Abstract
Lactococcus lactis MBP71 ΔthyA (thymidylate synthase) cannot synthesize dTTP de novo, and DNA replication is dependent on thymidine in the growth medium. In the nonreplicating state acidification by MBP71 was completely insensitive to bacteriophages (M. B. Pedersen, P. R. Jensen, T. Janzen, and D. Nilsson, Appl. Environ. Microbiol. 68:3010-3023, 2002). For nonreplicating MBP71 the biomass increased 3.3-fold over the first 3.5 h, and then the increase stopped. The rate of acidification increased 2.3-fold and then started to decrease. Shortly after inoculation the lactic acid flux was 60% of that of exponentially growing MBP71. However, when nonspecific ATPase activity was incorporated into MBP71, the lactic acid flux was restored to 100% but not above that point, indicating that control over the flux switched from ATP demand to ATP supply (i.e., to sugar transport and glycolysis). As determined by growing nonreplicating cells with high ATPase activity on various sugar sources, it appeared that glycolysis exerted the majority of the control. ATPase activity also stimulated the rate of acidification by nonreplicating MBP71 growing in milk, and pH 5.2 was reached 40% faster than it was without ATPase activity. We concluded that ATPase activity is a functional means of increasing acidification by nonreplicating L. lactis.
Lactic acid bacteria are employed in many types of food fermentation. In dairy fermentations the lactic acid bacteria convert the lactose of the milk into lactic acid, which in addition to its preservation effect is also important for flavor and texture development. However, during growth the cells are susceptible to bacteriophage infections, and this is one of the greatest problems in dairies today.
We have previously described construction of mutant MBP71 ΔthyA from Lactococcus lactis subsp. lactis CHCC373 (24). The thyA gene encodes thymidylate synthase, and without this enzyme thymidine must be present in the growth medium for the synthesis of dTTP (Fig. 1). Milk and M17 broth do not support propagation of MBP71; i.e., the number of CFU does not increase unless thymidine is added (24). When dTTP is not available inside the cell, DNA replication does not occur, and an infecting phage therefore is not able to replicate its genome and phage propagation is terminated. It was demonstrated previously that acidification by MBP71 was completely unaffected by phages at a multiplicity of infection of 0.1 when thymidine was not added (24). Naturally, the cells are not able to replicate their chromosome either, and they do not replicate and do not divide.
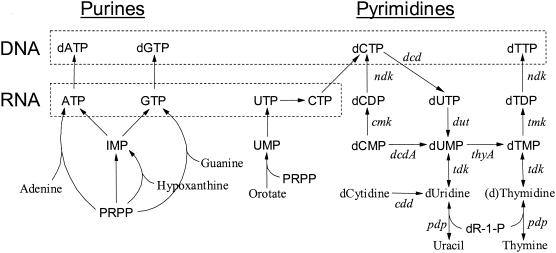
FIG. 1.
Overview of the nucleotide metabolism of L. lactis. Purine metabolism and pyrimidine metabolism are shown on the left and right, respectively. Only the interconversions of the deoxyribonucleotide pyrimidines and precursors are shown in detail, and other reaction arrows may represent more than one enzymatic reaction. Boxes surrounded by dashed lines indicate the triphosphate nucleotides utilized for synthesis of DNA and RNA, as indicated on the left. Genes: pdp, thymidine phosphorylase; cdd, cytidine deaminase; tdk, thymidine kinase; dcdA, dCMP deaminase; thyA, thymidylate synthase; cmk, CMP kinase; dut, dUTPase; tmk, thymidylate kinase; ndk, nucleotide diphosphate kinase; dcd, dCTP deaminase. Most of the genes indicated have been localized in the genome of L. lactis IL1403; the only exceptions are ndk and dcd (2). In the IL1403 genome sequence tdk is referred to as yfiG and tmk is referred to as yeaB. Abbreviations: PRPP, 5-phosphoribosyl 1-pyrophosphate; dR1P, deoxyribose 1-phosphate. Note that MBP71 ΔthyA can utilize thymidine, but not thymine, for synthesis of dTTP.
