Abstract
Postmortem records of wild-living birds in Norway with laboratory-confirmed findings of salmonella infection were summarized for the period from 1969 to 2000. Salmonella spp. were isolated from 470 birds belonging to 26 species. The salmonella-positive birds included 441 small passerines, 15 gulls, 5 waterfowl, 4 birds of prey, 3 doves, and 2 crows. The bullfinch (Pyrrhula pyrrhula) was by far the most frequently recorded species (54% of the cases). Salmonella enterica serover Typhimurium was recovered from all cases except from one hooded crow (Corvus corone), which yielded serovar Paratyphi-B var. Java. Variant O:4,12 comprised 96% (451 cases) of all serovar Typhimurium isolates, including all the passerines, while variant O:4,5,12 accounted for the remaining 4% (18 cases). The occurrence of salmonellae in small passerines showed a distinct seasonality, with a peak in February and March. Plasmid profile analysis of 346 isolates of serovar Typhimurium O:4,12 detected six profiles, of which two comprised 66 and 28% of the isolates, respectively. Phage typing of 52 randomly selected isolates of serovar Typhimurium O:4,12 from passerines detected four types: DT 40 (54%), U277 (35%), DT 99 (6%), and DT 110 (4%).
Bacteria belonging to the genus Salmonella are important causal agents of human enteritis throughout the world (13, 39-41). The major source of infection is meat products from domestic animals (45). In Norway, the number of recorded human cases has increased substantially in recent decades, in parallel with the trends noted elsewhere in the industrialized world (21, 23). However, in contrast to most other countries, the majority (>80%) of Norwegian patients acquired their infection abroad (23). The favorable domestic situation in Norway is obviously a consequence of the fact that Norwegian food-producing animals are virtually free from salmonella (21, 30a). However, analysis of data on human Salmonella enterica serovar Typhimurium infections during 1996 to 2001 shows that about 40% of Norwegian patients acquired the infection in Norway (30).
It has been suggested that serovar Typhimurium has established a reservoir in avian wildlife in Norway, and epidemiological and bacteriological evidence indicate that wild birds may transmit the infection to humans (20, 22). Recently, hedgehogs have also been recognized as a source of human serovar Typhimurium infection in Norway (14, 38, 42).
Surveys conducted in Norway have shown that gulls and crows may act as healthy carriers of a broad range of Salmonella serovars (1, 4, 6, 18, 31, 37, 43; T. Refsum, G. Holstad, and G. Kapperud, submitted for publication). Moreover, serovar Typhimurium is a well-known cause of fatal salmonellosis in small passerines (such as finches and sparrows) during winter (7). The purpose of the present study was to summarize historical records of Salmonella detections in wild birds in Norway and to compare the isolates by means of plasmid profile analysis and phage typing.
MATERIALS AND METHODS
All postmortem records covering wild birds at the National Veterinary Institute from 1969 to 2000 were examined, and all cases in which bacteriological examination had been performed and Salmonella bacteria had been isolated from one or more organs were included in this study. Most of the Salmonella-positive birds had been examined at the Section of Wildlife Disease at the main laboratory in Oslo (391 birds), whereas smaller numbers had been examined at the regional laboratories in western (10 birds), central (47 birds), and northern (22 birds) Norway. All included birds had been submitted spontaneously by members of the general public, except for 123 small passerines examined in a project carried out during 1998 to 2000. Prior to the receipt of these latter birds, the public had been encouraged to collect birds found dead at private feeding places. The project was carried out to study epidemiological and pathological aspects of fatal salmonellosis in small passerines’ feeding places during winter. Detailed results from this project will be published elsewhere (T. Refsum, T. Vikøren, K. Handeland, G. Kapperud, and G. Holstad, submitted for publication).
The methods used for isolation of Salmonella bacteria from dead birds included selective enrichment and plating on selective and differential agar media. From 1994, the presence of Salmonella was ascertained using the method recommended by the Nordic Committee on Food Analysis (29). The isolates were typed by the method of Popoff and Le Minor (33). For the great majority of the isolates, typing was conducted at the laboratory in Oslo and the results were confirmed by the National Salmonella Reference Laboratory at the Norwegian Institute of Public Health.
