Abstract
The virulence of different pulsed-field gel electrophoresis (PFGE) types of Listeria monocytogenes was examined by monitoring their ability to invade Caco-2 cells. Strains belonging to seven different PFGE types originating from both foods and humans were included. No significant differences in invasiveness were detected between strains isolated from humans and those isolated from food. Strains belonging to PFGE type 1 expressed a significantly lower ability to invade cells compared to strains belonging to other PFGE types. Although strains of PFGE type 2 also seemed to invade at a low level, this was not significant in the present study. PFGE types 1 and 2 as well as type 14 are more frequently found in food than the four other PFGE types examined and moreover have a relatively low prevalence in humans compared to their prevalence in food. Thus, the hypothesis that some PFGE types are less virulent than others is supported by this study showing that certain PFGE types of L. monocytogenes commonly found in food are less invasive than others to Caco-2 cells. In contrast to the differences in invasion, identical intracellular growth rates between the different PFGE types were observed. In vivo studies of the actual ability of the strains to invade the liver and spleen of cimetidine-treated rats following an oral dose of 109 L. monocytogenes cells were performed for isolates of PFGE types 1, 2, 5, and 15. After 2 days, equal amounts of bacteria were observed in the liver and spleen of the rats for any of the PFGE types tested.
Listeria monocytogenes is an intracellular pathogen of humans and animals that expresses differences in virulence within individual strains (7, 27). Among the 13 serotypes known for this species, most isolates from human cases of listeriosis seem to belong to only three serotypes (1/2a, 1/2b, and 4b). While serotyping has limited application in epidemiological studies, more discriminatory molecular typing methods, such as multilocus enzyme electrophoresis (12, 16), pulsed-field gel electrophoresis (PFGE) (18, 20), random amplification of polymorphic DNA (3, 4), ribotyping (23), and phage typing (14), have successfully been used to differentiate among isolates of L. monocytogenes.
PFGE has been shown to be highly discriminative for strains of L. monocytogenes (10, 11, 15), either alone or in combination with other methods. PFGE typing of 123 isolates of L. monocytogenes from ready-to-eat foods and 39 isolates from human clinical cases of infection showed a significant difference in the distribution of PFGE types among food isolates compared to human isolates (18), with some types having a higher prevalence among food isolates and vice versa.
Individual isolates of L. monocytogenes are known to vary in virulence (7, 9). Most earlier studies that have described the association between various subtypes and the virulence of the strains have focused on multiplication inside the host rather than invasion into the intestinal mucosa. The results are not clear, however. By this approach, Nørrung and Andersen (17) found that certain electrophoretic types (ET 2 and ET 4) were less virulent than others assayed by direct inoculation of bacteria into the allantoic sac of chick embryos. However, Brosch and coworkers (1) found no differences in virulence following direct intravenous injection of the bacteria into immunocompetent mice.
In vivo virulent L. monocytogenes invade enterocytes (or Peyer's patches) in the gut and multiply intracellularly (25). In vitro, virulent L. monocytogenes strains are able to invade the enterocyte-like cell line Caco-2 (8), and this characteristic has been used to measure the virulence of different strains of L. monocytogenes (5, 22, 26).
Virulence has also been studied with animals infected by injection or orally. This way, both mice (2) and rats (24) have been applied to study the virulence of L. monocytogenes with different means of infection, such as orally (24), intravenously (26), intraperitoneally (2, 19), and in the footpads (26), depending on the purpose of the study. Such studies have not, to our knowledge, correlated specific types of L. monocytogenes with the ability to actually invade the host.
The purpose of this study was to examine different PFGE types of L. monocytogenes with respect to their virulence. This was tested in vitro by the invasiveness and growth of the strain in Caco-2 cells as well as in vivo in orally infected cimetidine-treated rats. The effect of the origin of the isolate was also studied in vitro.
MATERIALS AND METHODS
Selection of bacterial isolates and culture conditions.
