Abstract
Many studies have demonstrated that bacteria, including Listeria monocytogenes, are capable of adapting to disinfectants used in industrial settings after prolonged exposure to sublethal concentrations. However, the consequent alterations of the cell surface due to sanitizer adaptation of this pathogen are not fully understood. Two resistant and four sensitive L. monocytogenes strains from different sources were progressively subcultured with increasing sublethal concentrations of a surfactant, benzalkonium chloride (BC). To evaluate the effects of acquired tolerance to BC, parent and adapted strains were compared by using several morphological and physiological tests. Sensitive strains showed at least a fivefold increase in the MIC, while the MIC doubled for resistant strains after the adaptation period. The hydrophobicities of cells of parent and adapted strains were similar. Serological testing indicated that antigen types 1 and 4 were both present on the cell surface of adapted cells. The data suggest that efflux pumps are the major mechanism of adaptation in sensitive strains and are less important in originally resistant isolates. A different, unknown mechanism was responsible for the original tolerance of resistant isolates. In an originally resistant strain, there was a slight shift in the fatty acid profile after adaptation, whereas sensitive strains had similar profiles. Electron micrographs revealed morphological differences after adaptation. The changes in cell surface antigens, efflux pump utilization, and fatty acid profiles suggest that different mechanisms are used by resistant and sensitive strains for adaptation to BC.
Quaternary ammonium compounds (QACs) are cationic biocides that are commonly used as disinfectants in both medical and food production environments (14, 20, 27). QACs act on general membrane permeability, causing cytolytic leakage of cytoplasmic material at low concentrations. At high concentrations, they target the carboxylic groups and cause general coagulation in the bacterial cytoplasm (28). QACs are primarily active against gram-positive bacteria, but they are also active against gram-negative bacteria, some viruses, fungi, and protozoans (16, 28). Benzalkonium chloride (BC) is a QAC that is widely used for medical disinfection and for sanitation in food-processing environments (22).
Due to regular exposure to the action of sanitizers, gram-positive bacteria, such as Staphylococcus aureus (29), exhibit adaptation and resistance to such chemicals. Tolerance to BC can be due to intrinsic resistance (19). Gram-negative bacteria, such as Pseudomonas spp., can also adapt to sanitizers used in the food-processing environment (6, 15). The gram-positive bacterium Listeria monocytogenes can tolerate QACs, yet little is known about the mechanism of resistance. As an adaptation to adverse environments, bacteria can use proton motive force-driven multidrug efflux pumps to eliminate and avoid accumulation of chemicals. The species in which the association between an efflux mechanism and resistance to BC has been studied most is S. aureus (10). It has also been shown that a similar efflux pump mechanism can be induced by BC in some strains of L. monocytogenes (1). A recent discovery showed that the mdrL gene of L. monocytogenes encodes a protein, MdrL, which shows some homology (21 to 24% identity) to the Bmr and Blt proteins of the Bacillus subtilis multidrug resistance efflux pump family, which allows elimination of substrates from the cells (11). Although the genetic elements for control and expression of efflux pumps appear to be present in L. monocyogenes (19), little is known about the regulation of these putative mechanisms, either at the level of gene expression or at the level of activity. However, the development of QAC resistance may be directly related to the efficiency of such an efflux pump system.
Intrinsic QAC resistance is directly associated with nontypeability by serological techniques, indicating that there are possible structural modifications on the cell surface of resistant cells (19). Modifications in the thickness and the degree of cross-linking of peptidylglycan in the cell wall are possible explanations for the tolerance of L. monocytogenes (18). Such changes can cause alterations in the cell surface charge and fatty acid profile, resulting in a different morphology. As an adaptive response to environmental stress, alteration of cell surface fatty acids can prevent the entrance of foreign chemicals into the cytoplasm. Modifications in the composition of cell wall lipids, especially lipopolysaccharides and lipids, help prevent the penetration of QACs into gram-negative cells (8). However, the alterations in gram-positive cells have not been investigated yet.
The objective of this study was to determine the chemical and morphological effects of prolonged exposure to sublethal levels of BC on L. monocytogenes. Initial levels of resistance were determined by MIC tests, and serological identities were confirmed by antiserum type 1 and 4 tests prior to collection of data. Utilization of efflux pumps and cell surface hydrophobicity tests were used to compare unadapted (parent) and adapted L. monocytogenes cells. Electron micrographs of thin sections were used to show differences in the appearance of cell surfaces.
MATERIALS AND METHODS
Bacteria and growth conditions.
Six L. monocytogenes isolates were selected from the culture collection of the Canadian Research Institute for Food Safety, University of Guelph (Table 1). As generally defined in the literature, a strain with an MIC of BC greater than or equal to 4 μg/ml is considered resistant, while sensitive strains tolerate less than 4 μg of BC/ml. Two resistant isolates (H7962 and H7764) were obtained from the Centers for Disease Control and Prevention (Atlanta, Ga.) and were from the Sara Lee Bil Mar outbreak of 1998. Two sensitive strains (C511 and LJH389) were from unknown sources. One strain (LJH381) was obtained from the Laboratory for Foodborne Zoonoses, Health Canada, Guelph, Ontario, Canada, and one strain (C712) was isolated from a meat-processing plant. Although isolates C511 and C712 were isolated at different times and places, they belong to the same ribogroup, as determined by automated ribotyping (RiboPrinter microbial characterization system; Dupont Qualicon, Wilmington, Del.). None of the other isolates are genetically related, as determined by ribotyping.
