Appl. Environ. Microbiol. 63:3946-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atwill, E. R., J. A. Harp, T. Jones, P. W. Jardon, S. Checel, and M. Zylstra. 1998. Evaluation of periparturient dairy cows and contact surfaces as a reservoir of Cryptosporidium parvum for calfhood infection. Am. J. Vet. Res. 59:1116-1121. [PubMed] [Google Scholar]
- 4.Atwill, E. R., E. Johnson, M. Das Graças, and C. Pereira. 1999. Association of herd composition, stocking rate, and calving duration with fecal shedding of Cryptosporidium parvum oocysts in beef herds. J. Am. Vet. Med. Assoc. 215:1833-1838. [PubMed] [Google Scholar]
- 5.Atwill, E. R., S. Maldonado Camargo, R. Phillips, L. H. Alonso, K. W. Tate, W. A. Jensen, J. Bennet, S. Little, and T. P. Salmon. 2001. Quantitative shedding of two genotypes of Cryptosporidium parvum in California ground squirrels (Spermophilus beecheyi). Appl. Environ. Microbiol. 67:2840-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awad-El-Kariem, F. M., H. A. Robinson, F. Petry, V. McDonald, D. Evans, and D. Casemore. 1998. Differentiation between human and animal isolates of Cryptosporidium parvum using molecular and biological markers. Parasitol. Res. 84:297-301. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn, W. H. 1984. Impacts of grazing intensity and specialized grazing systems on watershed characteristics and responses, p. 927-1000. In National Research Council/National Academy of Sciences (ed.), Developing strategies for rangeland management. Westview Press, Boulder, Colo.
- 8.Brush, C. F., M. F. Walter, L. J. Anguish, and W. C. Ghiorse. 1998. Influence of pretreatment and experimental conditions on electrophoretic mobility and hydrophobicity of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 64:4439-4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brush, C. F., W. C. Ghiorse, L. J. Anguish, J. Y. Parlange, and H. G. Grimes. 1999. Transport of Cryptosporidium parvum oocysts through saturated columns. J. Environ. Qual. 28:809-815. [Google Scholar]
- 10.Buckhouse, J. C., and G. F. Gifford. 1976. Water quality implications of cattle grazing on a semiarid watershed in southeastern Utah. J. Range Manag. 29:109-113. [Google Scholar]
- 11.Chow, V. T., and T. E. Harbaugh. 1965. Raindrop production for laboratory watershed experimentation. J. Geophys. Res. 70:6111-6119. [Google Scholar]
- 12.Coyne, M. S., R. A. Gilifillen, R. W. Rhodes, and R. L. Blevins. 1995. Soil and fecal coliform trapping by grass filter during simulated rainfall. J. Soil Water Conserv. 50:405-408. [Google Scholar]
- 13.das Graças, M., C. Pereira, E. R. Atwill, and T. Jones. 1999. Comparison of sensitivity of immunofluorescent microscopy to that of a combination of immunomagnetic separation and immunofluorescent microscopy for detection of Cryptosporidium parvum oocysts in adult bovine feces. Appl. Environ. Microbiol. 65:3236-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drozd, C., and J. Schwartzbrod. 1996. Hydrophobic and electrostatic cell surface properties of Cryptosporidium parvum. Appl. Environ. Microbiol. 62:1227-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Entry, J. A., R. K. Hubbard, J. E. Thies, and J. J. Fuhrmann. 2000. The influence of vegetation in riparian filterstrips on coliform bacteria. I. Movement and survival in water. J. Environ. Qual. 29:1206-1214. [Google Scholar]
- 16.Garber, L. P., M. D. Salman, H. S. Hurd, T. Keefe, and J. L. Schlater. 1994. Potential risk factors for Cryptosporidium infection in dairy calves. J. Am. Vet. Med. Assoc. 205:86-91. [PubMed] [Google Scholar]
- 17.Gary, H. L., S. R. Johnson, and S. L. Ponce. 1983. Cattle grazing impact on surface water quality in a Colorado front range stream. J. Soil Water Conserv. 38:124-128. [Google Scholar]
- 18.Gee, G. W., and J. W. Bauder. 1979. Particle size analysis by hydrometer: a simplified method for routine textural analysis and a sensitivity test of measurement parameters. Soil Sci. Soc. Am. J. 43:1004-1007. [Google Scholar]
- 19.Gifford, G. F., and R. H. Hawkins. 1978. Hydrologic impacts of grazing on infiltration: a critical review. Water Resour. Res. 14:305-313. [Google Scholar]
- 20.Grazyck, T. K., B. M. Evans, C. J. Shiff, H. J. Karreman, and J. A. Patz. 2000. Environmental and geographical factors contributing to watershed contamination with Cryptosporidium parvum oocysts. Environ. Res. 82:263-271. [DOI] [PubMed] [Google Scholar]
- 21.Haan, C. T., B. J. Barfield, and J. C. Hayes. 1994. Design hydrology and sedimentology for small catchments. Academic Press, New York, N.Y.
- 22.Hardin, J., and J. Hilbe. 2001. Generalized linear models and extensions, p. 141-158. Stata Press, College Station, Tex.