This study comprised 26 strains of L. monocytogenes (Table 1). Previously, they had been shown to belong to seven different PFGE types that could be divided into two groups: PFGE types 1, 2, and 14 consisted of strains predominantly isolated from food, and PFGE types 5, 6, 15, and 27 consisted of strains predominantly found in cases of human listeriosis (18). The original material (18) consisted of 39 strains representing all isolates from cases of clinical listeriosis in Denmark in 1998 collected by the Statens Serum Institute, Copenhagen, Denmark, combined with 123 strains isolated from ready-to-eat foods at the Municipal Food and Environmental Laboratories in Denmark during the first 6 months of 1998. The strains were serotyped and PFGE typed with ApaI for restriction digestion. Differences in two bands were used to discriminate between isolates obtaining a total of 41 different PFGE types. PFGE types were shared by both human and food isolates, but with different prevalences. This way, 1 of 10 isolates in PFGE type 1 was of clinical origin, 5 of 49 were in PFGE type 2, 3 of 5 were in PFGE type 5, 5 of 8 were in PFGE type 6, 1 of 11 was in PFGE type 14, 3 of 4 were in type 15, and 3 of 5 were in PFGE type 27. An equal number of isolates from each type and origin were tested when possible, resulting in 13 isolates of human origin and 13 isolates from ready-to-eat foods. All 26 strains were studied in the invasion assay, 7 of the strains were tested in the intracellular growth study, and 12 of the strains were tested in the per oral rat model. The strains were grown in brain heart infusion (BHI) broth (Difco) for 20 to 24 h at 37°C prior to testing. Stock cultures were maintained at −80°C in 15% (vol/vol) glycerol. The strains are presented in Table 1.
TABLE 1.
Number of L. monocytogenes cells invading Caco-2 cells
| Strain | Origin | PFGE type (serotype) | No. of intracellular bacteria (CFU/well) |
|---|---|---|---|
| 4666a,b | 30-yr-old male with a cutaneous abscess | 1 (1) | 125 |
| 7394a,b | Smoked ham | 1 (1) | 125 |
| 7286a,b | Crab meat | 1 (1) | 125 |
| 4459b | 64-yr-old female with septicemia | 2 (1) | 350 |
| 19b | 52-yr-old male with peritonitis | 2 (ND)c | 475 |
| 4629 | 58-yr-old female with septicemia | 2 (1) | 1,375 |
| 7430 | Ham/cheese | 2 (1) | 525 |
| 7324b | Kabob | 2 (1) | 175 |
| 4466 | 64-yr-old male with meningitis | 14 (1) | 900 |
| 7418 | Spreadable sauce | 14 (1) | 405 |
| 7813 | Spreadable cheese | 14 (1) | 3,775 |
| 4274a,b | 65-yr-old female with septicemia | 5 (4) | 2,075 |
| 7289a,b | Tomato sauce | 5 (4) | 2,300 |
| 6896a,b | Roast pork | 5 (1) | 4,575 |
| 4446a,b | 63-yr-old female with septicemia | 5 (4) | 24,500 |
| 5175 | 86-yr-old male with septicemia | 6 (1) | 1,160 |
| 7275 | Shrimp | 6 (1) | 910 |
| 4906 | 73-yr-old female with septicemia | 6 (ND) | 250 |
| 6895 | Ham | 6 (1) | 3,175 |
| 4473b | 80-yr-old female with meningitis | 15 (4) | 3,750 |
| 4651 | 76-yr-old female with septicemia | 15 (4) | 170 |
| 7291b | Pasta/chicken | 15 (4) | 3,750 |
| 4239 | 52-yr-old male with septicemia | 27 (1) | 150 |
| 5104 | 82-yr-old male with septicemia | 27 (1) | 2,825 |
| 7385 | Pickled pork | 27 (1) | 720 |
| 7386 | Smoked fillet | 27 (1) | 2,125 |
Isolate included in the intracellular growth studies.
Isolate included in the oral rat study.
ND, not determined.
Cell culture.