All available strains of serovar Typhimurium O:4,12 isolated since 1969 (n = 346; 342 isolates from small passerines and 4 isolates from other species) were characterized by plasmid profile analysis, using a small-scale modification of the alkaline lysis technique of Birnboim and Doly (5) as described by Maniatis et al. (27). Plasmid DNAs from Escherichia coli strains VA 517 (26) and J5 (R1) (17) were included as molecular weight standards.
In addition, phage typing of 52 randomly selected strains of serovar Typhimurium O:4,12 isolated from passerines from 1969 to 2000 was carried out. The typing scheme of Callow (8), as extended by Anderson et al. (3), was used.
RESULTS
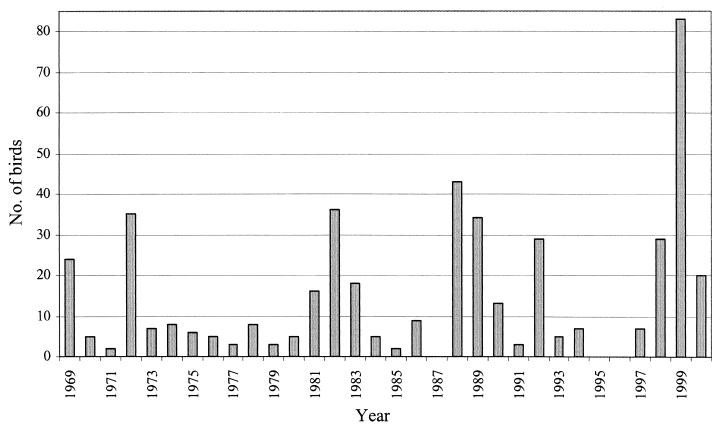
Salmonella was isolated from 470 bird carcasses representing 26 different species (Table 1). The total number of birds from which salmonellae were isolated according to year is given in Fig. 1. The great majority of cases (n = 441) occurred in small passerines found dead at private feeding places, representing 14 species and comprising 94% of the total material. The remaining cases (n = 29) occurred in 12 species of gulls, waterfowl, birds of prey, doves, and crows. The bullfinch (Pyrrhula pyrrhula) was by far the most frequently recorded species (54%), followed by the greenfinch (Carduelis chloris) (13%), the Eurasian siskin (Carduelis spinus) (11%), the common redpoll (Carduelis flammea) (7%), and the black-headed gull (Larus ridibundus) (3%). The remaining 21 species were represented by no more than 10 individuals.
TABLE 1.
Salmonella bacteria isolated from wild-living birds, by species, in Norway from 1969 to 2000
| Bird Species | No. of isolates of serovar:
|
Isolation frequencya | ||
|---|---|---|---|---|
| Typhimurium
|
Paratyphi B | |||
| O:4,12 | O:4,5,12 | |||
| Small passerines | ||||
| Bullfinch, Pyrrhula pyrrhula | 256 | 195/242 | ||
| European greenfinch, Carduelis chloris | 61 | 57/71 | ||
| Eurasian siskin, Carduelis spinus | 54 | 52/69 | ||
| Common redpoll, Carduelis flammea | 33 | 32/41 | ||
| Common sparrow, Passer domesticus | 10 | 7/31 | ||
| Great tit, Parus major | 8 | 6/87 | ||
| Blue tit, Cyanistes caeruleus | 4 | 3/14 | ||
| Yellow hammer, Emberiza citrinella | 4 | 2/15 | ||
| Chaffinch, Fringilla coelebs | 3 | 2/21 | ||
| Eurasian tree sparrow, Passer montanus | 3 | 3/6 | ||
| Brambling, Fringilla montefringilla | 1 | 1/16 | ||
| European robin, Erithacus rubecula | 1 | 0/1 | ||
| Hawfinch, Coccothraustes coccothraustes | 1 | 1/2 | ||
| Pied flycatcher, Ficedula hypoleuca | 1 | 0/8 | ||
| Willow tit, Poecile montanus | 1 | 1/8 | ||
| Total | 441 | 364/638 | ||
| Other species | ||||
| Black-headed gull, Larus ridibundus | 3 | 10 | ||
| Mallard, Anas platyrhynchos | 3 | |||
| Rock dove, Columba livia | 1 | 2 | 3/72 | |
| Black-billed magpie, Pica pica | 1 | 1/40 | ||
| Canada goose, Branta canadensis | 1 | |||
| Common murre, Uria aalge | 1 | |||
| Eurasian sparrow hawk, Accipiter nisus | 2 | |||
| Herring gull, Larus argentatus | 1 | |||
| Hooded crow, Corvus corone | 1 | 1/52 | ||
| Mew gull, Larus canus | 1 | |||
| Osprey, Pandion haliaetus | 1 | |||
| Tengmalm's owl, Aegolius funereus | 1 | |||
| Total | 10 | 18 | 1 | |
Number of cases/total number of birds submitted to the laboratory in Oslo.