TABLE 1.
L. monocytogenes strains used in this study
| Serogroup | Strain | Origin | Resistancea |
|---|---|---|---|
| 4 | H7962 | Sara Lee | Resistant |
| 1 | H7764 | Sara Lee | Resistant |
| 1 | C511 | Unknown | Sensitive |
| 4 | LJH389 | Unknown | Sensitive |
| 4 | LJH381 | Health Canada | Sensitive |
| 1 | C712 | CRIFS collection | Sensitive |
A resistant strain was defined as a strain having an initial tolerance of 4 μg of BC/ml of TSB; a sensitive strain was defined as a strain having an initial tolerance of <4 μg of BC/ml of TSB.
Parent L. monocytogenes strains were grown and maintained in tryptic soy broth (TSB) (Difco Laboratories, Sparks, Md.) at 37°C, whereas adapted strains were grown and maintained in TSB containing 1 to 10 μg of BC/ml at 37°C.
Adaptation to growth in the presence of QACs.
The tolerance of resistant and sensitive L. monocytogenes isolates was gradually increased by serially inoculating L. monocytogenes (100 μl) from a 24- to 48-h culture into TSB (10 ml) containing a range of concentrations (1 to 10 μg/ml) of BC (catalog no. B-1383; Sigma Chemical Co., St. Louis, Mo.). These cultures were incubated at 37°C for an additional 24 to 48 h. Additional subcultures were prepared from the tube containing the highest concentration of BC that resulted in turbidity after 24 h or 48 h. Strains were subcultured in tubes containing the same concentration and the next highest concentration of BC.
Determination of MIC.
The MIC of BC was determined by a microtiter plate assay (1, 29). A stock solution containing 500 μg of BC/ml in deionized water was prepared and stored at 4°C. Additional dilutions were prepared in TSB before each experiment as required. One hundred microliters of an L. monocytogenes culture containing approximately 104 CFU/ml (as determined from a standard curve relating absorbance at 600 nm to plate counts) was added in triplicate to 100-μl portions of TSB containing 0 to 10 μg of BC/ml. The MIC was defined as the lowest concentration of BC that completely prevented growth after incubation at 37°C for 24 h. The MICs of BC for all six parent and all six adapted strains were determined.
Determination of cell surface charge and hydrophobicity.
Cell surface charge and hydrophobicity were determined by the microbial adhesion to solvents (MATS) test based on a comparison of microbial cell affinities to polar and nonpolar solvents. The method originally proposed by Rosenberg et al. (24) and recently modified by Briandet et al. (3) was used to measure MATS. Two pairs of solvents were chosen based on their surface tension properties. Chloroform and hexadecane indicate the electron donor-acceptor interactions at the cell surface, and ethyl acetate and decane are indicators of hydrophobicity.
Log-phase cells of L. monocytogenes were harvested by centrifugation at 5,000 × g for 10 min, washed twice, and resuspended in 0.15 M NaCl at a concentration of approximately 108 CFU/ml. The cell suspension (2.4 ml) was vortexed with 0.4 ml of the appropriate solvent for 60 s and was allowed to stand for 15 min, which resulted in complete separation of the two phases. An aqueous-phase sample (1 ml) was obtained, and the absorbance at 400 nm was determined with a Pharmacia Novaspec II spectrophotometer (Amersham Pharmacia Biotech Inc., Uppsala, Sweden). The percentage of cells present in each solvent was calculated with the following equation: percent affinity = 100 × [1 − (A/A0)], where A0 is the absorbance of the suspension at 400 nm prior to mixing and A is the absorbance after vortexing. Each experiment was performed in triplicate with three independently prepared cultures.
Serology.
Listeria O antiserum types 1 and 4 (Difco Laboratories) were used for serological identification of L. monocytogenes by using the slide agglutination test according to the manufacturer's instructions.
Measurement of efflux pump activity.
Bacteria were grown at 37°C on tryptic soy agar (Difco Laboratories) plates containing 0.5 μg of ethidium bromide (EtBr) (Sigma Chemical Co.) per ml (29). The plates were inspected for fluorescence under UV light after 24 h of incubation at 37°C. Cells that accumulated EtBr fluoresced pink, whereas cells that partially accumulated EtBr were pale pink. Cells that did not accumulate EtBr were white.
Analysis of L. monocytogenes fatty acid profiles.
Three isolates, C712, LJH381, and H7962, were chosen for fatty acid profile analysis. Each parent L. monocytogenes strain grown in TSB and each adapted strain grown in TSB containing BC was inoculated into 10 ml of TSB. The tubes were incubated at 37°C. Cells were harvested in the mid-log phase by centrifugation at 5,000 × g and were washed three times with 0.15 M phosphate-buffered saline. The cell pellet was collected, and 40.0 ± 1 mg was placed into a sterile sample vial by using a 4-mm disposable loop. Analysis was performed with the MIDI 6850 GC Sherlock system (MIDI Inc., Newark, Del.) by using the fatty acid methyl ester preparation, the MIDI protocol, and the calibration assay described in the Microbial Identification System Operating Manual, version 4.93 (MIDI Inc.).