- 23.Harp, J. A., P. Jardon, E. R. Atwill, M. Zylstra, S. Checel, J. P. Goff, and C. Desimone. 1996. Field testing of prophylactic measures against Cryptosporidium parvum infection in calves in a California dairy herd. Am. J. Vet. Res. 57:1586-1588. [PubMed] [Google Scholar]
- 24.Harter, T., S. Wagner, and E. R. Atwill. 2000. Colloid transport and filtration of Cryptosporidium parvum in sandy porous media (soils and groundwater). Environ. Sci. Technol. 34:62-70. [Google Scholar]
- 25.Hershfield, D. M. 1961. Rainfall frequency atlas of the United States for duration from 30 minutes to 24 hours and return periods from 1 to 100 years. Technical paper no. 40. Department of Commerce, Washington, D.C.
- 26.Hoar, B., E. R. Atwill, and T. B. Farver. 2000. Estimating maximum possible environmental loading amounts of Cryptosporidium parvum attributable to adult beef cattle. Quant. Microbiol. 2:21-36. [Google Scholar]
- 27.Hoar, B. R., E. R. Atwill, C. Elmi, W. W. Utterback, and A. J. Edmondson. 1999. Comparison of fecal samples collected per rectum and off the ground for estimation of environmental contamination attributable to beef cattle. Am. J. Vet. Res. 60:1352-1356. [PubMed] [Google Scholar]
- 28.Holmgren, G. G. S. 1967. A rapid citrate-dithionite extractable iron procedure. Soil Sci. Soc. Am. Proc. 31:210-211. [Google Scholar]
- 29.Janitzky, P. 1986. Cation exchange capacity, p. 21-23. In M. J. Singer and P. Janitzky (ed.), Field and laboratory procedures used in a soil chronosequence study. U.S. Geological Survey bulletin 1648. U.S. Geological Survey, Washington, D.C.
- 30.Jenkins, M. B., M. J. Walker, D. D. Bowman, L. C. Anthony, and W. C. Ghiorse. 1999. Use of a sentinel system for field measurements of Cryptosporidium parvum oocyst inactivation in soil and animal waste. Appl. Environ. Microbiol. 65:1998-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuchzynska, E., and D. R. Shelton. 1999. Method for detection and enumeration of Cryptosporidium parvum oocysts in feces, manures, and soils. Appl. Environ. Microbiol. 65:2820-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen, R. E., J. R. Miner, J. C. Buckhouse, and J. A. Moore. 1994. Water quality benefits of having cattle manure deposited away from streams. Bioresour. Technol. 48:113-118. [Google Scholar]
- 33.Larsen, R. E., J. C. Buckhouse, J. A. Moore, and J. R. Miner. 1988. Rangeland cattle and manure placement: a link to water quality. Oreg. Acad. Sci. 24:7-15. [Google Scholar]
- 34.Lavkulich, L. M., and J. H. Wiens. 1970. Comparison of organic matter destruction by hydrogen peroxide and sodium hypochlorite and its effect on selected mineral constituents. Soil Sci. Soc. Am. Proc. 34:755-758. [Google Scholar]
- 35.Liacos, L. G. 1962. Water yield as influenced by degree of grazing in the California winter grasslands. J. Range Manag. 15:67-72. [Google Scholar]
- 36.Maldonado-Camargo, S., E. R. Atwill, J. A. Saltijeral-Oaxaca, and L. C. Herrera-Alonso. 1998. Prevalence of and risk factors for shedding of Cryptosporidium parvum in Holstein Freisian dairy calves in central México. Prev. Vet. Med. 36:95-107. [DOI] [PubMed] [Google Scholar]
- 37.Mawdsley, J. L., A. E. Brooks, and R. J. Merry. 1996. Movement of the protozoan Cryptosporidium parvum through three contrasting soil types. Biol. Fertil. Soil 21:30-36. [Google Scholar]
- 38.Mawdsley, J. L., A. E. Brooks, R. J. Merry, and B. F. Pain. 1996. Use of a novel titling table apparatus to demonstrate the horizontal and vertical movement of the protozoan Cryptosporidium parvum in soil. Biol. Fertil. Soil 23:215-220. [Google Scholar]
- 39.McLauchlin, J., C. Amar, S. Pedraza-Díaz, and G. L. Nichols. 2000. Molecular epidemiological analysis of Cryptosporidium spp. in the United Kingdom: results of genotyping Cryptosporidium spp. in 1,705 fecal samples from humans and 105 fecal samples from livestock animals. J. Clin. Microbiol. 38:3984-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohammed, H. O., S. E. Wade, and S. Schaaf. 1999. Risk factors associated with Cryptosporidium parvum infection dairy cattle in southeastern New York State. Vet. Parasitol. 83:1-13. [DOI] [PubMed] [Google Scholar]
- 41.Nelson, D. W., and L. E. Sommers. 1982. Total carbon, organic carbon, and organic matter, p. 539-579. In A. L. Page (ed.), Methods of soil analysis. Part 2. Chemical and microbiological properties. Agronomy monograph 9, 2nd ed. American Society of Agronomy, Madison, Wis.