FIG. 1.
Number of wild-living birds from which Salmonella bacteria were isolated, by year, in Norway from 1969 to 2000.
Information on the number of birds submitted for examination was available only for passerines received at the laboratory in Oslo (Table 1). The highest percentage of salmonellae in birds was noted among bullfinches (81%), followed by the Eurasian greenfinch (80%), the common redpoll (78%), and the Eurasian siskin (75%).
All isolates belonged to serovar Typhimurium, except for one isolate of serovar Paratyphi B var. Java from the hooded crow (Corvus corone) (Table 1). In small passerines, only serovar Typhimurium O:4,12 (also designated variant Copenhagen) was found, while both variants O:4,12 and O:4,5,12 were common in the nonpasserine species.
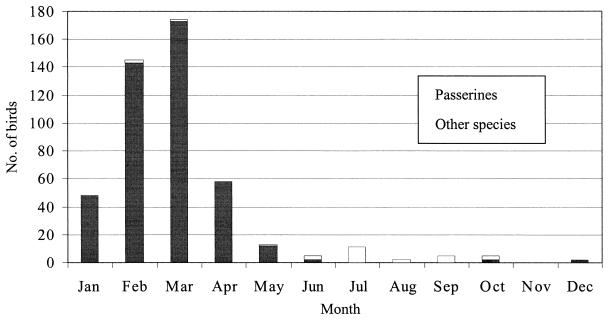
The seasonality of the cases is presented in Fig. 2. Salmonella isolations in small passerines were mainly recorded from January to April, with a distinct peak in February and March (Fig. 2). In other species, salmonellae were recorded in eight different months, without any apparent seasonality.
FIG. 2.
Number of wild-living birds from which Salmonella bacteria were isolated, by month, in Norway from 1969 to 2000.
The first recorded cases of Salmonella infection were detected in epizootics among passerines in southeastern Norway in 1969. Sporadic cases and epizootics occurred each winter in this region until the beginning of the 1980s, when carcasses were also submitted from western and central Norway. Since the 1980s, carcasses have been forwarded from all 19 counties in Norway.
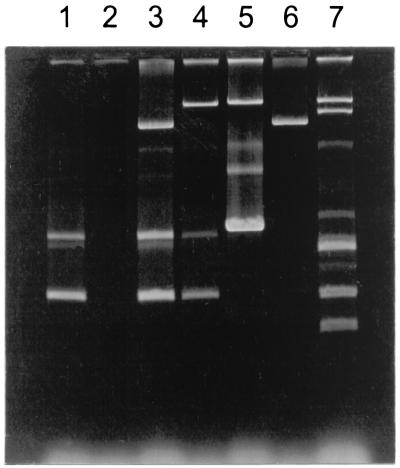
Plasmid profile analysis of 346 isolates of serovar Typhimurium O:4,12 detected six profile types (Fig. 3). Profile 1 was the most common, found in 228 of the cases (66%; 227 small passerines and 1 sparrow hawk, Accipiter nisus). The second most common profile was the plasmid-lacking profile 2, found in 97 of the cases (28%; 96 small passerines and 1 Tengmalm's owl, Aegolius funereus), whereas profiles 3, 4, 5, and 6 were present in 12 (3.5%; 12 small passerines), 4 (1.2%; small passerines), 4 (1.2%; 2 small passerines, 1 black-headed gull, Larus ridibundus, and 1 Canada goose, Branta canadensis), and 1 (0.3%, 1 bullfinch) of the isolates, respectively.