Electron microscopy of thin sections.
Thin sections of parent and adapted cultures of LJH381 and H7962 were prepared and examined by transmission electron microscopy. Cells were grown to the mid-log phase in TSB with BC (adapted strains) and without BC (parent strains) at 37°C. The cells were harvested by centrifugation (5,000 × g, 10 min, 4°C) and were washed by suspension and centrifugation in 0.15 M HEPES buffer (catalog no. H3375; Sigma Chemical Co.). Listeria cells were suspended in HEPES buffer with 2% glutaraldehyde for fixing; then they were washed with HEPES buffer (0.05 M, pH 6.8), fixed, and stained with osmium tetroxide at 4°C for at least 2 h. The washing procedure was then repeated with uranyl acetate. The cells were dehydrated with an ethanol series (25, 50, 75, and 95% ethanol, followed by two treatments with 100% ethanol). Prior to polymerization (1 h, 60°C), specimens were put in absolute ethanol-London Resin White (50:50, vol/vol) and in 100% London Resin White and left on a rotator for 8 h at 4°C. The cells were sectioned and then poststained with uranyl acetate and lead citrate for 5 and 1 min, respectively. They were examined with an electron microscope (LEO model 912 AB).
RESULTS AND DISCUSSION
Adaptation.
L. monocytogenes exhibited acquired tolerance to sublethal concentrations of BC and began to show gradual adaptation to BC after 1 week of incubation. Each L. monocytogenes parent was exposed to BC and was allowed to adapt to increasing concentrations until a point when 10 consecutive transfers resulted in no further adaptation. An unusual mode of growth in tubes incubated with shaking was observed for all strains during adaptation with BC at concentrations greater than 5 μg/ml. After overnight growth, cells were attached to the bottoms of the tubes. Only after 30 h of incubation did the strains begin to grow as planktonic cultures. It is known that gram-positive bacteria can produce extracellular lipoteichoic acid (9), which can be stimulated by adherent growth, and this may affect the permeability of the cells to BC. In addition, L. monocytogenes produces a surface-bound lipopolysaccharide-like substance that may decrease the cell envelope permeability (32).
The degree of adaptation to BC was confirmed with MIC tests by using the microtiter plate method. After the adaptation period, the MICs for initially resistant strains were at least double those for their parent strains, whereas the MICs for the initially sensitive strains were at least fivefold higher than the original MICs (Table 2). After the adaptation period, the MICs for all isolates were approximately equal, and based on the criteria of Aase et al. (1), who considered isolates with MICs of BC between 4 and 7 μg/ml resistant, all adapted strains were resistant. In agreement with previously described data (1), the initial MICs for sensitive strains were much lower than those for the resistant strains, the increases in the tolerance of the sensitive strains following adaptation were more significant, and the final MICs of BC for adapted cells of the initially sensitive strains were similar to those of the resistant strains. The MICs of BC for the adapted forms of the originally sensitive strains (strains with MICs of <4 μg/ml) remained virtually unchanged after further subculturing without BC for 10 months. However, the abilities of initially resistant strains (H7764 and H7962) to resist higher BC levels (8 to 10 μg/ml) were variable. This suggests that the acquired resistance of sensitive strains was not easily reversible and that the ability of the resistant strains (H7764 and H7962) to adapt further was limited.
TABLE 2.
MICs of BC and serotypes of parent and adapted strains
| Strain | Resistancea | Parent strain
|
Adapted strain
|
||
|---|---|---|---|---|---|
| MIC (μg/ml) | Serotype | MIC (μg/ml) | Serotype | ||
| H7962 | R | 4 | 4 | 8 | 1 and 4 |
| H7764 | R | 4 | 1 | 8 | 1 and 4 |
| C511 | S | 1 | 1 | 5 | 1 and 4 |
| LJH389 | S | 1 | 4 | 6 | 1 and 4 |
| LJH381 | S | 1 | 4 | 5 | 1 and 4 |
| C712 | S | 1 | 1 | 5 | 1 and 4 |
R, resistant; S, sensitive.
Serology.
Serological identities were confirmed with Bacto-Listeria O antiserum types 1 and 4. As expected, parent strains were either type 1 or type 4 positive (Table 2). However, all adapted strains showed reactions with both type 1 and type 4 antisera, with stronger antigen-antibody agglutination to the parent serotypes (100% of the cells clumped) and weaker reactions with the alternative serotypes (approximately 50% of the cells clumped). No agglutination of the cells was observed in saline in the absence of antisera. The identities of the isolates were confirmed after adaptation by automated ribotyping, and serotyping was carried out with isolated colonies to ensure that the results were not the result of cross-contamination. Parent and adapted isolates had identical riboprint patterns, yet in all cases the adapted strains showed some level of agglutination with both types of antisera. This suggests that the presentation of cell surface chemicals, possibly antigenic determinants (epitopes), can induce antigen-antibody reactions with both antigen 1 and antigen 4 after adaptation to BC. A possible explanation for the presence of both type 1 and type 4 antigenic activities after adaptation involves modification of teichoic acid. This acid is an important anionic polymer in the Listeria cell wall that determines serotype. Glycosylation of teichoic acid with different sugar substitutes can alter the antigenic characteristics and pathogenicity of Listeria (7, 13, 2-30).