Synthesis of RNA and protein is not affected directly in a thyA mutant (Fig. 1), and therefore genes may still be expressed, which increases the metabolic capacity of the cells. Even though the cells can synthesize new enzymes, they should have a lower rate of macromolecule synthesis (8) and consume less ATP than exponentially growing cells. This leads to a lower lactic acid flux.
One method of increasing ATP consumption is to introduce nonspecific ATPase activity. The F1 unit of the membrane-bound H+-ATPase catalyzes interconversion of free energy between ATP and the membrane proton gradient. The atpAGD genes, encoding the α, γ, and β subunits, respectively, specifically express part of the F1 unit of the H+-ATPase enzyme complex, which has been shown to have strong ATPase activity in vitro (7). The ATPase hydrolyzes ATP and causes glycolysis to occur at a higher rate under conditions under which a relatively high ATP/ADP ratio may limit the glycolytic flux. We have previously shown that nonspecific ATPase activity may be introduced into Escherichia coli (19) and L. lactis (18) by overexpressing the atpAGD genes.
In E. coli growing exponentially under aerobic conditions, the glycolytic flux was increased by 70% by incorporating ATPase activity (19). This indicates that there is substantial overcapacity for catabolism. Conversely, in L. lactis growing exponentially under anaerobic conditions, ATPase activity increased the lactic acid flux only marginally (18). The fact that the glycolytic flux can be increased for E. coli but not for L. lactis may be because aerobically growing E. coli generates ATP through oxidative phosphorylation, and therefore a substantially lower glycolytic flux is required.
In this study, we investigated the importance of synthesis of new enzymes for the rate of acidification of nonreplicating L. lactis. We also investigated how incorporating nonspecific ATPase activity into nonreplicating cells, which have a relatively low ATP demand, may be used to increase the lactic acid flux.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
L. lactis subsp. lactis MBP71 ΔthyA was constructed from CHCC373 (Chr. Hansen Culture Collection, Hørsholm, Denmark) as described previously (24). Construction of a library of pAK80 (15) derivatives with random constitutive promoters cloned in front of lacLM and atpAGD from L. lactis subsp. cremoris MG1363 (12) subsequently cloned in between the promoters and lacLM was described previously (18) (see Fig. 3A). Transformation of this library into MBP71 was carried out as described previously (14).
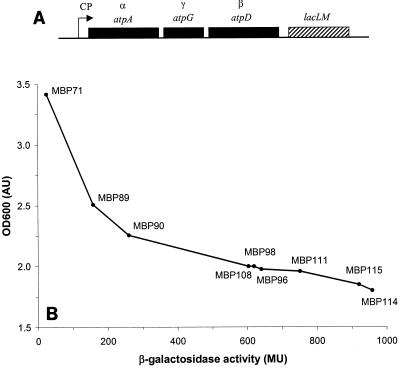
FIG. 3.
Genetic organization of plasmids expressing atpAGD and stationary yield as a function of β-galactosidase activity for MBP71 derivatives with these plasmids. (A) Genetic organization of the plasmids expressing atpAGD. CP, synthetic constitutive promoters. The lacLM gene encodes β-galactosidase. (B) Stationary yields as a function of β-galactosidase activity for MBP71 derivatives with the plasmids described above. Strains were grown overnight to the stationary phase in LM17 containing thymidine and erythromycin (erythromycin was not added for MBP71). The OD600 was measured, and the cultures were inoculated into fresh medium to determine the β-galactosidase activity. For each data point the strain designation is shown.
Cultures of L. lactis were routinely grown at 30°C in M17 broth (25) with 0.5% lactose; this medium is referred to below as LM17. LM17 does not support propagation of MBP71 ΔthyA and its derivatives (24). Hence, 20 mg of thymidine (ICN Biomedicals Inc., Aurora, Ohio) per liter was added for propagation. For strains with pAK80 derivatives the medium was supplemented with 5 mg of erythromycin (Sigma-Aldrich Denmark A/S, Vallensbk Strand, Denmark) per liter. When present, the membrane proton gradient uncoupler monensin (Sigma-Aldrich) was added to a final concentration of 3 μM. For acidification experiments in milk, 9.5% (wt/vol) reconstituted skim milk (RSM) (Arla Food Ingredients, Viby, Denmark) boiled for 30 min was used.
