Abstract
Efficient fermentation of maltotriose is a desired property of Saccharomyces cerevisiae for brewing. In a standard wort, maltotriose is the second most abundant sugar, and slower uptake leads to residual maltotriose in the finished product. The limiting factor of sugar metabolism is its transport, and there are conflicting reports on whether a specific maltotriose permease exists or whether the mechanisms responsible for maltose uptake also carry out maltotriose transport. In this study, radiolabeled maltotriose was used to show that overexpression of the maltose permease gene, MAL61, in an industrial yeast strain resulted in an increase in the rate of transport of maltotriose as well as maltose. A strain derived from W303-1A and lacking any maltose or maltotriose transporter but carrying a functional maltose transport activator (MAL63) was developed. By complementing this strain with permeases encoded by MAL31, MAL61, and AGT1, it was possible to measure their specific transport kinetics by using maltotriose and maltose. All three permeases were capable of high-affinity transport of maltotriose and of allowing growth of the strain on the sugar. Maltotriose utilization from the permease encoded by AGT1 was regulated by the same genetic mechanisms as those involving the maltose transcriptional activator. Competition studies carried out with two industrial strains, one not containing any homologue of AGT1, showed that maltose uptake and maltotriose uptake were competitive and that maltose was the preferred substrate. These results indicate that the presence of residual maltotriose in beer is not due to a genetic or physiological inability of yeast cells to utilize the sugar but rather to the lower affinity for maltotriose uptake in conjunction with deteriorating conditions present at the later stages of fermentation. Here we identify molecular mechanisms regulating the uptake of maltotriose and determine the role of each of the transporter genes in the cells.
The three principal sugars available to Saccharomyces cerevisiae during the fermentation of alcoholic beverages are glucose, maltose, and maltotriose. Efficient fermentation requires the rapid and complete utilization of all of these sugars (18). Maltotriose, which is the second most abundant sugar in wort, is formed from the breakdown of complex sugars during mashing (19). A common problem in the brewing industry is incomplete utilization, resulting in a beer with residual maltotriose. The incomplete fermentation represents a waste of fermentable extract and a loss in revenue and leads to a product with higher carbohydrate levels and potentially an atypical flavor profile (36). Explanations for poor maltotriose utilization are varied; Findlay (12) believed that maltotriose was not a viable carbon source for brewing strains, whereas Casey et al. (4) suggested that with the advent of high-gravity brewing, increased stress on the yeast cells together with poor nutritional supplementation led to less efficient fermentation. Hornsey (18) proposed that the mutation and/or loss of maltotriose permease occurred during brewing, while Zheng et al. (36) considered that the conditions experienced during fermentation were responsible for incomplete maltotriose utilization.
Previous genetic analysis focused mainly on the maltose fermentation system, revealing in laboratory strains a series of five unlinked telomere-associated MAL loci, any of which will allow for fermentation. Each locus is a cluster of three functionally distinct genes; a composite of these is required for maltose fermentation. MALx1 encodes the permease (the x denoting the locus of origin); MALx2 encodes maltase, the enzyme responsible for intracellular maltose breakdown. The regulator, MALx3, is the MAL activator responsible for the inducible expression of the structural genes (8). Genes in the MAL loci have a high degree of homology; a gene in one locus is capable of complementing a mutation of the corresponding gene in another MAL locus (6).
An essential step in the utilization of maltotriose is its transport into the cell; for the metabolism of sugars this is the rate-limiting step (34). Using basic physiological tests as indicators of the uptake of maltose and maltotriose, Harris and Thompson (15) indicated the possibility of the presence of a specific maltotriose permease. Virsuri and Kirsop (33) and Kotyk and Michaljanicova (23) showed that maltose and maltotriose competitively inhibit each other and therefore proposed that the two sugars presumably are transported by the same mechanism. In a recent study by Han et al. (14), a general α-glucoside permease was characterized and considered responsible for maltotriose transport.
Charron and Michels (7) identified the gene, AGT1 (α-glucoside transporter 1), a naturally occurring allele of MAL11 which encodes a permease with a substrate specificity different from that of the common Mal11p. The AGT1 transport system involves active proton symport (28), with a reported substrate range including the α-glucosides maltotriose, maltose, isomaltose, melezitose, α-methylglucoside, sucrose, palatinose, trehalose, and turanose (14). AGT1 can be induced by maltose, probably under the control of the MALx3 transcriptional activator binding to a maltose-specific enhancer (UASMAL) located upstream of the AGT1 structural gene sequence (14). Stambuk et al. (28) found that α-methylglucoside induced the expression of Agt1p to the greatest extent and that trehalose was the preferred substrate; however, this work was done without testing maltotriose.
In this study, we have endeavored to improve the understanding of maltotriose utilization by an analysis of expression and regulation, revealing a shared system of transport and regulation for maltotriose and maltose.
MATERIALS AND METHODS
Materials.
d-[U-14C]maltotriose was obtained from American Radiochemical Company, and d-[U-14C] maltose was obtained from ICN Biomedicals. The purity of labeled and nonlabeled sugars was confirmed by high-pressure liquid chromatography with a C18 column and an aqueous mobile phase as described by Robyt and White (25). Medium components were supplied by Oxoid. The nonlabeled sugars and other chemicals used in this study were obtained from Sigma Chemical Company unless otherwise stated.
Yeast and bacterial strains and plasmids.
The S. cerevisiae strains used in this study are listed in Table 1. Escherichia coli JM101 was used for transformation and plasmid amplification and preparation. Plasmids in the series pRS40x, pRS41x, and pRS42x (Stratagene; x represents 3, 4, 5, or 6, which corresponds to the detectable marker histidine, tryptophan, leucine, or uracil, respectively) were used for genetic manipulation unless otherwise stated. For cloning of PCR products, pGEM-Teasy (Promega) was used in accordance with the manufacturer's instructions.
TABLE 1.