Other researchers have reported that QAC resistance was associated with nontypeability with bacteriophage, which may also be related to cell surface changes that alter the structure of phage receptor sites (19).
Efflux pump activity.
Sundheim et al. (29) showed that staphylococci were capable of using efflux pumps to eliminate foreign chemicals from the cytoplasm. To illustrate the role of efflux pumps in adaptation of L. monocytogenes to external chemical stress, parent and adapted cells were examined by using tryptic soy agar plates containing EtBr. All parent strains showed bright pink fluorescence when they were examined under UV light, indicating that EtBr accumulated in the cells. In contrast, adapted strains of originally sensitive strains had pale pink (LJH389) or white colonies (C511, LJH389, and C712), showing that efflux pumps were activated. Following adaptation, resistant strains H7962 and H7764 did not show induction of efflux pumps and had levels of fluorescence similar to those of the parent strains. Thus, the efflux pump was more active in three of the four sensitive strains than in the remaining sensitive strain and was absent in the two resistant strains. Previous studies have also shown that an increase in resistance to both EtBr and BC was related to induction of an active efflux mechanism in L. monocytogenes (1) and other species (10). This energy-linked efflux system is not substrate specific and can be initiated by chemicals other than BC (21), indicating that BC and EtBr could be substrates for the same efflux system in Listeria. In this study, the sensitive strains exhibited more dramatic increases in tolerance to BC than the resistant strains exhibited, which indicated that there is a close correlation between the tolerance to BC and the use of efflux pumps as an adaptation mechanism. These results suggest the utilization of efflux pumps contributes significantly to the ability of L. monocytogenes to adapt to BC. However, the parent strains (H7962 and H7764) that were originally scored as resistant to BC (MIC, ≥4 μg/ml) were not able to completely exclude EtBr by using efflux pumps, suggesting that a different resistance mechanism is responsible for the intrinsic tolerance of these isolates to BC.
Changes in cell surface properties.
As an adaptive response, bacterial cell surfaces may undergo chemical changes to repel the water-soluble compound BC. These changes may result in a change in cell surface hydrophobicity. Such changes have been shown to occur in Lactobacillus spp. (23), but no studies have been carried out with L. monocytogenes. In order to examine whether changes in the cell surface characteristics of L. monocytogenes occurred as a result of adaptation to BC, the MATS test was conducted with cells before and after adaptation.
In this study, there was no major difference in cell surface hydrophobicity between parent strains and the corresponding adapted strains (Table 3). Both parent and adapted strains showed higher affinity to chloroform than to hexadecane, indicating the hydrophilic nature of all strains. The affinity to decane was higher than the affinity to ethyl acetate, showing the weak electron-accepting properties of L. monocytogenes in general. The microbial adhesion to chloroform and the microbial adhesion to ethyl acetate were also determined (Table 3) in order to assess the Lewis electron donor-electron acceptor properties of Listeria. All strains showed higher affinity for the acidic compound chloroform and lower affinity for the basic compound ethyl acetate, demonstrating that Listeria strains were strong electron donors and weak electron acceptors, which agreed with their hydrophilic cell surface properties. The results showed that all the strains had cell surfaces that were characterized as strongly basic and weakly acidic. Listeria could possess chemical groups, such as the —COO− and HSO3− present on the cell surface of Lactobacillus spp. (23), responsible for the strong electron donor character, but this needs to be investigated further. In gram-negative bacteria, a hydrophilic cell surface is associated with the presence of polysaccharides, whereas hydrophobic surfaces contain more (glyco-)proteinaceous material (5). The lack of these surface materials in Listeria suggested that modification of the cell surface is largely due to teichoic acids, which are responsible for antigenic determination (30) and also serve as phage receptors (31), or due to the peptidoglycan itself.
TABLE 3.