Determination of cell dry weight.
Type HAWP membrane filters with a diameter of 47 mm and a pore size of 0.45 μm (Millipore Corporation, Bedford, Mass.) were dried initially in a microwave oven set at 15% of the maximum power for 10 min and then were immediately weighed. Between 10 and 60 ml of a culture, depending on the cell density, was added directly to a wetted filter, and the medium was removed with a vacuum. The cells were then washed twice with 10 ml of water, and the filter was dried in the microwave oven as described above and was immediately reweighed.
β-Galactosidase assay for determination of promoter strength.
A 950-μl portion of a cell suspension (optical density at 600 nm [OD600], 0.3 to 0.7 absorbing units [AU]), harvested from LM17 containing 5 μg of erythromycin per ml, was added to 50 μl of a solution containing 2 mg of chloramphenicol (Merck, Darmstadt, Germany) per ml. The β-galactosidase activity assay was performed as described by Miller (22).
Fermentation in LM17 and RSM.
Stationary-phase cultures of MBP71 grown in 100 ml of LM17 were washed with an isotonic solution to remove residual thymidine. The cells were then resuspended in fresh isotonic solution so they were concentrated about fivefold compared to the stationary-phase culture. A stationary-phase culture of MBP71 has an OD600 of about 3.4 AU. The level of inoculation was expressed as the percentage of a stationary-phase culture that was used for inoculation. An inoculation level of 100% was equivalent to an OD600 of 3.0 AU. For both M17 and RSM 200-ml cultures were used. For experiments in M17 the cultures either were stirred by gently shaking the flasks before each sample was taken or were stirred with a magnetic stir bar at 300 rpm for 5 s every 1 min. No differences were observed in the results obtained with the two methods.
One-milliliter samples were used to determine OD600. The samples were measured directly when the OD600 was <0.4 AU. When the OD600 was >0.4 AU, 0.5-, 0.2-, or 0.1-ml samples were diluted in 1 ml of medium, so that the OD600 was always <0.4 AU. The pH was monitored automatically or manually over time. For RSM cultures there was no agitation.
Correlation among cell dry weight, OD600, CFU, and inoculum.
The correlation factor for OD600 and cell dry weight could be determined by measuring these two parameters. The correlation factor was determined by dividing the cell dry weight (in grams per liter) by the OD600 (in AU); thus, we determined to how much cell dry weight (in grams) per liter an OD600 of 1.0 AU corresponded.
With regard to CFU, it was determined previously that an OD600 of 1.0 AU corresponds to 6 × 108 CFU/ml (24). Furthermore, for the inoculations used in this study, as described above, a 100% inoculum corresponded to an OD600 of 3.0 AU.
Determination of lactic acid production in M17.
In M17 the pH decreases linearly from 6.8 to 5.8 when HCl is added. The correlation factor is 37.7 mM/pH unit. By using this linear correlation, measured pH values were converted into amounts of lactic acid produced.
Calculation of the lactic acid flux.
Sixth-order polynomial fits were made to the curves for lactic acid production (millimolar). By differentiating the resulting polynomials. the rate of acidification (millimolar per hour) was calculated for a specific time point (hour). When the OD600 of the culture was measured, the corresponding concentration of cells (in grams [dry weight] per liter) was calculated as described above. By dividing the rate of acidification by the cell dry weight the specific rate of lactic acid production or lactic acid flux (in millimoles per gram [dry weight] per hour) was obtained.
Screening for ATPase mutants with higher growth rates.
Derivatives of MBP71 with incorporated ATPase activity were grown to the stationary phase in 10 ml of LM17 containing thymidine and erythromycin. The following day the cultures were diluted, plated on the same medium, and incubated. After overnight incubation the few mutant colonies that were larger than the rest of the colonies were isolated.