Yeast strains used in this work
| Strain | Relevant genotype | Sourcea |
|---|---|---|
| PB1 | mal0suc0his4 pIBID | 16 |
| PB1+MAL63 | mal0suc0his4 pIBID | 16 |
| PB1+VH31 | mal0suc0his4 pIBID | 16 |
| PB1+VH50 | mal0suc0his4 pIBID | 16 |
| L38 | Industrial strain | 17 |
| L38+PDC | Industrial strain | 17 |
| Lager | Industrial strain | Carlton and United Breweries |
| Ale | Industrial strain | Carlton and United Breweries |
| 600-1B | MAL11 MAL12 MAL13 | ATCC |
| W303-1A | AGT1 MAL12 mal13 MAL31 MAL32 mal33 | ATCC |
| W303R | AGT1 MAL12 mal13 MAL31 MAL32 mal33 (pRS406-MAL63) | 11 |
| W303RKO | agt1::TRP1 MAL12 mal13 mal31::LEU2 MAL32 mal33 (pRS406-MAL63) | 11 |
| W303R/AGT1 | agt1::TRP1 MAL12 mal13 mal31::LEU2 MAL32 mal33 (pRS406-MAL63) (pRS413-AGT1) | 11 |
| W303R/MAL31 | agt1::TRP1 MAL12 mal13 mal31::LEU2 MAL32 mal33 (pRS406-MAL63) (pRS413-MAL31) | 11 |
| W303R/MAL61 | agt1::TRP1 MAL12 mal13 mal31::LEU2 MAL32 mal33 (pRS406-MAL63) (pRS413-MAL61) | 11 |
ATCC, American Type Culture Collection.
Growth conditions.
Bacteria were grown on Luria-Bertani medium (1% yeast extract, 2% peptone, 2% NaCl) at 37°C. Yeast strains were grown on YEP medium (1% yeast extract, 2% Bacto Peptone, 2% sugar) at 30°C with aeration. α-Glucosides were filter sterilized through 0.22-μm-pore-size membranes to avoid hydrolysis and were added after autoclaving (15 kPa, 15 min). When necessary, strains and transformants were grown on minimal medium (0.17% yeast nitrogen base, 0.5% ammonium sulfate, 2% sugar) supplemented with appropriate auxotrophic requirements.
Growth kinetics.
Cells were grown to an optical density at 600 nm (OD600) of 1.0 in YEP— 2% sugar medium; a 1-ml aliquot was pelleted (3,000 × g, room temperature, 5 min), washed twice with sterile H2O, and resuspended in 1 ml of sterile H2O. An aliquot of this culture (10 μl) was used to inoculate 50 ml of minimal medium containing appropriate auxotrophic requirements, and cell density (OD600) readings were taken in duplicate at regular intervals for a period of 80 h. Plotted data are the average of the replicates. Duplicate growth readings did not vary by more than 15%.
Substrate specificity.
Cells were pregrown on either YEP-2% glucose or YEP-2% maltose medium and streaked on solid minimal medium supplemented with auxotrophic requirements. The plates were incubated at 30°C for up to 3 days. Results were confirmed by monitoring growth (by measurement of the cell density at OD600) under the same conditions in liquid medium.
Sugar uptake assays.
Cells were grown to an OD600 of 0.4, harvested by centrifugation (2,500 × g at 4°C for 10 min), washed twice with cold H2O, and resuspended in 0.2 M potassium phosphate buffer (pH 4.5) to a cell density of 0.4 g of dry weight/ml. The transport of radiolabeled maltotriose or maltose (at a concentration of 0.5 mM) was assayed by the method of Zheng et al. (37). Competition assays were carried out in a manner similar to that of the standard transport assays but in the presence of 10 mM unlabeled substrate. Permease kinetics were analyzed over a concentration range of 0.1 to 100 mM, and kinetic constants were determined by computer analysis with Enzyme Kinetics, version 1.11 (Trinity Software). Transport assays were performed in triplicate, and standard errors for transport activities were less than 15%.
Radioactive compounds retained inside yeast cells were analyzed by the method of Stambuk et al. (30). Each extract was chromatographed on silica gel 60 thin-layer chromatography (TLC) plates (Merck) with a mobile phase of n-butanol- acetic acid-water (6:2:1 [vol/vol]) (27). The positions of the sugars were detected by autoradiography with solutions of labeled glucose, maltose, and maltotriose as controls.
Northern hybridization.
Cells were grown to an OD600 of 0.5, and RNA was extracted with Trizol (Life Technologies) according to the manufacturer's instructions. A second purification step was introduced after the cellular material was removed; the supernatant was centrifuged through a PhaseLock gel system (Eppendorf) to ensure that there was no contamination with cellular material. The resolution of RNA, transfer to membranes, and the preparation of the membranes for hybridization were carried out by the methods outlined by Sambrook et al. (26). The membranes (Pall Gellman) were probed for MALx1, AGT1, and ACT1. MALx1 and AGT1 probes were PCR-generated fragments of unique regions within the open reading frames of each gene (Table 2). A 2-kb EcoRI-HindIII fragment containing the yeast actin gene, ACT1, was used as a control. Probes were labeled by using a Megaprime kit (Amersham Pharmacia) and purified by using Bio-Rad spin columns to remove unincorporated deoxynucleoside triphosphates, limiting nonspecific binding to the membranes. Transcript levels were quantified by using a PhosphorImager (Molecular Dynamics).
TABLE 2.
PCR primers used in this worka
| Gene | Purpose | Direction | Primer sequence (5′ to 3′) |
|---|---|---|---|
| AGT1 | Probe | F | CTCGCTGTTTTATGCTTGAGG |
| R | TTGCTTTACAATGGATTTGGC | ||
| Disruption | F | TATTTCTTCCAAAAAAAAAAAAACAACCCTTTTACTTAACATTTAGCAGATTGTACTGAGAGTGC | |
| R | AGAAGAACATCAAACAACTAAAAAAATAGTATAATATGAAAAATACTGTGCGGTATTTCACACCG | ||
| Cloning | F | GCGATTGTGAAACTATGTGA | |
| R | ACCCCATATCTTGTTGAGGA | ||
| MALx1 | Probe | F | TAATGATGCACCACAGGAGC |
| R | GGAGCTTTCTATGCCCTGC | ||
| Disruption | F | TAAATAACGCCGTATCTACCTACTGGCTAAAAAAATCATTTGTTGCAGATTGTACTGAGAGTGC | |
| R | ACAATAAGAATTACATCCAAGACTATTAATTAACTATGAAGGGACTGTGCGGTATTTCACACCG | ||
| Cloning | F | GGGCAGCTTACTAATATAAAT | |
| R | CTTTTAAATCACCCCAGCCA |
All primers were generated by using the Saccharomyces genome database primer design program (http://genome-www2.stanford.edu/cgi-bin/SGD/web-primer). F, forward; R, reverse. In the primers used for disruption, the underlined section matches the pRS42x vector; the other sections match the targeted gene. Note that the MALx1 primers represent a region that is common to both MAL31 and MAL61.