MATS test: hydrophobicity results for parent and adapted strains
| Strain | % Affinity (mean ± SD) to:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Chloroform
|
Hexadecane
|
Decane
|
Ethyl acetate
|
|||||
| Parent strain | Adapted strain | Parent strain | Adapted strain | Parent strain | Adapted strain | Parent strain | Adapted strain | |
| H7962 | 94.82 ± 1.40 | 85.14 ± 0.80 | 56.02 ± 11.15 | 55.43 ± 1.06 | 65.53 ± 1.69 | 55.17 ± 2.58 | 49.46 ± 1.69 | 42.99 ± 2.24 |
| H7764 | 82.80 ± 6.43 | 77.86 ± 1.24 | 79.41 ± 3.40 | 44.37 ± 4.37 | 53.12 ± 3.73 | 46.61 ± 6.23 | 38.24 ± 5.79 | 38.38 ± 1.40 |
| C511 | 83.71 ± 2.83 | 92.48 ± 0.70 | 70.85 ± 3.81 | 57.32 ± 8.67 | 25.83 ± 9.03 | 81.78 ± 1.34 | 21.55 ± 6.11 | 28.36 ± 1.93 |
| LJH389 | 91.90 ± 0.75 | 87.39 ± 0.52 | 84.12 ± 4.23 | 43.02 ± 2.16 | 51.51 ± 1.12 | 59.12 ± 1.29 | 47.72 ± 1.82 | 52.77 ± 3.37 |
| LJH381 | 94.44 ± 1.24 | 92.13 ± 1.05 | 50.06 ± 9.13 | 70.44 ± 2.16 | 47.00 ± 2.96 | 78.34 ± 1.19 | 33.39 ± 0.54 | 29.12 ± 3.70 |
| C712 | 90.59 ± 1.78 | 95.72 ± 0.62 | 63.34 ± 11.25 | 67.78 ± 2.63 | 55.53 ± 9.38 | 83.47 ± 2.67 | 23.19 ± 1.07 | 46.15 ± 4.62 |
Thus, this study indicates that a change in cell surface hydrophobicity does not appear to be involved in the adaptation response of L. monocytogenes to BC.
Changes in cellular fatty acid composition.
To determine the relationship between modification of the outer membrane and resistance, the fatty acid profiles of parent and adapted H7962, LJH381, and C712 strains were determined by using the MIDI automated gas chromatographic instrument. By comparing sample strains with reference standards, cell surface fatty acids ranging from 10:0 decanoic acid to anteiso-19:0 fatty acids were recognized, and their relative amounts were calculated. The predominant fatty acids present in all L. monocytogenes strains were iso-15:0 to anteiso-17:0 fatty acids (Table 4), which is consistent with the expected profiles for L monocytogenes (2). For the sensitive strains, the parent and adapted cells had similar fatty acid profiles. Pseudomonas aeruginosa strains adapted to QACs showed no significant shift between long-chain and short-chain fatty acids but did exhibit a decrease in the overall percentage of hydroxy fatty acids present (12). However, this change in the proportion of hydroxy fatty acids was not seen in any of the L. monocytogenes strains used in this study. In the H7962 resistant strain, there was a slight shift from shorter to longer fatty acids upon exposure to sublethal concentrations of BC (Table 4). Rather than having a more flexible outer lipopolysaccharide layer like gram-negative microorganisms, L. monocytogenes possesses a thicker and more rigid peptidoglycan layer as a barrier to the external environment. A modification resulting in a larger proportion of longer fatty acids in the cell membrane would result in a decrease in fluidity. Such a change in fluidity may play a role in the increased resistance of L. monocytogenes upon exposure to BC.
TABLE 4.
Fatty acid profiles as determined by the MIDI system for three parent and three adapted L. monocytogenes isolates
| Fatty acid | % of total fatty acids ina:
|
|||||
|---|---|---|---|---|---|---|
| C712
|
LJH381
|
H7962
|
||||
| Parent strain | Adapted strain | Parent strain | Adapted strain | Parent strain | Adapted strain | |
| 10:0 (decanoic) | 0 | 0.20 | 0 | 0 | 0.20 | 0 |
| 12:0 (dodecanoic) | 0.49 | 0.46 | 0 | 0 | 0.59 | 0 |
| Unknown 13.565 | 0.33 | 0.49 | 0.55 | 0.38 | 0.45 | 0 |
| 14:0 iso | 0.82 | 0.79 | 0.77 | 0.76 | 1.27 | 0.45 |
| 14:0 (tetradecanoic) | 0.55 | 0.83 | 0.31 | 0.28 | 0.74 | 0.16 |
| 15:0 isob | 13.08 | 13.97 | 14.15 | 13.39 | 16.77 | 8.32 |
| 15:0 anteisob | 39.47 | 44.72 | 36.75 | 35.15 | 38.67 | 21.50 |
| 14:0 iso 30H | 1.31 | 2.02 | 1.99 | 1.63 | 1.72 | 0.30 |
| 14:0 20H | 0 | 0.28 | 0.25 | 0 | 0.15 | 0 |
| 16 iso | 4.52 | 0 | 4.77 | 4.95 | 4.89 | 6.66 |
| Unknown 15.669 | 1.44 | 2.06 | 1.88 | 1.69 | 1.58 | 1.74 |
| 16:0 (hexadecanoic) | 2.96 | 2.31 | 1.89 | 2.03 | 3.78 | 2.43 |
| 17:0 isob | 6.28 | 5.51 | 7.75 | 8.20 | 6.38 | 13.93 |
| 17:0 anteisob | 27.76 | 25.34 | 27.43 | 30.27 | 21.67 | 42.24 |
| 18 iso | 0 | 0 | 0 | 0 | 0 | 0.36 |
| 18:0 (octadecanoic) | 0.34 | 0.26 | 0.26 | 0.22 | 0.31 | 0.26 |
| 19:0 iso | 0 | 0 | 0 | 0 | 0 | 0.34 |
| 19:0 anteiso | 0.31 | 0.28 | 0.54 | 0.68 | 0.23 | 1.12 |
| Total | 90.87 | 84.17 | 86.61 | 87.22 | 90.47 | 91.20 |
The total amounts of the fatty acids in the C712 parent, C712 adapted, LJH381 parent, LJH381 adapted, H7962 parent, and H7962 parent strains were 294,431, 285,514, 254,898, 290,319, 267,746, and 421,656, respectively.