FIG. 3.
Plasmid profiles of serovar Typhimurium O:4,12 isolates from wild-living birds in Norway. The six plasmid profiles detected are shown in lanes 1 to 6. The size markers in lane 7 are standard plasmids (from top to bottom, 62.0, 35.8, 4.8, 3.4, 2.0, and 1.4 MDa).
Phage typing of 52 randomly selected passerine isolates of serovar Typhimurium O:4,12 detected four phage lysis patterns. The most common phage types were DT 40 and U 277, found in 54% (n = 28) and 35% (n = 18) of the isolates, respectively. The other phage types found were DT 99 (6%, n = 3) and DT 110 (4%, n = 2), whereas one isolate was termed RDNC (routine dilution, no conformity). The relationship between phage types and plasmid profiles is shown in Table 2.
TABLE 2.
Relationship between phage types and plasmid profiles of serovar Typhimurium O:4,12
| Phage type | No. of phage types with plasmid profilea:
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 5 | |
| DT 40 | 15 | 12 | 1 | |
| U277 | 13 | 4 | 1 | |
| DT 99 | 3 | |||
| DT 110 | 2 | |||
| RDNC | 1 | |||
Isolates with plasmid profiles 4 and 6 were not represented in the random selection of isolates for phage type analysis.
DISCUSSION
Our findings strongly indicated that serovar Typhimurium is endemically present in the avian fauna in Norway. In the present study, all but 1 of a total of 470 avian Salmonella isolates belonged to serovar Typhimurium variant O:4,12 or O:4,5,12. This is in accordance with a Swedish study in which these variants accounted for 98.5% of avian salmonellae (7). Small passerines were invariably associated with serovar Typhimurium O:4,12, a finding supported by studies from Sweden (7) and Germany (11, 24, 36). The isolates from other bird species included both variants O:4,12 and O:4,5,12.
This study has shown that the bacterium has caused fatal disease among small passerines during most winters in Norway since 1969 (Fig. 1). A similar seasonality, as visualized in Fig. 2, has also been reported from Sweden (7, 16), Great Britain (9, 32), Germany (11), and North America (10, 12, 44). Finches (Fringillidae) like the bullfinch, Eurasian greenfinch, Eurasian sisikin, and common redpoll, were most often affected; the same species predominated in the Swedish investigation (7).
Six plasmid profiles and four phage types were detected among the small-passerine isolates in the present study. However, plasmid profiles 1 and 2 and phage types DT 40 and U277 constituted more than 90% of the isolates examined. The few plasmid profiles and phage types obtained in our study may indicate that only a few strains are circulating in the passerine fauna.
The most common phage type found in small passerines was DT 40. This is in accordance with reports from Sweden (7, 28, 35). This phage type has also been detected in passerines in Great Britain (25) and North America (44). Phage type DT 40 was previously denoted DT 1 and DT 9 in Sweden and U165 in Great Britain and North America. Our findings support the assumption that DT 40 is the most important phage type in passerines and has an extensive geographical distribution (10, 32). Phage type U277, which was the second most common, is also common in Sweden (A. Gunnarsson, personal communication). The distribution of this phage type outside the Scandinavian peninsula is unknown.
Previous bacteriological and epidemiological investigations indicate that passerines are an important source of serovar Typhimurium O:4,12 in human salmonellosis in Norway. Sporadic cases of domestically acquired human infections caused by the plasmid profile 1 variant and cases of fatal salmonellosis among passerines were most often reported at the same time of year, thus indicating an epidemiological link (22). Moreover, a case-control study identified contact with ill or dead birds or their droppings as risk factors (22). An extensive human outbreak due to contaminated chocolate bars was reported in Norway in 1987 (20). Isolates of serovar Typhimurium O:4,12 from human patients, chocolate, and wild-living passerines belonged to the same plasmid profile (profile 1) and phage type (U277) (19). In the present study, profile 1 was the most common plasmid profile and U277 was the second most common phage type detected from passerines. In addition, recent molecular epidemiological studies have shown that 32% of the isolates recovered from human patients in Norway belonged to clones detected in the small-passerine fauna (15, 34).