Other methods.
Nucleotide sequences of yeast genes were obtained from the Saccharomyces genome database (http://genome-www2.stanford.edu/cgi-bin/SGD), and protein sequences were retrieved from the yeast proteome database (http://www.proteome.com/databases/YPD). Sequence analysis and alignment of sequences were conducted by using WebANGIS (http://www.angis.org.au/WebANGIS).
RESULTS
The Malx3p regulator is responsible for the induction of maltotriose transport.
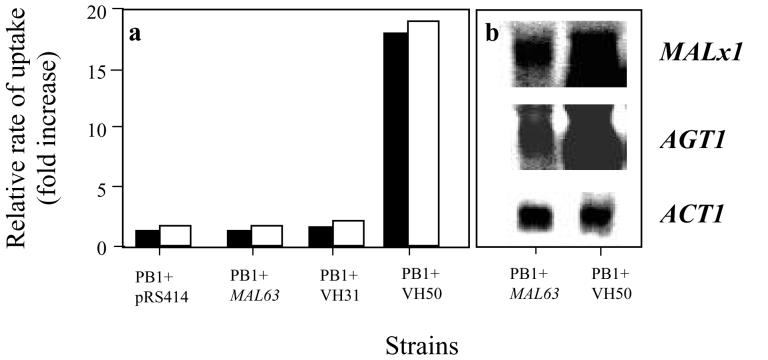
Previous work showed that the AGT1 gene encodes an α-glucoside transporter and is a member of the maltose permease gene family, so the question arises as to whether its expression is regulated in the same manner as that of the other members of the family. The regulation of AGT1 and MALx1 expression was investigated by using a set of strains that were isogenic, except that each one carried a different allele of the MAL63 gene. The parent strain, PB1, is malx3, and so is unable to ferment maltose. Centromeric plasmids containing different MAL63 alleles were introduced into this strain (16). Strain PB1+MAL63 carries a wild-type regulator, and maltose utilization is tightly controlled and is induced by maltose. Strain PB1+VH31 carries a similar tightly controlled regulator generated by domain swapping of different MAL63 alleles (16). Strain PB1+VH50 carries the same allele as PB1+VH31 but has a single amino acid substitution that results in a regulator that is loosely controlled, such that the level of expression of the MAL loci in this strain is constitutively high (16). Strain PB1 transformed with pRS414 (vector alone) was used as a control.
Maltotriose and maltose permease activities were assayed under noninducing conditions, i.e., in cells grown on galactose, by using radiolabeled sugars at a concentration of 0.5 mM (Fig. 1a). The level of transport of maltotriose in PB1+VH50 was high, with a comparable elevation in maltose permease activity. However, the permease activities of PB1(pRS414), PB1+MAL63, and PB1+VH31 were low (5% the level found in PB1+VH50), as expected under noninducing conditions. Since the only difference between the systems was the nature of the Mal63p regulator, this transcription factor is implicated in the regulation of the maltotriose fermentation system. The question then arises as to which transport system is responsible for the elevated maltotriose uptake. Northern analysis of these strains (Fig. 1b) indicated that both AGT1 and MALx1 were present. The level of expression of AGT1 and MALx1 in strain PB1+VH50 was approximately 15-fold higher than that in the other strains, and these expression data correlated with the elevated permease activity seen under the same conditions of growth. In the tightly controlled strains (PB1+MAL63 and PB1+VH31), expression was present at a basal level similar to that seen in the vector-only control strain. These data showed that AGT1 expression was regulated by the same control system as that of the maltose permeases. This finding is not surprising, since AGT1 is divergently transcribed from a promoter region shared with the maltase gene, MAL12; this is the classical architecture of the other MAL loci. Since the expression of the AGT1 maltose transporter and the expression of the MALx1 maltose transporter were induced similarly under these conditions, it was not possible to assess the extent to which either or both were involved in maltotriose transport.
FIG. 1.
Regulation of permeases. (a) Cells were grown under noninducing conditions, and maltose (white) and maltotriose (black) permease activities were assayed. Activity is represented as the fold increase, with the basal level as the reference point. (b) Northern hybridization analysis of strains PB1+MAL63 and PB1+VH50 with probes to MALx1, AGT1, and ACT1 (RNA control).
Mal61p can act as a maltotriose transporter.
In order to determine the possible role of other members of the maltose permease family in the transport of maltotriose, an initial study was performed with a system involving the enhanced expression of the permease encoded by MAL61.
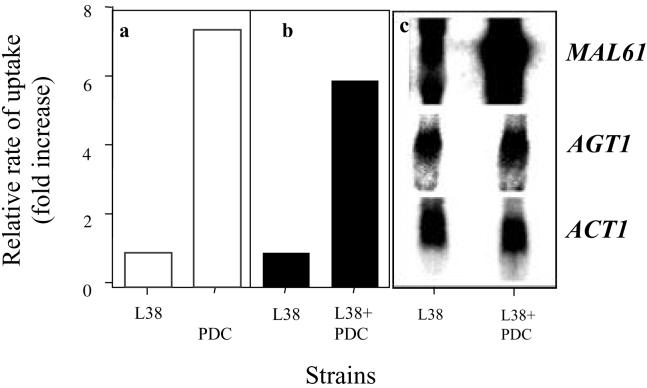
Industrial strain L38 has a very low level of maltose permease activity under noninducing conditions. Strain L38+PDC was constructed by inserting an integrating plasmid (at the TRP1 locus of L38) that carried the MAL61 structural gene fused to the PDC1 promoter (17). Since the PDC1 promoter is highly expressed in cells grown on any sugar, this process conferred constitutive expression of MAL61. Maltotriose and maltose permease activities were assayed in cells of these strains grown on galactose, i.e., under noninducing conditions (Fig. 2a). Under the control of the PDC1 promoter, the uptake of both sugars increased at least sixfold. This result indicated that maltotriose is a substrate of the Mal61p permease and that this permease can contribute significantly to the uptake of maltotriose.