RESULTS
Correlation between optical density and cell dry weight for nonreplicating cells.
For exponentially growing cells it is known that a constant correlation factor between the optical density reading of a spectrophotometer (in AU) and the cell dry weight can be obtained, which is useful for determining metabolic fluxes. It is, however, not obvious that a similar correlation exists for nonreplicating cells as they increase greatly in size but not in number (24), with a concomitant decrease in the surface-to-volume ratio. When MBP71 was grown in LM17, which does not support propagation of this mutant (24), the optical density increased linearly to 3.3 times the initial value (Fig. 2A).
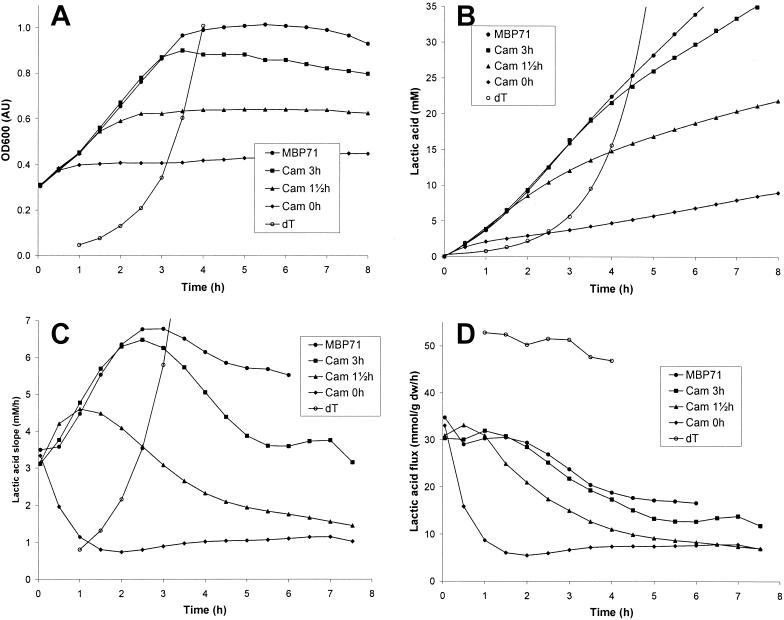
FIG. 2.
Growth, lactic acid production, slopes, and fluxes for MBP71. Stationary-phase cultures of MBP71 were washed and inoculated at an OD600 of 0.3 AU into four 200-ml LM17 cultures at 30°C. For three of the cultures protein synthesis was blocked at 0, 1.5, and 3 h by addition of 100 mg of chloramphenicol (Cam) per liter to the medium. In a fifth culture MBP71 was inoculated at an OD600 of 0.03 AU into LM17 containing thymidine (dT). OD600 and the pH were monitored over time. (A) OD600. When thymidine was added, the specific growth rate was 1.03 h−1. (B) Lactic acid produced. (C) Rate of lactic acid production. (D) Lactic acid flux. Only data points at which the OD600 was less than 1.0 and the pH was more than 5.8 are shown. dw, dry weight.
MBP71 was inoculated at various levels into LM17 with or without thymidine (Table 1). OD600 and cell dry weight were determined at three different times. At time zero (sample 1) the correlation factors were 0.26, 0.26, and 0.30 g (dry weight)/liter/AU. Around the time that the optical density stopped increasing (sample 2) the correlation factors for the three nonreplicating cultures were 0.34, 0.29, and 0.32 g (dry weight)/liter/AU. After 24 h (sample 3) the correlation factors were 0.68, 0.75, and 0.54 g (dry weight)/liter/AU. These relatively high values were caused by cell lysis, as the optical densities for sample 3 were lower than the maximal values found around the time that sample 2 was obtained (Table 1). For the exponentially growing culture, which did not lyse, the correlation factors for samples 2 and 3 were 0.28 and 0.33 g (dry weight)/liter/AU, respectively. The average value for the eight nonlysed samples was 0.30 g (dry weight)/liter/AU, and it is clear that this value may be used both for nonreplicating and exponentially growing cultures.