Enterocyte-like Caco-2 cells obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) were cultured in Eagle's minimum essential medium (MEM) enriched with Glutamax and HEPES (Invitrogen, Inc., Tåstrup, Denmark), supplemented with 20% heat-inactivated (30 min at 56°C) fetal bovine serum (Invitrogen), 0.1 mM nonessential amino acids (Invitrogen A/S), and 0.5 ml of gentamicin (50 mg/ml) (Invitrogen) in a water jacket incubator with 5% CO2 at 37°C. Cells were used at passages 10 to 15.
Invasion assay and intracellular growth studies.
Cells were trypsinized, and the cell concentration was adjusted to 5 × 105 cells per ml. Cells were grown without gentamicin to a monolayer (68 to 72 h at 37°C) in Eagle's MEM supplemented with 10% heat-inactivated fetal bovine serum and 0.1 mM nonessential amino acids. The medium was changed every 24 h. L. monocytogenes isolates were grown on blood agar (BA) plates (Oxoid) at 37°C 24 h prior to infection. The bacteria were harvested, adjusted to approximately 3 × 106 bacteria per ml, and added to each well, resulting in a multiplicity of infection of about 30 bacteria per cell. Following 1 h of incubation at 37°C, the cells were washed three times with peptone water containing 8.5 g of NaCl per liter and 0.5 g of Bacto Peptone per liter (Difco) at pH 7.2. To kill extracellular bacteria, 2 ml of MEM with gentamicin (10 μg/ml) was added to the wells, and the mixture was then incubated for 2 h at 37°C with 5%CO2 (modified according to reference 8). After incubation, the cells were lysed immediately with 1 ml of ice-cold 0.1% Triton X-100, and the bacteria were serially diluted before being plated onto BA to determine the number of viable intracellular bacteria. Plates were counted following 2 days of incubation at 37°C. The invasiveness of an isolate was measured as the number of CFU per well. For the intracellular growth studies, cells were lysed as described above 2, 4, 6, 12, and 24 h after gentamicin had been applied to the infected cells. Each isolate was measured in duplicate on two separate occasions. The isolates tested over time are marked in Table 1. In each run, a control strain (isolate 4274) was included for both types of cell assays.
Oral rat model.
A model of food-borne L. monocytogenes infection in rats using oral inoculation as described by Schlech et al. (24) was used in this study. Twelve of the 26 (marked in Table 1) strains of L. monocytogenes were examined in the rat model. Twenty-four juvenile (body weights of 125 to 150 g, 30 to 40 days old) Sprague-Dawley rats (M & B A/S, Ry, Denmark) raised under conventional conditions were used in the experiment. Each L. monocytogenes strain was administered to two rats (a male and a female). Inocula of L. monocytogenes were prepared by overnight growth in 10 ml of BHI broth at 37°C. Five milliliters of the overnight cultures was centrifuged (7,500 × g, 10 min), and the pellet was resuspended in 5 ml of physiological saline. One milliliter of this suspension was used as the inoculum. The inoculum size was confirmed by plating on BHI agar and was found to be around 109 cells per animal. This inoculum dose was chosen because a preliminary study with three animals with inocula of 107, 108, and 109 cells per animal showed that invasion of spleen and liver only occurred in the animal that received 109 cells. Each strain of L. monocytogenes (inoculum) was administered to one male and one female rat by a feeding tube placed in the esophagus. One hour prior to inoculation, the rats were given an intraperitoneal injection of 50 mg of cimetidine per kg of body weight in 0.5 ml of saline in order to lower the gastric pH. Animals were placed in individual cages and were given free access to water and standard food.
Forty-eight hours after inoculation, the rats were anesthetized with CO2 and decapitated. Samples of liver and spleen were obtained, weighed, and homogenized. Serial dilutions of homogenates were plated on BA and incubated at 37°C overnight. The BA plates were examined for purity, and all small colonies with hemolysis were counted as L. monocytogenes.
Experimental design and statistics of invasion assay.
The invasion experiment was designed as a three-way variance analysis (Table 2), examining the effects of the origin (human or food), PFGE type, and the run (days I to VII). The isolates measured in each run were chosen randomly among the 14 combinations of PFGE type or origin in order to average out the potential block effect from the runs. Measurement of an isolate consisted of two duplicates, each measured twice, for a total of four measurements per isolate.