FIG. 2.
Enhanced expression of Mal61p. (a and b) Strains L38 and L38+PDC1 were grown on YEP-2% galactose medium, i.e., under noninducing conditions. The uptake of maltose (a) and maltotriose (b) (concentration, 0.5 mM) was measured. The rates of uptake of maltose and maltotriose in L38+PDC1 were 51 and 32 nmol min−1 mg of dry weight−1, respectively. (c) Northern hybridization analysis with probes to MALx1, AGT1, and ACT1 (RNA control).
Northern analysis showed that the expression of MAL61 in L38+PDC1 was at least fivefold higher than that in L38, correlating with the increased uptake of maltotriose and maltose. In both L38 and L38+PDC1, the expression of AGT1 was present at similar basal levels (Fig. 2b). Taken together, these results indicate that Mal61p, not Agt1p, mediates the increase in maltotriose transport.
Strains expressing MAL61, MAL31, and AGT1 grow on maltotriose and maltose.
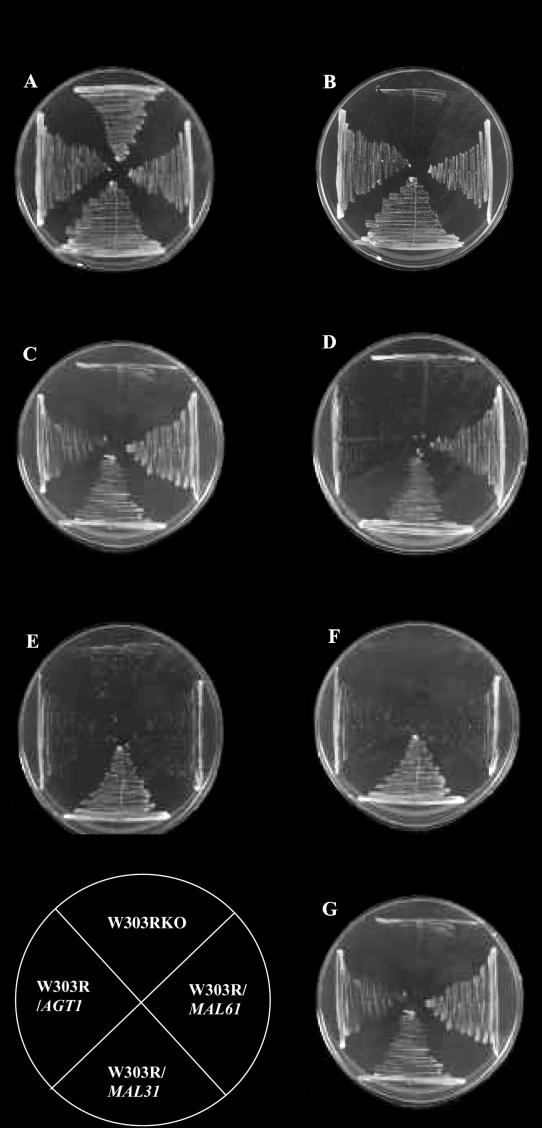
In view of the preceding data, it was necessary to define and confirm the role of the other members of the maltose permease family in the transport of α-glucosides. A model strain was needed in which there was no α-glucoside activity (i.e., lacking all genes of this family), so that the individual genes could then be introduced and fully characterized. In order to create this ideal strain, permease genes MAL31 and AGT1 were deleted from the haploid laboratory strain W303-1A (2). Since this strain lacks a functional MAL3 regulator, it was also necessary to integrate a maltose regulator gene (MAL63) as previously described (11). The final strain (W303RKO) was unable to grow on either maltose or maltotriose (Fig. 3) and was not able to transport either sugar at significant rates (Fig. 4).
FIG.3.
Growth of W303R/MAL31, W303R/MAL61, W303R/AGT1, and W303RKO on different sugars. The cells were pregrown on YEP-2% maltose medium and streaked on solid minimal media supplemented with the appropriate auxotrophic requirements and containing the following sugar: glucose (A), maltose (B), maltotriose (C), α-methylglucoside (D), melezitose (E), trehalose (F), and turanose (G). The plates were incubated at 30°C for up to 3 days. The key to strain locations is given in the lower left-hand corner of the figure.
FIG. 4.
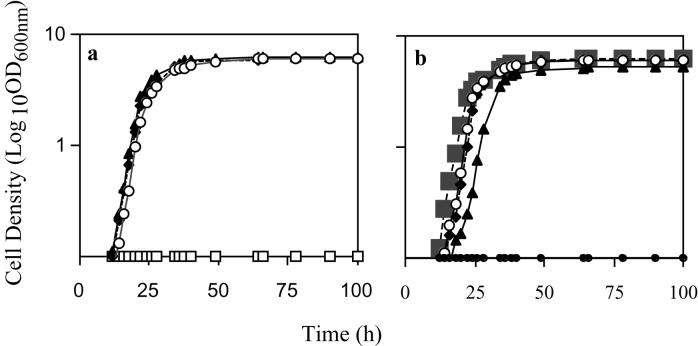
Growth kinetics of W303R/MAL31, W303R/MAL61, W303R/AGT1, and W303RKO. Symbols: ♦, W303R/MAL31; ▴, W303R/MAL61; ○, W303R/AGT1; •, W303RKO; □, W303R/MAL61 with a permease but no integrated regulator; ▪, W303R/MAL61 grown with glucose. Results for W303RKO in panel a are the same as those for W303R/MAL61 with a permease but no integrated regulator. Strains were inoculated on minimal medium with auxotrophic requirements and maltose (a) or maltotriose (b). Cell density measurements (OD600) were taken at regular intervals.