TABLE 1.
Correlation between OD600 and cell dry weighta
| Inoculum (%) | Harvest time (h)
|
OD600 (AU)
|
Cell dry wt (g/liter)
|
Correlation factor (g[dry wt]/liter/AU)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | |
| 5 | 0.00 | 6.00 | 23.75 | 0.131 | 0.446 | 0.132 | 0.034 | 0.150 | 0.090 | 0.26 | 0.34 | (0.68) |
| 15 | 0.00 | 4.00 | 24.00 | 0.372 | 1.028 | 0.472 | 0.095 | 0.302 | 0.353 | 0.26 | 0.29 | (0.75) |
| 25 | 0.00 | 3.50 | 24.75 | 0.630 | 1.420 | 1.143 | 0.460 | 0.620 | 0.32 | (0.54) | ||
| 5(+dT)b | 0.00 | 5.83 | 25.00 | 0.125 | 3.375 | 3.087 | 0.038 | 0.960 | 1.033 | 0.30 | 0.28 | 0.33 |
The OD600 and cell dry weight were determined at three time points (samples 1, 2, and 3) for both exponentially growing cultures (with thymidine) and nonreplicating cultures of MBP71 in LM17. The correlation factor was determined by dividing the cell dry weight by the OD600. The values in parentheses indicate that cultures had lysed.
dT, thymidine.
Nonreplicating MBP71 ΔthyA synthesizes new enzymes, and the rate of acidification increases.
It has been shown previously that nonreplicating MBP71 is able to acidify milk down to pH 5.2 in 6 h at 37°C when it is inoculated at a level of 17% (24). Although the cells are nonreplicating, they should still be able to synthesize new enzymes, since synthesis of RNA and protein is not directly affected in a thyA mutant (8) (Fig. 1). Hence, we investigated the importance of protein synthesis for biomass formation and acidification.
MBP71 was inoculated into LM17 at an initial OD600 of 0.3 AU in four parallel cultures. For three of the cultures protein synthesis was blocked by addition of chloramphenicol at 0, 1.5, and 3 h. As Fig. 2A shows, the OD600 of the MBP71 culture containing no chloramphenicol increased to 1.0 AU over the first 3.5 h and leveled off after a 3.3-fold increase. After 1 h the increase was linear until 3.5 h. For the three other cultures the addition of chloramphenicol ended the increase in biomass after a transition period of 0.5 to 1.0 h (Fig. 2A). The maximum OD600 for the three cultures to which chloramphenicol was added at 0, 1.5, and 3 h were 0.4, 0.6, and 0.9 AU, respectively.
Figure 2B shows how acidification was affected by the addition of chloramphenicol. The slope of the curve for lactic acid production by the MBP71 culture without chloramphenicol increased for the first 2 to 3 h, and then it appeared to decrease slightly (Fig. 2B). For the three other cultures, addition of chloramphenicol greatly reduced acidification. Figure 2C shows the slopes of the acidification curves (i.e., the rates of acidification over time). For the MBP71 culture without chloramphenicol, the rate increased from about 3 mM/h to almost 7 mM/h over the first 3 h. This increase was concomitant with the increase in OD600. Interestingly, the culture continued acidifying at a rate greater than 5.5 mM/h for 3 h, after the biomass concentration had stopped increasing (Fig. 2A and C).
The lactic acid flux of nonreplicating MBP71 was 60% of the lactic acid flux of exponentially growing MBP71.
Since nonreplicating cells are in a physiological state in which DNA replication is blocked, we investigated how metabolically active such cells were compared to exponentially growing cells. The acidification by MBP71 in LM17 was compared to the acidification in a culture to which thymidine was added.