TABLE 2.
Study design for comparison of PFGE type versus origin of L. monocytogenes strainsa
| Origin | PFGE type
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
14
|
5
|
6
|
15
|
27
|
||||||||
| Strain | Run | Strain | Run | Strain | Run(s) | Strain | Run(s) | Strain | Run | Strain | Run | Strain | Run(s) | |
| Food | 7494 | I | 7430 | II | 7418 | IV, VII | 7289 | I | 7275 | IV | 7291 | III | 7385 | IV |
| 7286 | II | 7324 | III | 7813 | VI | 6896 | II | 6895 | V | 7386 | VI | |||
| Human clinical | 4666 | I | 4459 | I | 4466 | IV, VII | 4446 | V | 5175 | III | 4473 | II | 4239 | IV, VII |
| 19 | II | 4274 | I-VII | 4906 | VI | 4651 | III | 5104 | VI | |||||
| 4629 | III | |||||||||||||
Roman numerals describe in which run a subexperiment was performed.
The data were log transformed, and the residual plot indicated a normal distribution of data. The normality was a prerequisite for carrying out the variance analysis. The variance analysis was carried out by the GLM procedure in SAS (SAS Institute, Cary, N.C.). All data including those for the control strain were used in the statistical analysis. A full model with potential interactions was tested and reduced when possible—that is, when effects were not significant. Duncan's multiple range test was subsequently employed to identify whether any type within the whole group was significantly different from the others. Due to the incomplete and unbalanced experimental design, the means had to be adjusted before use in the Duncan's test.
RESULTS
An overview of the results from the invasion assay is presented in Fig. 1; the actual numbers are presented in Table 1. Neither the interactions nor the effect of the origin was found to be significant (results not shown) by statistical analysis. Thus, the model was reduced to a two-way variance analysis with the PFGE types and runs being the only significant effects (P = 0.001 and P = 0.0045, respectively), as shown in Table 3. The variation of the control strain used in each run is shown in Fig. 2.
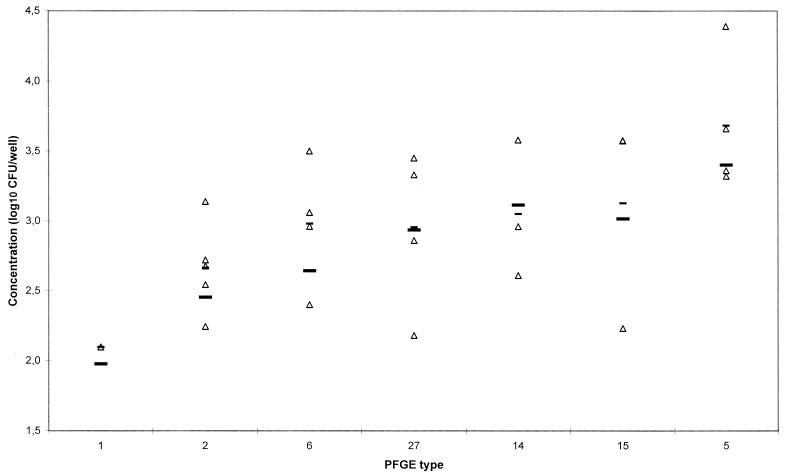
FIG. 1.
Distribution of the invasion of isolates from PFGE types 1, 2, 5, 6, 14, 15, and 27 sorted with increasing rates of invasion. Each triangle represents the mean of an isolate. A short bar represents the mean of the data unadjusted for the run effect, and a long bar represents the mean of the adjusted data for each group.
TABLE 3.