Predominant fatty acid in all strains.
The total amounts of fatty acids recovered from all of the parent and adapted strains were fairly consistent, with one important exception. The adapted strain of the originally resistant isolate H7962 exhibited a 57% increase in the total fatty acid content compared to the unadapted parent strain (Table 4). The difference cannot be accounted for on the basis of the original sample size. It has previously been demonstrated that an increase in total fatty acid content was responsible for adaptation to QACs in Serratia marcescens (4). It is possible that an increase in the fatty acid content in the cell membrane was responsible for the increased BC tolerance observed following adaptation of the originally resistant isolates. Further study is necessary to determine if this is a common phenomenon.
Morphological changes.
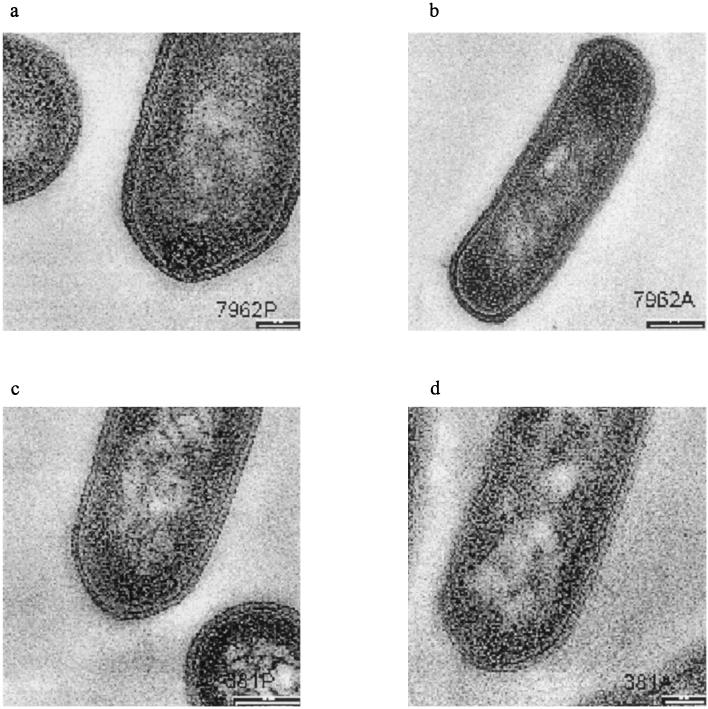
Transmission electron microscopy was used to examine the morphological changes in strains H7962 and LJH381 resulting from exposure to sublethal concentrations of BC. Previous studies showed that native resistance and acquired resistance to environmental stresses of typical wild-type Listeria strains were directly related to the length of the cells and the roughness of the cell surface (25, 26). Results obtained in this study agreed with these findings since the cells were elongated and filamentous after adaptation (Table 5). Electron micrographs of thin sections of parent and adapted L. monocytogenes cells grown in the presence of BC showed that the outer layers of adapted cells were more undulating and rougher than the outer layers of the parent cells (Fig. 1). Internally, the membrane-bound materials in the cytoplasm of adapted cells did not differ from the membrane-bound materials in the cytoplasm of unadapted cells, but there was shedding of unknown cell surface material in adapted cells, while shedding was absent in the unadapted cells.
TABLE 5.
Measurements for single L. monocytogenes cells, as determined with thin sectionsb
| Strain | Typea | Length 1 (nm) | Length 2 (nm) | Mean length (nm) | Width 1 (nm) | Width 2 (nm) | Mean width (nm) |
|---|---|---|---|---|---|---|---|
| H 7962 | P | 1,127.23 | 1,218.36 | 1,172.80 | 420.54 | 413.80 | 417.17 |
| H 7962 | A | 1,734.78 | 1,589.47 | 1,662.13 | 482.28 | 476.15 | 479.22 |
| LJH 381 | P | 1,179.11 | 1,152.91 | 1,166.01 | 421.86 | 480.01 | 450.94 |
| LJH 381 | A | 1,436.24 | 1,779.31 | 1,607.78 | 422.58 | 451.77 | 437.18 |
P, parent strain; A, adapted strain.
Two cells were measured for each isolate (length 1, width 1 and length 2, width 2).
FIG. 1.
Electron micrographs of thin sections of L. monocyotgenes. (a) Resistant parent strain H7962P grown in TSB. Scale bar = 100 nm. (b) Adapted resistant strain H7962A grown in the presence of 8 μg of BC per ml. Scale bar = 200 nm. (c) Sensitive parent strain LJH381P grown in TSB. Scale bar = 100 nm. (d) Adapted sensitive strain LJH381A grown in the presence of 5 μg of BC per ml. Scale bar = 100 nm. Compared with the parent strains, adapted strains were elongated and slimmer. Waviness and shedding of unknown materials were apparent in adapted strains.