- 42.Okhuysen, P. C., C. L. Chappell, J. H. Crabb, C. R. Sterling, and H. L. DuPont. 1999. Virulence of three different Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 180:1275-1281. [DOI] [PubMed] [Google Scholar]
- 43.Orr, H. K. 1960. Soil porosity and bulk density on grazed and protected Kentucky bluegrass range in the Black Hills. J. Range Manag. 13:80-86. [Google Scholar]
- 44.Packer, P. E. 1953. Effect of trampling disturbance on watershed condition, runoff and erosion. J. For. 51:28-31. [Google Scholar]
- 45.Pearce, R. A., M. J. Trlica, W. C. Leininger, D. E. Mergen, and G. Frasier. 1998. Sediment movement through riparian vegetation under simulated rainfall and overland flow. J. Range Manag. 51:301-308. [Google Scholar]
- 46.Peng, M. M., L. Xiao, A. R. Freeman, M. J. Arrowood, A. A. Escalante, A. C. Weltman, C. S. L. Ong, W. R. MacKenzie, A. A. Lal, and C. B. Beard. 1997. Genetic polymorphism among Cryptosporidium isolates: evidence of two distinct human transmission cycles. Emerg. Infect. Dis. 3:567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perryman, L. E., S. J. Kapil, M. L. Jones, and E. L. Hunt. 1999. Protection against calves against cryptosporidiosis with immune bovine colostrum induced by a Cryptosporidium parvum recombinant protein. Vaccine 17:2142-2149. [DOI] [PubMed] [Google Scholar]
- 48.Pinheiro, J. C., and D. M. Bates. 2000. Mixed effects model in S and S-Plus, p. 57-96. Springer, New York, N.Y.
- 49.Rauzi, F., and C. L. Hanson. 1966. Water intake and runoff as affected by intensity of grazing. J. Range Manag. 19:351-356. [Google Scholar]
- 50.Robertson, L. J., A. T. Campbell, and H. V. Smith. 1992. Survival of Cryptosporidium parvum oocysts under various environmental stressors. Appl. Environ. Microbiol. 58:3494-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosen, B. H., R. Croft, E. R. Atwill, S. Wade, and S. Stehman. 2000. Waterborne pathogens in agricultural watersheds. Technical note 2, p. 1-64. Watershed Science Institute, University of Vermont, Burlington, Vt.
- 52.Scholl, M. A., A. L. Mills, J. S. Herman, and G. M. Hornberger. 1990. The influence of mineralogy and solution chemistry on the attachment of bacteria to representative aquifer materials. J. Contam. Hydrol. 6:321-336. [Google Scholar]
- 53.Sischo, W. M., E. R. Atwill, L. E. Lanyon, and J. George. 2000. Cryptosporidia on dairy farms and the role these farms may have in contaminating surface water supplies in the northeastern United States. Prev. Vet. Med. 43:253-267. [DOI] [PubMed] [Google Scholar]
- 54.Spano, F., L. Putignani, J. McLauchlin, D. P. Casemore, and A. Crisantil. 1997. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrari and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol. Lett. 150:209-217. [DOI] [PubMed] [Google Scholar]
- 55.Tate, K. W. E. R. Atwill, M. R. George, N. K. McDougald, and R. E. Larsen. 2000. Cryptosporidium parvum transport from cattle fecal deposits on California rangeland. J. Range Manag. 53:295-299. [Google Scholar]
- 56.Teunis, P. F. M., G. J. Medema, L. Kruidnier, and A. H. Havelaar. 1997. Assessment of the risk of infection by Cryptosporidium or Giardia in drinking water from a surface water source. Water Res. 31:1333-1346. [Google Scholar]
- 57.Uga, S., J. Matsuo, E. Kono, K. Kimura, M. Inoue, S. K. Rai, and K. Ono. 2000. Prevalence of Cryptosporidium parvum infection and pattern of oocyst shedding in calves in Japan. Vet. Parasitol. 94:27-32. [DOI] [PubMed] [Google Scholar]
- 58.Whittig, L. D., and W. R. Allardice. 1986. X-ray diffraction techniques, p. 331-362. In A. Klute (ed.), Methods of soil analysis. Part 1. Physical and mineralogical methods. Agronomy monograph 9, 2nd ed. American Society of Agronomy, Madison, Wis.
- 58a.Xiao, L., U. M. Morgan, J. Limor, A. Escalante, M. Arrowood, W. Shulaw, R. C. A. Thompson, R. Fayer, and A. A. Lal. 1999. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol. 65:3386-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young, R. A., T. Hundtrods, and W. Anderson. 1980. Effectiveness of vegetated buffer strips in controlling pollution from feedlot runoff. J. Environ. Qual. 9:483-487. [Google Scholar]
- 60.Younos, T. M., A. Mendez, E. R. Collins, and B. B. Ross. 1998. Effects of a dairy loafing lot-buffer strip on stream water quality. J. Am. Water Res. Assoc. 34:1061-1069. [Google Scholar]