Results of variance analysis for log-transformed data
| Source of data | No. of degrees of freedom | Sum of squares | Mean of squares | F statistic | Probability > F |
|---|---|---|---|---|---|
| PFGE type | 6 | 5.732 | 0.955 | 7.60 | 0.0001 |
| Run | 6 | 3.188 | 0.531 | 4.23 | 0.0045 |
| Error | 19 | 3.141 | 0.126 | ||
| Total | 25 | 13.956 |
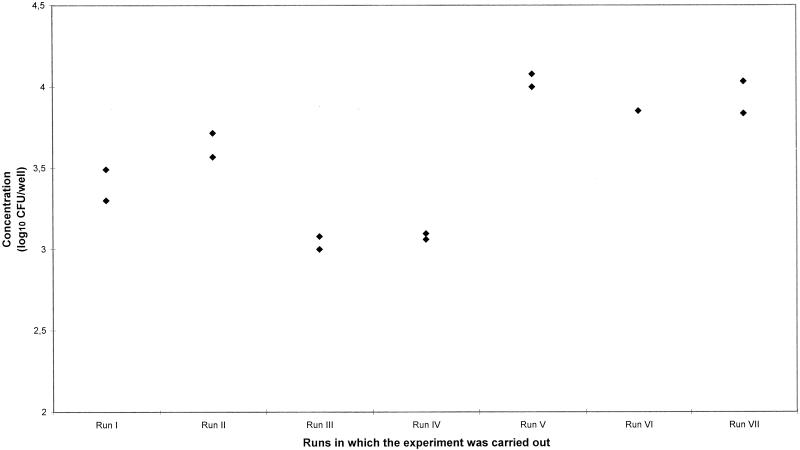
FIG. 2.
Variation of the control data (isolate 4274) in runs I to VII of the intracellular study of 26 isolates of L. monocytogenes. The standard deviation for each measurement is shown.
Prior to employing Duncan's multiple range test for the PFGE types, some adjustment was carried out due to the significant run effect. All measurements were adjusted by subtracting the estimate of the run-specific effect (the estimates were given by variance analysis). In Fig. 1, the results from adjusting the data can be seen in the estimate of the mean for each PFGE type (long bar). The results showed that the mean rate of invasion of PFGE type 1 was significantly lower than those of types 5, 6, 14, 15, and 27, but was not significantly lower than that of type 2, at a significance level of 5% (results not shown). Type 2 also seems to invade at a relative low rate, supporting the study by Nørrung et al. (18), who found that types 1, 2, and 14 have a higher prevalence among the food isolates than among human isolates. In this study, however, type 14 was not found to have a particularly low rate of invasion.
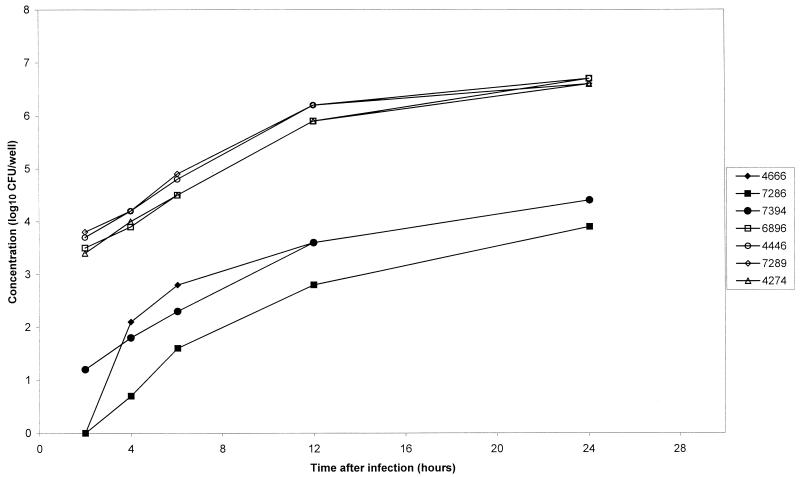
Studying the growth of L. monocytogenes strains 4666, 7286, and 7394 (all PFGE type 1) inside the cell and comparing it to the intracellular growth of strains 4274, 4446, 6896, and 7289 (all PFGE type 5) appear to demonstrate that, despite the difference in their abilities to invade the Caco-2 cells, once inside the cell, all strains tested seemed to grow equally well. The strains belonging to PFGE type 1 never reached the same concentration inside the cells as PFGE type 5 in the 24-h period tested (Fig. 3). The run effect for this study could not easily be statistically tested. However, even if adjusted for the run effect by using the controls, the overall results do not change: the growth rates for the two types are about the same, whereas the invasion rate for type 5 is higher than that for type 1, matching the results of the invasion experiments.