In conclusion, the results obtained in this study demonstrate that L. monocytogenes exhibits morphological and physicochemical changes in the bacterial cell surface following adaptation to BC. After exposure to sublethal concentrations of BC, the extents of these changes were different in different strains. In this study, the resistant and sensitive strains appeared to use different mechanisms to adapt to environmental chemical stress. All adapted variants of the originally sensitive strains showed induction of an efflux pump mechanism after the adaptation period; the efflux pump activity was absent in originally resistant strains that were adapted. Development of resistance in normally susceptible bacteria usually involves enzymatic inactivation mechanisms or overexpression of the efflux system rather than a decrease in cell membrane permeability. However, the changes in cell surface hydrophobicity were not sufficient to demonstrate a difference between parent and adapted cells for both resistant and sensitive strains. Alterations in the fatty acid profile were demonstrated only for the resistant strain tested and were not apparent in sensitive strains. An increase in the total amount of fatty acids in the cell membrane may be the mechanism responsible for increased tolerance of originally resistant isolates following adaptation. Morphological changes to adapted cells, resulting in changes in shape, size, and roughness of the cell surfaces, were obvious.
In order to quantitatively compare the differences between resistant and sensitive strains, a larger sample size and the use of more strains are necessary. In order to increase the effectiveness of sanitation, food companies often use a combination of antimicrobial agents. The interactions among different disinfectants on L. monocytogenes need to be studied. In this study, cells were grown under optimal conditions. The effects of other stresses on adaptation also should be examined. Attached L. monocytogenes cells are capable of forming biofilms and generating a glycocalyx layer that protects the cells and prevents direct contact with QACs. Although not tested in this study, the possible development of biofilms in response to sanitizer adaptation and the role of the growth phase of L. monocytogenes are factors that should be considered (17).
This study contributed to our understanding of L. monocyotgenes adaptation to a QAC. The underlying hazards associated with prolonged exposure to a low concentration of a single disinfectant should be a focus in industrial sanitation.
Acknowledgments
We acknowledge the financial assistance of Sara Lee Corporation and the Beef Industry Development Fund of the Alberta Agricultural Research Institute. Stacy Favrin acknowledges the financial support of the Canadian Food Inspection Agency through its President's Scholarship program. The continued financial support of Dairy Farmers of Ontario and the Natural Sciences and Engineering Research Council of Canada is appreciated by Mansel W. Griffiths.
We are also grateful to Bob Harris and Kokying Lee for their technical assistance with the transmission electron microscopy and MIDI analyses, respectively.
REFERENCES
- 1.Aase, B., G. Sundheim, S. Langsrud, and L. V. Rørvik. 2000. Occurrence of and a possible mechanism for resistance to a quaternary ammonium compound in Listeria monoctyogenes. Int. J. Food Microbiol. 62:57-63. [DOI] [PubMed] [Google Scholar]
- 2.Annous, B. A., L. A. Becker, D. O. Bayles, D. P. Labeda, and B. J. Wilkinson. 1997. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 63:3887-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briandet, R., T. Meylheuc, C. Maher, and M. N. Bellon-Fontaine. 1999. Listeria monocytogenes Scott A: cell surface charge, hydrophobicity, and electron donor and acceptor characteristics under different environmental growth conditions. Appl. Environ. Microbiol. 65:5328-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaplin, C. E. 1952. Bacterial resistance to quaternary ammonium disinfectants. J. Bacteriol. 63:453-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuperus, P. L., H. C. van der Mei, G. Reid, A. W. Bruce, A. H. Khoury, P. G. Rouxhet, and H. J. Busscher. 1993. Physiochemical surface characteristics of urogenital and poultry lactobacilli. J. Colloid Interface Sci. 156:319-324. [Google Scholar]
- 6.Davis, J. G. 1962. Iodophors as detergent-sterilizers. J. Appl. Bacteriol. 25:195-201. [Google Scholar]
- 7.Fiedler, F., J. Seger, A. Schrettenbrunner, and H. P. R. Seeliger. 1984. The biochemistry of murein and cell wall teichoic acids in the genus Listeria. Syst. Appl. Microbiol. Rev. 5:360-376. [Google Scholar]
- 8.Guerin-Mechin, L., F. Dubois-Brissonnet, B. Heyd, and J. Y. Leveau. 2000. Quaternary ammonium compound stresses induce specific variation in fatty acid composition of Pseudomonas aeruginosa. Int. J. Food Microbiol. 55:157-159. [DOI] [PubMed] [Google Scholar]
- 9.Hammond, S. M., P. A. Lambert, and A. N. Rycroft. 1984. Walls of Gram-positive bacteria, p. 29-56. In The bacterial cell surface. Kapitan Szabo Publishers, Washington, D.C.