We generated a set of isogenic strains each expressing a single and specific permease and determined the ability of the strains to grow on various sugar substrates. The α-glucosides tested were maltose, maltotriose, melezitose, α-methylglucoside, trehalose, and turanose. All strains, including W303R/MAL61, could grow on maltose, maltotriose, and turanose (Fig. 3); hence, it was confirmed that maltose, maltotriose, and turanose are substrates of Mal61p. The α-glucoside range of Mal31p had not previously been defined. Not surprisingly, the substrate range was the same as that for Mal61p, which shares 99% identity with Mal31p. Strain W303R/AGT1 transported all α-glucosides tested, as previously shown (14). The negative control, W303R/403 (containing only the vector), containing no functional α-glucoside permease genes, did not transport any of these sugars.
Confirmation of these growth results was obtained by measuring the growth kinetics of each strain (Fig. 4). All strains grew on glucose, and only the strains with the introduced permeases grew on maltose (Fig. 4a) and maltotriose (Fig. 4b). It is notable that when maltotriose was the sole carbon source, strain W303R/MAL61 had a longer lag phase (Fig. 4b) and stationary phase was reached at an OD600 of 5.1, whereas the other strains entered exponential phase 6 h earlier and stationary phase occurred at an OD600 of 6.0. Strains without an integrated regulator did not grow on maltose or maltotriose, indicating that all permeases were under the control of the regulator. There was no discernible difference in the abilities of the strains to use maltose. The slower growth of the Mal61p-containing strain when maltotriose was the sole carbon source may indicate that this permease has a lower affinity for this sugar than Agt1p and Mal31p.
Analyses of maltose and maltotriose transport.
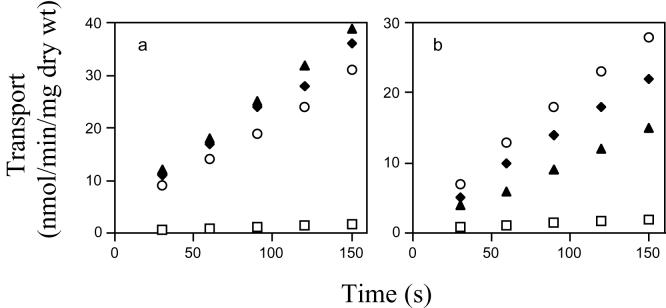
In order to determine the affinity of each permease for different sugars, it was necessary to study the kinetics of maltose and maltotriose transport in the different W303R strains. Figure 5 illustrates the rates of uptake of maltose and maltotriose by strains W303R/AGT1, W303R/MAL31, and W303R/MAL61. It is evident that all of these strains transported maltose at similar rates (Fig. 5a), whereas the rate of maltotriose transport by Mal61p, although relatively high, was lower (Fig. 5b) than that observed for Mal31p and Agt1p and thus correlated with the growth results. The transport of both maltose and maltotriose was linear over the period measured (150 s) for all permeases.
FIG. 5.
Transport of maltose and maltotriose in W303R/MAL31, W303R/MAL61, W303R/AGT1, and W303RKO. Strains were grown in YEP-2% glucose medium; cells were harvested at an OD600 of 0.4, washed twice, resuspended in an equal volume of YEP- 2% maltose medium, and harvested after 4 h. Maltose (a) and maltotriose (b) (0.5 mM) permease activities of strains W303R/MAL31 (♦), W303R/MAL61 (▴), and W303R/AGT1 (○) were assayed over a period of 150 s. W303RKO (a) and W303R/MAL61 (b) cells were grown in YEP- 2% glucose medium, and permease activity was assayed over a period of 150 s (□).
Kinetic analyses were performed to define the affinities of the permeases for maltose and maltotriose (Table 3) by using the Hanes-Woolf system, which has been shown to minimize experimental artifacts (9). It was established that the permeases were saturable and were active transport systems. Mal61p and Mal31p had a greater affinity for maltose than for maltotriose. Agt1p had the greatest affinity for maltotriose and a comparable affinity for maltose.
TABLE 3.
Kinetics of generated strainsa
| Strain | Maltotriose
|
Maltose
|
||
|---|---|---|---|---|
| Km (mM) | Vmax nmol min−1 mg of dry wt−1 | Km (mM) | Vmax nmol min−1 mg of dry wt−1 | |
| W303R/AGT1 | 4 ± 0.7 | 39 | 5.1 ± 0.6 | 41 |
| W303R/MAL31 | 5.7 ± 1.0 | 41 | 4.2 ± 1.1 | 42 |
| W303R/MAL61 | 7.2 ± 0.9 | 40 | 2.7 ± 0.6 | 36 |
Determined with a Hanes-Woolf plot generated with Enzyme Kinetics, version 1.11.
Low-affinity transport is an experimental artifact.
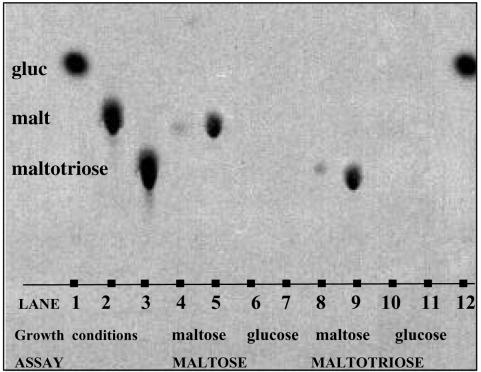
The biphasic transport of α-glucosides has been well documented (29, 37, 24). Typically, there are a high-affinity system and a low-affinity system, although there has been opposition to the idea of a low-affinity system (13, 3). The isogenic strain set provided an ideal system with which to test the low-affinity component, since there was some apparent residual uptake of radioactivity when the uptake of labeled maltose and maltotriose was assayed with glucose-grown cells (Fig. 5). In order to determine whether this was due to sugar entering the cell, TLC was used to analyze extracts (27). Strain W303R/MAL61 (which was used as a representative strain) showed no evidence of radiolabeled sugar in extracts of glucose-grown cells (Fig. 6), indicating that the low-affinity transport system evident in these tests is an experimental artifact.
FIG. 6.