FIG. 3.
Invasion and growth inside Caco-2 cells of L. monocytogenes PFGE type 5 isolates 4446, 6896, 7289, and 4274 and PFGE type 1 isolates 4666, 7286, and 7394.
In the oral rat model, no differences in rates of invasion of the liver and spleen for the strains of L. monocytogenes PFGE types 1, 2, 5, and 15 tested were observed (Table 4).
TABLE 4.
Number of L. monocytogenes cells in liver and spleen 48 h after peroral infection of cimetidine-treated Sprague-Dawley rats
| Strain and PFGE type | Invasion (log CFU/g)
|
Avg invasion for PFGE type (log CFU/g)
|
||
|---|---|---|---|---|
| Spleen | Liver | Spleen | Liver | |
| Type 1 | ||||
| 4666 | 4.17 | 3.54 | 4.33 | 3.68 |
| 7394 | 4.35 | 3.74 | ||
| 7286 | 4.40 | 3.74 | ||
| Type 2 | ||||
| 4459 | 4.51 | 3.42 | 4.54 | 4.18 |
| 19 | 4.57 | 4.40 | ||
| 7324 | 4.52 | 4.24 | ||
| Type 5 | ||||
| 4274 | 4.18 | 3.86 | 4.42 | 3.82 |
| 7289 | 4.36 | 3.42 | ||
| 4446 | 3.98 | 3.40 | ||
| Type 15 | ||||
| 6896 | 4.75 | 4.14 | 4.38 | 3.72 |
| 4473 | 3.48 | 3.65 | ||
| 7291 | 4.65 | 3.78 | ||
DISCUSSION
Virulence of L. monocytogenes is dependent on the organism's ability to invade the enterocytes in the intestines, multiply, and spread to neighboring cells of different origin. It has been suggested that some strains are more virulent than others (9, 24), which has been investigated by different methods.
The objective of this study was to compare the levels of virulence of seven different PFGE types of L. monocytogenes originating from both human clinical cases of infection and food. Invasiveness into and growth in a human epithelial cell line and invasiveness into the spleens of orally infected rats were used as virulence models. Since the various PFGE types seemed to be dominated by either clinical or food-related isolates, it was interesting to see whether the virulence differed between the various PFGE types and/or the origin of the isolates. From each of these seven PFGE types, when possible, equal numbers of strains originating from clinical samples as well as food were examined.
To our knowledge, this is the first study showing a significant correlation between PFGE type and invasion into Caco-2 cells. PFGE type 1 expressed a statistically significant lower invasiveness than types 5, 6, 14, 15, and 27. At the other end of the scale, PFGE type 5 showed the highest invasion rate. These observed differences in invasiveness might explain why certain PFGE types seem to be dominant in clinical cases of listeriosis. In the original material (18), the clinical cases are equally represented in the seven PFGE types analyzed here, whereas 36% of the food isolates in the original material are PFGE type 2.
When looking at the growth inside the Caco-2 cell over 24 h, the lower infection rate did not seem to influence the growth rate. Despite the observed variation in the abilities of the different PFGE types to invade Caco-2 cells, 100% infection of the liver and spleen of orally infected rats was observed 2 days after infection for all PFGE types measured. Both cell and rat models suggest that the isolates share the ability to establish a generalized infection once inside the cell. However, infection of rats has been shown to be dose dependent (24), and a lower dose level of 107 cells was tried out first without the rats becoming infected (results not shown). Therefore, the rats were infected at a higher level. This high level could be the reason why the infections in the liver and spleen were almost identical in this study, regardless of the PFGE studies. Also the pH in the stomach of the rats was modified, making it easier to reach the intestines unaffected by the passage of the stomach. In this sense, variable tolerance of isolates of L. monocytogenes to low pH has been demonstrated in vitro (6, 21) and could have an influence on the level of invading bacteria. Future investigations will show whether invasion of these isolates is influenced by stress both in vitro and in vivo.