- 10.Heir, E., G. Gundheim, and A. L. Holck. 1998. The Staphylococcus qacH gene product: a new member of the SMR family encoding multidrug resistance. FEMS Microbiol. Lett. 163:49-56. [DOI] [PubMed] [Google Scholar]
- 11.Huillet, E., S. Larin, P. Pardon, and P. Berche. 1999. Identification of a new locus in Listeria monocytogenes involved in cellobiose-dependent repression of hly expression. FEMS Microbiol. Lett. 174:265-272. [DOI] [PubMed] [Google Scholar]
- 12.Jones, M. V., T. M. Herd, and H. J. Christie. 1989. Resistance of Pseudomonas aeruginosa to amphoteric and quaternary ammonium biocides. Microbios 58:49-62. [PubMed] [Google Scholar]
- 13.Kamisango, K., H. Fujii, H. Okumura, I. Saiki, Y. Araki, Y. Yamamura, and I. Azuma. 1983. Structural and immunochemical studies of teichoic acid of Listeria monocytogenes. J. Biochem. 93:1401-1409. [DOI] [PubMed] [Google Scholar]
- 14.Krysinski, E. P., L. J. Brown, and T. J. Marchisello. 1992. Effect of cleaners and sanitizers on Listeria monocytogenes attached to product contact surfaces. J. Food Prot. 55:246-251. [DOI] [PubMed] [Google Scholar]
- 15.Langsrud, S., and G. Sundheim. 1997. Factors contributing to the survival of poultry associated Pseudomonas spp. exposed to a quaternary ammonium compound. J. Appl. Microbiol. 82:705-712. [DOI] [PubMed] [Google Scholar]
- 16.Lopes, J. A. 1986. Evaluation of dairy and plant sanitizers against Salmonella typhimurium and Listeria monocytogenes. J. Dairy Sci. 69:2791-2796. [DOI] [PubMed] [Google Scholar]
- 17.Luppens, S. B. I., T. Abee, and J. Oosterom. 2001. Effect of benzalkonium chloride on viability and energy metabolism in exponential- and stationary-growth-phase cells of Listeria monocytogenes. J. Food Prot. 64:476-482. [DOI] [PubMed] [Google Scholar]
- 18.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mereghetti, L., R. Quentin, N. Marquet-van Der Mee, and A. Audurier. 2000. Low sensitivity of Listeria monocytogenes to quaternary ammonium compounds. Appl. Environ. Microbiol. 66:5083-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merianos, J. J. 1991. Quaternary ammonium antimicrobial compounds, p. 225-255. In S. S. Block (ed.), Disinfection, sterilization and preservation, 4th ed. Lea & Febiger, Malvern, Pa.
- 21.Midgley, M. 1994. Characteristics of an ethidium efflux system in Enterococcus hirae. FEMS Microbiol. Lett. 120:119-123. [DOI] [PubMed] [Google Scholar]
- 22.Mustapha, A., and M. B. Liewen. 1989. Destruction of Listeria monocytogenes by sodium hypochlorite and quaternary ammonium sanitizers. J. Food Prot. 52:306-311. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier, C., C. Bouley, C. Cayuela, S. Bouttier, P. Bourlioux, and M. Bellon-Fontaine. 1997. Cell surface characteristics of Lactobacillus casei subsp. casei, Lactobacillus paracasei subsp. paracasei, and Lactobacillus rhamnosus strains. Appl. Environ. Microbiol. 63:1725-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg, M., D. Gutnick, and E. Rosenberg. 1980. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 9:29-33. [Google Scholar]
- 25.Rowan, N. J., and J. G. Anderson. 1998. Effects of above-optimum growth temperature and cell morphology on thermotolerance of Listeria monocytogenes cells suspended in bovine milk. Appl. Environ. Microbiol. 64:2065-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowan, N. J., J. G. Anderson, and A. A. G. Candlish. 2000. Cellular morphology of rough forms of Listeria monocytogenes isolated from clinical and food samples. Lett. Appl. Microbiol. 31:319-322. [DOI] [PubMed] [Google Scholar]
- 27.Russell, A. D., S. D. Hammond, and J. R. Morgan. 1986. Bacterial resistance to antiseptics and disinfectants. J. Hosp. Infect. 7:213-225. [DOI] [PubMed] [Google Scholar]
- 28.Russell, A. D., W. B. Hugo, and G. A. J. Ayliffe. 1999. Principles and practice of disinfection, preservation, and sterilization, 3rd ed. Blackwell Science, University Press, Cambridge, United Kingdom.
- 29.Sundheim, G., T. Hagtvedt, and R. Dainty. 1992. Resistance of meat associated staphylococci to a quaternary ammonium compound. Food Microbiol. 9:161-167. [Google Scholar]
- 30.Uchikawa, K., J. Sekikawa, and I. Azuma. 1986. Structural studies on teichoic acids in cell walls of several serotypes of Listeria monocytogenes. J. Biochem. 99:315-327. [DOI] [PubMed] [Google Scholar]
- 31.Wendlinger, G., M. J. Loessner, and S. Scherer. 1996. Bacteriophage receptors on Listeria monocytogenes cells are the N-acetylglucosamine and rhamnose substituents of teichoic acids or the peptidoglycan itself. Microbiology 142:985-992. [DOI] [PubMed] [Google Scholar]
- 32.Wexter, H., and J. D. Oppenheim. 1979. Isolation, characterization, and biological properties of an endotoxin-like material from the gram-positive organism Listeria monocytogenes. Infect. Immun. 23:845-857. [DOI] [PMC free article] [PubMed] [Google Scholar]