Analysis of intracellular material of W303R/MAL61. Strains were grown in YEP-2% glucose medium; cells were harvested at an OD600 of 0.4, washed twice, resuspended in an equal volume of YEP-2% maltose or YEP-2% glucose medium, and harvested after 4 h. Maltose and maltotriose permease activity assays were performed. The intracellular material was extracted and run on a silica gel 60 TLC plate; the solvent used was n-butanol-acetic acid-water (6:3:1). Control samples were run by using solutions of radiolabeled sugars. The chromatogram was visualized by using a PhosphorImager. A repeat of labeled glucose is contained in lane 12.
Maltose is the preferred substrate of brewing strains.
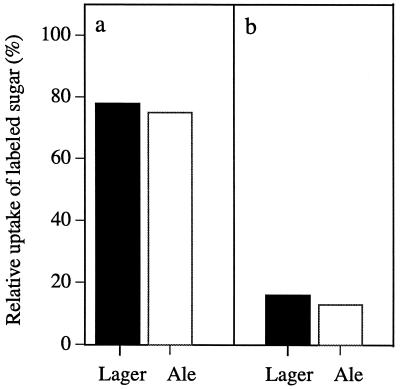
In studying the laboratory strains and the specific permeases, it has been established that all of the permeases tested transport maltotriose. It is important that this finding be examined in an industrial context. Thus, the following question arises: if S. cerevisiae has multiple ways of transporting maltotriose, then why are residual amounts left in beer? A standard fermentation profile illustrates that glucose is utilized quickly, resulting in a lack of glucose-mediated repression either by Mig1p binding or proteolysis of the permeases, so that only maltose and maltotriose remain (18). Since the two principal sugars in the wort are maltose and maltotriose, it is likely that the presence of two such similar sugars that have been shown to enter the cell via the same system would result in competition and in turn affect the ability of the cells to utilize either sugar. The competition of maltose and maltotriose for transport in a lager strain and an ale strain (Fig. 7) was measured by using a nonlabeled competitor at a concentration 10-fold (5 mM) higher than that of the labeled sugar (0.5 mM). The ability of the strains to transport labeled maltose in the presence of maltotriose was 75% the normal capacity (Fig. 7a). The transport of labeled maltotriose in the presence of competing maltose showed a fivefold decrease (20% the normal capacity) in both the lager and the ale strains (Fig. 7b). These competition data indicated that maltose is the preferred substrate for both of the industrial strains tested, and this finding is probably the reason that maltotriose is not used until the later stages of fermentation.
FIG. 7.
Effect of competing sugars on maltose and maltotriose permease activities in an industrial strain. A lager strain (black) and an ale strain (white) were grown on YEP-2% maltose medium and used to measure the uptake of 0.5 mM radiolabeled maltose (a) and maltotriose (b) in the presence of 5 mM maltotriose and maltose, respectively. The rate of uptake at 100% corresponds to activity in the presence of no competitior (only reaction buffer). (a) Maltose permease activities in the presence of no competitor in the lager and ale strains, 10 and 7.2 nmol min−1 mg of dry weight−1, respectively. (b) Maltotriose permease activities in the presence of no competitor in the lager and ale strains, 6 and 3.5 nmol min−1 mg of dry weight−1, respectively. Results are the mean of triplicate determinations.
DISCUSSION
The incomplete utilization of maltotriose in industrial brewing has led to the suggestion that a separate maltotriose uptake system distinct from that for maltose exists in S. cerevisiae. Early attempts to assess this possibility with physiological tests as indicators of maltose and maltotriose uptake were inconclusive and often conflicting (15, 23, 33). An initial molecular genetic analysis indicated that the maltose permease allele MAL61 was not capable of facilitating maltotriose transport and that a gene designated AGT1 was considered responsible for maltotriose transport (5, 7, 14). The AGT1 gene was found to be present in almost all brewing strains (21). Therefore, it was surprising that an industrial strain that was capable of utilizing maltotriose as a sole carbon source did not posses the AGT1 gene (11). This finding indicates either that there was no specific maltotriose uptake system or that this strain contained a maltotriose permease that had yet to be characterized.
We report for the first time the use of radiolabeled [14C]maltotriose to study the characteristics of maltototriose transport in S. cerevisiae. Previous studies on maltotriose utilization did not directly measure maltotriose transport but rather monitored growth and fermentation (14, 22) or assayed the proton uptake rate as a measure of the corresponding active H+ symport activity of strains (29, 31, 35). An analysis of the maltotriose uptake of an industrial strain overexpressing the MAL61 gene showed an increase in the transport of maltotriose. This finding contrasts with that of a previous report showing that the overexpression of this gene in an industrial strain did not increase its fermentation rate in a medium containing maltotriose as the sole source of carbon (22). Whether this finding was due to a lack of enhanced maltotriose transport or other possible factors, such as the inability to hydrolyze maltotriose more efficiently, was not established, since maltotriose uptake was not measured in this strain.
Whereas Han et al. (14) found that strains containing MAL61 could not grow on maltotriose and Stambuk and de Araujo found that MAL21 could not facilitate H+ symport activity (29), we showed that the maltose permease genes MAL61 and MAL31 facilitated high-affinity transport of maltotriose similar to that facilitated by AGT1. Transport was reflected by the ability of these strains to grow on maltotriose as the sole source of carbon. By using defined genetically constructed strains, we were able to measure the kinetic parameters of maltotriose uptake for each of the transporters. The analyses of transport kinetics for the various permeases (encoded by MAL61, MAL31, and AGT1) in these genetically defined strains revealed the affinity (Km values) for maltose to be similar to previously reported values (14, 28). Apart from maltotriose, the pattern of specificity of MAL61 and AGT1 for growth on other α-glucosides corresponded to that reported previously (14).
The MAL61 and MAL31 alleles used in these experiments were sequenced to determine whether sequence variations could explain the differences in results between laboratories, but it was found that the MAL61 sequence was essentially the same as that of the published MAL61 allele in the S. cerevisiae genome database (results not shown). Another possible explanation for the unexpected utilization of maltotriose by strains carrying the MAL61 and MAL31 alleles may have been the degradation of maltotriose to maltose and glucose. However, high-pressure liquid chromatography analyses of the maltotriose used in the media for growth assays (results not shown) and TLC analyses of the radiolabeled maltotriose used in uptake assays (Fig. 6) determined that no degradation had occurred, confirming that maltotriose transport was being measured.