The lactic acid flux of exponentially growing MBP71 was about 50 mmol/g (dry weight)/h (Fig. 2D). This value was more or less constant as the cells were in the exponential growth phase. In contrast, the lactic acid flux for the nonreplicating culture of MBP71 was not constant. It was around 30 mmol/g (dry weight)/h for the first 1.5 h but then decreased gradually, and after 6 h it had decreased to 17 mmol/g (dry weight)/h. Therefore, the maximum lactic acid flux of nonreplicating MBP71 was 30 mmol/g (dry weight)/h or 60% of the value for exponentially growing MBP71.
Incorporation of ATPase activity decreased the biomass yield of exponentially growing cells.
The fact that nonreplicating cells have a lower lactic acid flux than exponentially growing cells indicates that there is potential for increasing the flux.
Previously, a plasmid library was constructed in which the atpAGD genes of L. lactis MG1363 were cloned between a series of synthetic constitutive promoters and the promoterless lacLM gene of pAK80 for determination of the promoter strength (18) (Fig. 3A). This library was transformed into MBP71, and various clones were isolated and designated MBP90 through MBP115. Strain MBP89 was constructed separately by using a plasmid with a specific constitutive promoter, CP34 (16), but with the same genetic organization.
When nonspecific ATPase activity is incorporated, the biomass yield should be affected, since some of the ATP destined for biomass formation is wasted (18). Indeed, when the derivatives were grown in LM17 containing thymidine and erythromycin, the stationary-phase OD600 was reduced from 3.4 AU for MBP71 (no erythromycin added) to 1.8 AU for MBP114, which had the highest ATPase activity (Fig. 3B). The reduction in the biomass yield correlated well with the measured β-galactosidase activity. The stationary-phase yield was found to be most sensitive to the ATPase activity at the lower values; e.g., for MBP89, which had just 150 Miller units (MU) of activity, the stationary-phase yield was only 2.5 AU (Fig. 3B). The final pH of all the cultures was 4.8, which shows that the amount of lactic acid produced was independent of the ATPase activity.
ATPase activity in nonreplicating MBP71 restores the lactic acid flux to the level of exponentially growing MBP71.
The following three strains shown in Fig. 3B were selected for further investigation (the level of β-galactosidase or ATPase activity is indicated in parentheses): MBP89 (150 Miller units; low activity), MBP90 (250 Miller units; medium activity), and MBP98 (600 Miller units; high activity). These three strains and MBP71 were inoculated into LM17, and the OD600 and pH were monitored over time. As a control, MBP71 was inoculated into LM17 containing thymidine. Apparently, it would have been more appropriate to use MBP71(pAK80) in this experiment. However, pAK80 reduces the specific growth rate of MBP71 by almost 10% in M17-based medium, whereas it does not influence the nonreplicating growth of MBP71.
Figure 4A and B show the growth curves and amounts of lactic acid produced. It is clear that the derivatives with ATPase activity acidify faster than MBP71. However, it is more relevant to look at the lactic acid fluxes calculated over time, and these are shown in Fig. 4C.
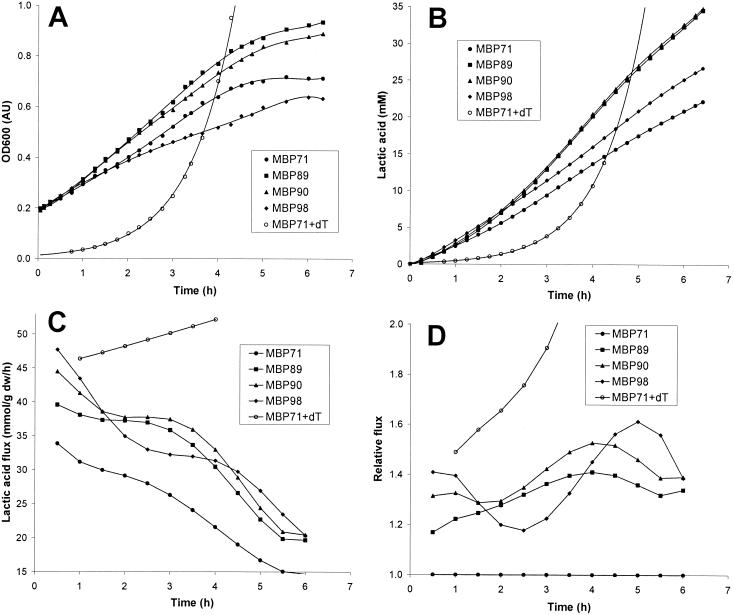
FIG. 4.