Serovar Typhimurium O:4,5,12 was the most common variant found in bird species other than small passerines, mainly gulls, in the present study. In recent years, this variant has been responsible for three extensive outbreaks of human salmonellosis in Norway. The sources of infection in the outbreaks in eastern Norway in 1996 and in western Norway in 2000 were traced to heavily infected hedgehog populations (14). The Norwegian hedgehog was suggested to be a reservoir host for serovar Typhimurium O:4,5,12. Further, in a waterborne variant O:4,5,12 outbreak in northwestern Norway in 1999, gulls were suggested as the most likely source of infection (2; Refsum, Holstad, and Kapperud, submitted). However, it has been speculated whether the gulls might have become infected from Salmonella-infected hedgehogs (14). This is supported by recent molecular epidemiological analyses of hedgehog and avian isolates (14, 34). The molecular epidemiological studies also indicate that different clones of serovar Typhimurium predominate among passerines, gulls, and pigeons.
Acknowledgments
We thank Astrid Stovner for invaluable guidance in retrieving historical files and Kerstin Nordby and Traute Vardund for performing the plasmid profile analysis. We are also grateful for the data contributed by the regional laboratories of the National Veterinary Institute, which helped make the overview as complete as possible.
Financial support was provided by Sørlie's Foundation.
REFERENCES
- 1.Aalvik, B., and L. Rossebø. 1969. Studies of the occurrence of salmonella in intestinal contents and deposited feces from seagulls in Norwegian coastal regions. Nor. Veterinærtidsskr. 21:389-393. (In Norwegian.) [Google Scholar]
- 2.Aavitsland, P., and T. Hofshagen. 1999. Outbreak of salmonellosis in Herøy municipality. Nytt fra miljø- og samfunnsmedisin, vol. 3, report no. 12. National Institute of Public Health, Oslo, Norway. (In Norwegian.).
- 3.Anderson, E. S., L. R. Ward, M. J. Saxe, and J. D. de Sa. 1977. Bacteriophage-typing designations of Salmonella typhimurium. J. Hyg. 78:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aurstad, K., and E. S. Vaadal. 1991. The presence of Salmonella bacteria in gulls at Tranamarka refuse dump, Steinkjer municipality, August-September 1991. Municipal Food Control Authorities, Innherred, Norway.
- 5.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bø, G. 1980. The occurrence of salmonella in gulls at Grønmo refuse dump in Oslo. Viltrapport no. 10, p. 147-153. The Directorate for Nature Management (DN), Oslo, Norway. (In Norwegian.)