There have been conflicting reports on whether a low-affinity transport system for maltose does actually exist in S. cerevisiae or whether the results are only experimental artifacts (14, 24). As expected, no high-affinity transport of maltose or maltotriose was evident in a strain grown in glucose, because it is highly repressive for MAL gene expression. The results, however, did indicate the apparent existence of a low-affinity transport system active for maltose and maltotriose uptake similar to that found for maltose uptake by Lagunas (24) and Han et al. (14). However, the inability to detect radiolabeled maltose or maltotriose in extracts of these cells analyzed by TLC (Fig. 6) supports the view that the low-affinity transport of maltose and maltotriose is an experimental artifact.
Members of the maltose permease family have strongly overlapping but nevertheless distinct functions. The multiplicity of this family is eclipsed only by that of the hexose transporter family, with 18 members. This evolution of multigene sugar families indicates that sugar uptake is very important and that the presence of multiple gene copies likely is a selective advantage for S. cerevisiae. It reflects the successful adaptation to the variety of environmental conditions to which yeast cells are exposed; such adaptability is very important in a unicellular organism.
The α-glucoside transporters differ in substrate range. This fact may reflect the different α-glucosides that are present in nature or that result from the enzymatic degradation of starch. In an industrial context, strong selection pressure may have led to the different permease specificities seen with Agt1p and Malx1p. Among the maltose permease family, two putative permeases, Mph2p and Mph3p, were investigated in another study (11). These two permeases share 100% identity with each other, 75% identity with the Malx1p permeases, and 55% identity with Agt1p. They seem to occupy the middle ground between the maltose permeases and the more general α-glucoside permeases with a substrate range of maltose, maltotriose, α-methylglucoside, and turanose. Since transport is an important step in controlling the rate of α-glucoside metabolism, the elucidation of the functions of this multitude of related proteins is a fundamentally important task. This task also is relevant when considering the curious outcome found in this work, that Mal31p and Mal61p, which share 99% identity, differ significantly in their ability to transport maltotriose (but not maltose). This finding indicates that at least some of the eight amino acid differences between Mal31p and Mal61p are important for maltotriose uptake but not maltose uptake. All the alterations, except for Ala247Thr (which lies in the center of transmembrane domain 5), lie in regions that are cytoplasm associated: Thr315Iso, Iso484Met, Asp608Pro, and Ser610Pro are in the cytoplasmic regions of the protein; Met167Val, Phe291Leu, and Iso415Val lie on the interface of the membrane and the cytoplasm. Most of the alterations in Mal61p favor an amino acid more hydrophobic than that in the Mal31p counterpart, again with the exception of the Ala247Thr substitution (an interesting substitution, as the membrane domains are predominantly hydrophobic). These and other such regions in Mal31p, Mal61p, and the other members of the family are potentially responsible for the affinities of the permeases and need to be investigated, as they represent crucial points in the formation and subsequent functions of the proteins.
The issues of maltotriose being the last sugar utilized and its incomplete fermentation have been addressed to some extent in this work. All the permeases tested transported maltotriose; the competition data indicated that maltose is the preferred substrate, a selective advantage, as maltose is the most abundant sugar found in a standard wort (which is usually composed of 70% maltose and 15% maltotriose). This situation results in maltotriose not being utilized until the later stages of fermentation, and even then, the rate of utilization is low (18). Preliminary work performed by us and to some extent expounded in the literature involves the investigation of the impact of the fermentation environment on yeast cells (1, 4, 10, 20, 32, 37). It has been indicated that in the later stages of fermentation, nutritional and environmental stresses are likely to compromise the ability of the yeast cells to ferment any sugar, and this scenario may explain why the residual sugar can be removed by a second fermentation (36). The maltose genes are under the tight control of glucose repression (8, 16), and gene expression analysis in this report showed that maltotriose utilization is also under the same control. This finding indicates that the use of strains that utilize maltose in the presence of glucose (16, 17, 22) may enable maltotriose to be utilized earlier in the fermentation, before the concentration of secondary metabolites becomes inhibitory.
Acknowledgments
We thank Geoff Kornfeld for helpful discussions and assistance with computing analysis.
This work was supported by an ARC SPIRT grant in collaboration with Carlton and United Breweries. R. E. Day was supported by an Australian postgraduate award (industry).
REFERENCES
- 1.Attfield, P. V. 1997. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat. Biotechnol. 15:1351-1357. [DOI] [PubMed] [Google Scholar]
- 2.Baudin, A., O. Ozier-Kalogeropoulos, A. Danouel, F. Lacroute, and C. Cullin. 1993. A simple and efficient method for direct gene disruption in Saccharomyces cerevisiae. Nucleic Acids Res. 21:3329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benito, B., and R. Lagunas. 1992. The low-affinity component of Saccharomyces cerevisiae maltose transport is an artifact. J. Bacteriol. 174:3065-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey, G. P., C. A. Magnus, and W. M. Ingledew. 1984. High-gravity brewing: effects of nutrition on yeast composition, fermentative ability, and alcohol production. Appl. Environ. Microbiol. 48:639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, Y. S., R. A. Dubin, E. Perkins, C. A. Michels, and R. B. Needleman. 1989. Identification and characterization of the maltose permease in genetically defined Saccharomyces strain. J. Bacteriol. 171:6148-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charron, M. J., E. Read, S. R. Haut, and C. A. Michels. 1989. Molecular evolution of the telomere associated MAL loci of Saccharomyces. Genetics 122:307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charron, M. J., and C. A. Michels. 1988. The naturally occurring alleles of MAL1 in Saccharomyces species evolved by various mutagenic processes including chromosomal rearrangement. Genetics 120:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, Q., and C. A. Michels. 1991. MAL11 and MAL61 encode the inducible, high-affinity maltose transporter of Saccharomyces cerevisiae. J. Bacteriol. 173:1817-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornish-Bowden, A. 1989. Fundamentals of enzyme kinetics. Butterworths, London, United Kingdom.