The significant variations in the control strain from run to run emphasize how difficult cell assays are to perform and how important it is to always measure a control strain in each run, and these variations should be considered when designing the experiments. This way, the run effect can be accounted for and potential significant effects will become easier to detect.
When comparing the infection rates of rats, all isolates seemed to perform equally well regardless of the PFGE type, showing 100% infection of the liver and spleen. These results suggest that all of the isolates share the ability to establish a generalized infection once inside the cell.
However, rats might not be the best in vivo model to use (13), since the surface protein internalin cannot interact with the rat E-cadherin. This infection mechanism cannot be the only route, though, since this and other studies report oral infection of rats. The possibility that the rat is infected by alternative routes from those in humans could be the reason why the results of the human cell assay cannot be compared with the results of the oral rat model. This needs to be confirmed in future studies with guinea pigs, whose E-cadherin seems to be comparable with human E-cadherin (13).
In conclusion, this study has shown that strains of different PFGE types differ significantly in their ability to invade Caco-2 cells, although they do show the same growth potential once inside the cell. The results from the invasion studies could not be reproduced in an oral rat model for the PFGE types tested, and another model could be tried. When working with cells, it seems very important to control the assay by testing a control strain in every run. Combining studies of differences in the expression of virulence genes with the present study could give more information on the complexity of virulence of L. monocytogenes.
Acknowledgments
Support for this work by The Centre for Advanced Food Studies is greatly appreciated.
REFERENCES
- 1.Brosch, R., B. Catimel, G. Milon, C. Buchrieser, E. Vindel, and J. Rocourt. 1993. Virulence heterogeneity of Listeria monocytogenes strains from various sources (food, human, animal) in immunocompetent mice and its association with typing characteristics. J. Food Prot. 56:296-301. [DOI] [PubMed] [Google Scholar]
- 2.Conner, D. E., V. N. Scott, S. S. Sumner, and D. T. Bernard. 1989. Pathogenicity of foodborne, environmental and clinical isolates of Listeria monocytogenes in mice. J. Food Sci. 54:1553-1556. [Google Scholar]
- 3.Czajka, J., and C. A. Batt,. 1994. Verification of causal relationships between Listeria monocytogenes isolates implicated in food-borne outbreaks of listeriosis by random amplified polymorphic DNA patterns. J. Clin. Microbiol. 32:1280-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czajka, J., N. Bsat, M. Piani, W. Russ, K. Sultana, M. Wiedmann, R. Whitaker, and C. A. Batt. 1993. Differentiation of Listeria monocytogenes and Listeria innocua by 16S rDNA genes and intraspecies discrimination of Listeria monocytogenes strains by random amplified polymorphic DNA polymorphisms. Appl. Environ. Microbiol. 59:304-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Corral, F., R. L. Buchanan, M. M. Bencivengo, and P. H. Cooke. 1990. Quantitative comparison of selected virulence associated characteristics in food and clinical isolates of Listeria. J. Food Prot. 53:1003-1009. [DOI] [PubMed] [Google Scholar]
- 6.Dykes, G. A., and S. M. Moorhead. 2000. Survival of osmotic and acid stress by Listeria monocytogenes strains of clinical or meat origin. Int. J. Food Microbiol. 56:161-166. [DOI] [PubMed] [Google Scholar]
- 7.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaillard, J.-L., P. Berche, J. Mounier, S. Richard, and P. Sansonetti. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hof, H., and J. Rocourt. 1992. Is any strain of Listeria monocytogenes detected in food a health risk? Int. J. Food Microbiol. 16:173-182. [DOI] [PubMed] [Google Scholar]