- 7.Borg, K. 1985. Spread of infection through wild animals—review of studies carried out over a 35-year period. Svensk Veterinärtidn. 37:111-128. (In Swedish.) [Google Scholar]
- 8.Callow, B. R. 1959. A new phage-typing scheme for Salmonella typhi-murium. J. Hyg. 57:346-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelius, L. W. 1969. Field notes on salmonella infection in greenfinches and house sparrows. Wildl. Dis. 5:142-143. [DOI] [PubMed] [Google Scholar]
- 10.Daoust, P. Y., D. G. Busby, L. Ferns, J. Goltz, S. McBurney, C. Poppe, and H. Whitney. 2000. Salmonellosis in songbirds in the Canadian Atlantic provinces during winter-summer 1997-98. Can. Vet. J. 41:54-59. [PMC free article] [PubMed] [Google Scholar]
- 11.Englert, H. K., K. Haass, J. Schneider, and M. Schnetter. 1967. Enzootic salmonellosis in birds in Baden. Berl. Münch. Tierärztl. Wochenschr. 80:277-279. (In German.) [PubMed] [Google Scholar]
- 12.Faddoul, G. P., G. W. Fellows, and J. Baird. 1966. A survey on the incidence of salmonellae in wild birds. Avian Dis. 10:90-94. [Google Scholar]
- 13.Gomez, T. M., Y. Motarjemi, S. Miyagawa, F. K. Käferstein, and K. Stöhr. 1997. Foodborne salmonellosis. World Health Stat. Q. 50:81-89. [PubMed] [Google Scholar]
- 14.Handeland, K., G. Kapperud, T. Refsum, B. Strøm Johansen, G. Holstad, G. Knutsen, I. Solberg, and J. Schulze. 2002.. Prevalence of Salmonella Typhimurium in Norwegian hedgehog populations associated with two human disease outbreaks. Epidemiol. Infect. 128:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heir, E., B.-A. Lindstedt, I. Nygård, T. Vardund, V. Hasseltvedt, and G. Kapperud. 2002. Molecular epidemiology of Salmonella Typhimurium isolates from human sporadic and outbreaks cases. Epidemiol. Infect. 128:373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurvell, B. 1973. Salmonella typhi-murium infections in wild passerines in Sweden. Svensk Veterinärtidn. 25:683-687. (In Swedish.) [Google Scholar]
- 17.Jacob, A. E., J. A. Shapiro, L. Yamamoto, D. I. Smith, S. N. Cohen, and D. Berg. 1977. Plasmids studied in Escherichia coli and other enteric bacteria, p. 607. In A. I. Bukhari, J. A. Shapiro, and S. L. Adhya, (ed.), DNA insertion elements, plasmids, and episomes. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 18.Kapperud, G., and O. Rosef. 1983. Avian wildlife reservoir of Campylobacter fetus subsp. jejuni, Yersinia spp., and Salmonella spp. in Norway. Appl. Environ. Microbiol. 45:375-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapperud, G., J. Lassen, K. Dommarsnes, B. E. Kristiansen, D. A. Caugant, E. Ask, and M. Jahkola. 1989. Comparison of epidemiological marker methods for identification of Salmonella typhimurium isolates from an outbreak caused by contaminated chocolate. J. Clin. Microbiol. 27:2019-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapperud, G., S. Gustavsen, I. Hellesnes, A. H. Hansen, J. Lassen, J. Hirn, M. Jahkola, M. A. Montenegro, and R. Helmuth. 1990. Outbreak of Salmonella typhimurium infection traced to contaminated chocolate and caused by a strain lacking the 60-megadalton virulence plasmid. J. Clin. Microbiol. 28:2597-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapperud, G., J. Lassen, S. Aasen, and V. Hasseltvedt. 1994. Global transmission of Salmonella—increased risk in Norway. Tidsskr. Nor. Lægeforen. 114:2125-2129. (In Norwegian.) [PubMed] [Google Scholar]
- 22.Kapperud, G., H. Stenwig, and J. Lassen. 1998. Epidemiology of Salmonella typhimurium O:4-12 infection in Norway—evidence of transmission from an avian wildlife reservoir. Am. J. Epidemiol. 147:774-782. [DOI] [PubMed] [Google Scholar]
- 23.Kapperud, G., J. Lassen, and V. Hasseltvedt. 1998. Salmonella infections in Norway: descriptive epidemiology and a case-control study. Epidemiol. Infect. 121:569-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kösters, J., and M. Scheer. 1967. Salmonellosis in wild living finches. Tierärztl. Umsch. 22:66-71. (In German.) [Google Scholar]
- 25.MacDonald, J. W., and L. W. Cornelius. 1969. Salmonellosis in wild birds. Br. Birds 62:28-30. [Google Scholar]
- 26.Macrina, F. L., D. J. Kopecko, K. R. Jones, D. J. Ayers, and S. M. McCowen. 1978. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid 1:417-420. [DOI] [PubMed] [Google Scholar]
- 27.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual, p. 368-369. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Mr̊tensson, L., T. Holmberg, B. Hurvell, L. Rutqvist, K. Sandstedt, and M. Wierup. 1984. Salmonella isolated from animals and feed stuffs in Sweden during 1978-1982. Nord. Vet. Med. 36:371-393. [PubMed] [Google Scholar]
- 29.Nordic Committee on Food Analysis (NMKL). 1991. Salmonella: detection in food. Method no. 71, 4th ed. National Veterinary Institute, Oslo, Norway.