- 10.Cunningham, S., and G. G. Stewart. 1998. Effects of high-gravity brewing and acid washing on brewers' yeast. J. Am. Soc. Brew. Chem. 56:12-18. [Google Scholar]
- 11.Day, R. E., V. J. Higgins, P. J. Rogers, and I. W. Dawes. Characterisation of the putative maltose transporters encoded by YJR160c and YDL247w. Yeast, in press. [DOI] [PubMed]
- 12.Findlay, W. P. K. 1971. Modern brewing technology. Macmillan Press, London, United Kingdom.
- 13.Fuhrmann, G. F., and B. Volker. 1993. Misuse of graphical analysis in non-linear sugar transport kinetics by Eadie-Hofstee plots. Biochim. Biophys. Acta 1145:180-182. [DOI] [PubMed] [Google Scholar]
- 14.Han, E. K., F. Cotty, C. Sottas, H. Jiang, and C. A. Michels. 1995. Characterisation of AGT1 encoding a general α-glucoside transporter from Saccharomyces. Mol. Microbiol. 17:1097-1107. [DOI] [PubMed] [Google Scholar]
- 15.Harris, G., and C. C. Thompson. 1960. Uptake of nutrients. II. Maltotriose permease and the utilisation of maltotriose by yeasts. J. Inst. Brew. 66:293-298. [Google Scholar]
- 16.Higgins, V. J., M. Braidwood, P. Bissinger, I. W. Dawes, and P. V. Attfield. 1999. Leu343Phe substitution in the Malx3 protein of Saccharomyces cerevisiae increases the constitutivity and glucose insensitivity of MAL gene expression. Curr. Genet. 35:491-498. [DOI] [PubMed] [Google Scholar]
- 17.Higgins, V. J., M. Braidwood, P. Bell, P. Bissinger, I. W. Dawes, and P. V. Attfield. 1999. Genetic evidence that high noninduced maltase and maltose permease activities, governed by MALx3-encoded transcriptional regulators, determine efficiency of gas production by baker's yeast in unsugared dough. Appl. Environ. Microbiol. 65:680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornsey, I. S. 1999. Brewing. RSC Paperbacks, Cambridge, United Kingdom.
- 19.Hough, J. S., D. E. Briggs, R. Stevens, and I. W. Young. 1982. Malting and brewing sciences, vol. 2. Cambridge University Press, Cambridge, United Kingdom.
- 20.Ivorra, C., J. E. Perez-Ortin, and M. L. del Olmo. 1999. An inverse correlation between stress resistance and stuck fermentations in wine yeasts. A molecular study. Biotechnol. Bioeng. 64:698-708. [DOI] [PubMed] [Google Scholar]
- 21.Jesperson, L., L. B. Cesar, P. G. Meaden, and M. Jakobsen. 1999. Multiple α-glucoside transporter genes in brewer's yeast. Appl. Environ. Microbiol. 65:450-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodama, Y., N. Fukui, T. Ashikari, and Y. Shibano. 1995. Improvement of maltose fermentation efficiency: constitutive expression of MAL genes in brewing yeasts. J. Am. Soc. Brew. Chem. 53:24-29. [Google Scholar]
- 23.Kotyk, A., and D. Michaljanicova. 1979. Uptake of trehalose by Saccharomyces cerevisiae. J. Gen. Microbiol. 110:323-329. [DOI] [PubMed] [Google Scholar]
- 24.Lagunas, R. 1993. Sugar transport in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 104:229-242. [DOI] [PubMed] [Google Scholar]
- 25.Robyt, J. F., and B. J. White. 1987. Biochemical techniques: theory and practice. Wavelend Press, Prospect, Ill.
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Stahl, E. 1965. Thin layer chromatography—a laboratory handbook. Springer-Verlag, Berlin, Germany.
- 28.Stambuk, B. U., M. A. da Silva, A. D. Panek, and P. S. de Araujo. 1999. Active α-glucoside transport in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 170:105-110. [DOI] [PubMed] [Google Scholar]
- 29.Stambuk, B. U., and P. S. de Araujo. 2001. Kinetics of active α-glucoside transport in Saccharomyces cerevisiae. FEMS Yeast Res. 1:73-78. [DOI] [PubMed] [Google Scholar]
- 30.Stambuk, B. U., A. D. Panek, J. H. Crowe, L. M. Crowe, and P. S. de Araujo. 1998. Expression of high-affinity trehalose-H+ symport in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1379:118-128. [DOI] [PubMed] [Google Scholar]
- 31.Stambuk, B. U., P. S. de Araujo, A. D. Panek, and R. Serrano. 1996. Kinetics and energetics of trehalose transport in Saccharomyces cerevisiae. Eur. J. Biochem. 237:876-881. [DOI] [PubMed] [Google Scholar]
- 32.Stewart, G. G., I. Russell, and A. M. Sills. 1983. Factors that control the utilisation of wort carbohydrates by yeast. Tech. Q. Master Brew. Assoc. Am. 20:1-8. [Google Scholar]
- 33.Visuri, K., and B. H. Kirsop. 1970. The influence of pH and of selected cations on the fermentation of maltose and maltotriose. J. Inst. Brew. 76:362-366. [Google Scholar]
- 34.Wieczorke, R., S. Krampe, T. Weierstall, K. Friedal, C. P. Hollenberg, and E. Boles. 1999. Concurrent knockout of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 464:123-128. [DOI] [PubMed] [Google Scholar]
- 35.Zastrow, C. R., M. A. Mattos, C. Hollatz, and B. U. Stambuk. 2000. Maltotriose metabolism by Saccharomyces cerevisiae. Biotechnol. Lett. 22:455-459. [Google Scholar]
- 36.Zheng, X., T. D'Amore, I. Russell, and G. G. Stewart. 1994. Factors influencing maltotriose utilisation during brewery wort fermentations. J. Am. Soc. Brew. Chem. 52:41-47. [Google Scholar]
- 37.Zheng, X., T. D'Amore, I. Russell, and G. G. Stewart. 1994. Transport kinetics of maltotriose in strains of Saccharomyces. J. Ind. Microbiol. 13:159-166. [Google Scholar]