- 10.Howard, P. J., K. D. Harsono, and J. B. Luchansky. 1992. Differentiation of Listeria monocytogenes, Listeria innocua, Listeria ivanovii, and Listeria seeligeri by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 58:709-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerouanton, A., A. Brisabois, E. Denoyer, F. Dilasser, J. Grout, G. Salvat, and B. Picard. 1998. Comparison of five typing methods for the epidemiological study of Listeria monocytogenes. Int. J. Food Microbiol. 43:61-71. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence, L. M., and A. Gilmour. 1995. Characterization of Listeria monocytogenes isolated from poultry products and from the poultry-processing environment by random amplification of polymorphic DNA and multilocus enzyme electrophoresis. Appl. Environ. Microbiol. 61:2139-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loessner, M. J. 1991. Improved procedure for bacteriophage typing of Listeria strains and evaluation of new phages. Appl. Environ. Microbiol. 57:882-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louie, M., P. Jayaratne, I. Luchsinger, J. Devenish, J. Yao, W. Schlech, and A. Simor. 1996. Comparison of ribotyping, arbitrarily primed PCR, and pulsed-field gel electrophoresis for molecular typing of Listeria monocytogenes. J. Clin. Microbiol. 34:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nørrung, B., and N. Skovgaard. 1993. Application of multilocus enzyme electrophoresis in studies of the epidemiology of Listeria monocytogenes in Denmark. Appl. Environ. Microbiol. 59:2817-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nørrung, B., and J. K. Andersen. 2000. Variations in virulence between different electrophoretic types of Listeria monocytogenes. Lett. Appl. Microbiol. 30:228-232. [DOI] [PubMed] [Google Scholar]
- 18.Nørrung, B., B. Ojeniyi, and M. Badaki. 1999. Pulsed field gel electrophoresis of Listeria monocytogenes isolated from foods and human listeriosis in Denmark, p. 576-579. In A. C. J. Tuijtelaars, R. A. Samson, F. M. Rombouts, and S. Notermans (ed.), Food microbiology and food safety into the next millenium. Proceedings of the 17th International Conference of the International Committee on Food Microbiology and Hygiene. Foundation for Food Microbiology '99, TNO, Zeist, The Netherlands.
- 19.O'Driscoll, B., C. G. M. Gahan, and C. Hill. 1996. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl. Environ. Microbiol. 62:1693-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ojeniyi, B., H. Wegener, N. E. Jensen, and M. Bisgaard. 1996. Listeria monocytogenes in poultry and poultry products: epidemiological investigations in seven Danish abattoirs. J. Appl. Bacteriol. 80:395-401. [DOI] [PubMed] [Google Scholar]
- 21.Phan-Than, L., F. Mahouin, and S. Aligé. 2000. Acid responses of Listeria monocytogenes. Int. J. Food Microbiol. 55:121-126. [DOI] [PubMed] [Google Scholar]
- 22.Pine, L., S. Kathariou, F. Quinn, V. George, J. D. Wenger, and R. E. Weaver. 1991. Cytopathogenic effects in enterocytelike Caco-2 cells differentiate virulent from avirulent Listeria strains. J. Clin. Microbiol. 29:990-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryser, E. T., S. M. Arimi, M. M. C. Bunduki, and C. W. Donnelly. 1996. Recovery of different Listeria ribotypes from naturally contaminated, raw refrigerated meat and poultry products with two primary enrichment media. Appl. Environ. Microbiol. 62:1781-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlech, W. F., D. P. Chase, and A. Badley. 1993. A model of food-borne Listeria monocytogenes infection in the Sprague-Dawley rat using gastric inoculation—development and effect of gastric-acidity on infective dose. Int. J. Food Microbiol. 18:15-24. [DOI] [PubMed] [Google Scholar]
- 25.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Langendonck, N., E. Bottreau, S. Bailly, M. Tabouret, J. Marly, P. Pardon, and P. Velge. 1998. Tissue culture assays using Caco-2-cell line differentiate virulent from non-virulent Listeria monocytogenes strains. J. Appl. Microbiol. 85:337-346. [DOI] [PubMed] [Google Scholar]
- 27.Wiedmann, M., J. L. Bruce, C. Keating, A. E. Johnson, P. L. McDonough, and C. A. Batt. 1997. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect. Immun. 65:2707-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]