- 30.Norwegian Institute of Public Health. 1996-2001. The Norwegian Surveillance System for Communicable Diseases, MSIS. Norwegian Institute of Public Health, Oslo.
- 30a.Norwegian Zoonosis Centre. 1999-2000. Annual reports. Norwegian Zoonosis Centre, Oslo.
- 31.Olsvik, Ø. 1980. Salmonella in crows. Viltrapport no. 10, p. 157. Directorate for Nature Management (DN), Oslo, Norway. (In Norwegian.).
- 32.Pennycott, T. W., H. M. Ross, I. M. McLaren, A. Park, G. F. Hopkins, and G. Foster. 1998. Causes of death of wild birds of the family Fringillidae in Britain. Vet. Rec. 143:155-158. [DOI] [PubMed] [Google Scholar]
- 33.Popoff, M. Y., and L. Le Minor. 1992. Antigenic formulas of the Salmonella serovars. WHO Collaborating Centre for References and Research on Salmonella. Institut Pasteur, Paris, France.
- 34.Refsum, T., E. Heir, G. Kapperud, T. Vardund, and G. Holstad. 2002. Molecular epidemiology of Salmonella enterica serovar Typhimurium isolates determined by pulsed-field gel electrophoresis: comparison of isolates from avian wildlife, domestic animals, and the environment in Norway. Appl. Environ. Microbiol. 68:5600-5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandstedt, K., A. Gunnarsson, B. Hurvell, B. Nordblom, L. Rutqvist, and O. Söderlind. 1980. Salmonella isolated from animals and feed stuffs in Sweden during 1973-1977. Nord. Vet. Med. 32:57-74. [PubMed] [Google Scholar]
- 36.Schaal, E., and H. Ernst. 1967. Enzootic occurrence of salmonellosis in local wild birds. Berl. Münch. Tierärztl. Wochenschr. 80:13-16. (In German.) [PubMed] [Google Scholar]
- 37.Skjølaas, O. 1969. Salmonella in kituwakes and other bird species in the North Atlantic area. Thesis. Die Tierärztliche Hochschule Hannover, Hanover, Germany. (In German.)
- 38.Søbstad, Ø., J. Blinkenberg, E. Bergesen, A. Digranes, I. Tveit, E. Heir, G. Kapperud, T.-L. Stavnes, V. Hasseltvedt, and B. G. Iversen. 2000. Transmission of salmonellosis through hedgehogs in Norway. Eurosurveillance Weekly, vol. 4, no. 38.
- 39.Tauxe, R. V. 1997. Emerging foodborne diseases: an evolving public health challenge. Emerg. Infect. Dis. 3:425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorns, C. J. 2000. Bacterial food-borne zoonoses. Rev. Sci. Technol. 19:226-239. [DOI] [PubMed] [Google Scholar]
- 41.Todd, E. C. 1997. Epidemiology of foodborne diseases: a worldwide review. World Health Stat. Q. 50:30-50. [PubMed] [Google Scholar]
- 42.Vidar, G., and M. Haugum. 1997. Outbreak of salmonellosis in Moss during the autumn 1996. Norsk Veterinærtidsskr. 109:33-35. (In Norwegian.) [Google Scholar]
- 43.Willumsen, B., and S. Hole. 1987. Presence of Campylobacter spp. and Salmonella spp. in seagulls and crows and its association with human enteritidis in the Bodø area. Norsk Veterinærtidsskr. 99:277-282. (In Norwegian.) [Google Scholar]
- 44.Wobeser, G. A., and M. C. Finlayson. 1969. Salmonella typhimurium infection in house sparrows. Arch. Environ. Health 19:882-884. [DOI] [PubMed] [Google Scholar]
- 45.Wray, C., and A. Wray. 2000. Salmonella in domestic animals. CABI Publishing, Wallingford, United Kingdom.