Growth, lactic acid production, fluxes, and relative fluxes for MBP71 and derivatives with ATPase activity. Stationary-phase cultures of MBP71, MBP89 (low activity), MBP90 (medium activity), and MBP98 (high activity) were inoculated at an OD600 of 0.3 AU into 200 ml of fresh LM17 (erythromycin was not added, since it is not required for plasmid stability in the nonreplicating cells). MBP71 was also inoculated at an OD600 of 0.03 AU into LM17 containing thymidine (dT). OD600 and pH were monitored over time. (A) OD600. When thymidine was added to the MBP71 culture, the specific growth rate was 1.03 h−1. The curves connecting the points are sixth-order polynomial fits. (B) Lactic acid produced. (C) Lactic acid flux. (D) Fluxes from panel C relative to the MBP71 culture. Only data points at which the OD600 was less than 1.0 and the pH was more than 5.8 are shown.
The data show that the three strains with ATPase activity, MBP89 (low activity), MBP90 (medium activity), and MBP98 (high activity), all have higher fluxes than the mother strain, MBP71. For MBP98 the glycolytic flux was almost 50 mmol/g (dry weight)/h at the beginning of fermentation, and the ATPase thus restored the flux to the level of exponentially growing MBP71. Figure 4D shows the fluxes relative to the flux of nonreplicating MBP71, and it is clear that ATPase activity increased the flux by 20 to 60%, with variations over time and depending on the ATPase activity.
If the cells in a stationary-phase culture with ATPase activity were smaller than the cells in a culture without ATPase activity, one would inoculate a preparation with more cells per optical density unit and thus more chromosomes. This would lead to faster acidification, but the faster acidification would not be caused directly by the ATPase activity. However, CFU measurements did not indicate that this was the case (data not shown), although if cells with ATPase activity have a greater tendency to grow in chains, the result would be obscured. To verify that it is indeed possible to increase the lactic acid flux for nonreplicating MBP71, we performed an experiment with the membrane proton gradient uncoupler monensin, which increases the glycolytic flux for exponentially growing Streptococcus bovis (3) but not for exponentially growing L. lactis (10). For nonreplicating MBP71, monensin at a concentration of 3 μM resulted in growth, flux, and relative flux curves similar to the curves for MBP98 shown in Fig. 4A, C, and D, respectively, when the data were compared to the data for a culture to which monensin was not added (data not shown).
If the ATPase activity is high, catabolism apparently becomes limiting.
Figure 4A shows that the rate of biomass formation for MBP71 was slightly lower than that for MBP89 (low activity) or MBP90 (medium activity), which is not what we initially expected, since there is an engineered drain of ATP in MBP89 and MBP90. In other experiments the rate of biomass formation for MBP71 was slightly higher than that for MBP89 or MBP90, yet in some experiments the rate of biomass formation was the same (data not shown). The differences are believed to be due to slight variations in the ages of the stationary cultures from experiment to experiment. However, the slight variations from experiment to experiment were not found to greatly affect the relative fluxes shown in Fig. 4D (data not shown).
For MBP89 and MBP90, the ATPase activity was in general too low to retard biomass formation, and biomass formation proceeded as fast as it did for MBP71. At the same time, more lactic acid was produced due to an increase in the lactic acid flux. Hence, under these conditions the lactic acid flux was controlled by the demand for ATP. For MBP98 (high activity) the rate of biomass formation was lower than the rates for the three other cultures (Fig. 4A) in all experiments carried out (data not shown). This shows that the ATPase activity was so high that biomass formation was retarded due to a shortage of ATP; i.e., catabolism became limiting.
ATPase activity also increased acidification by nonreplicating MBP71 in milk.
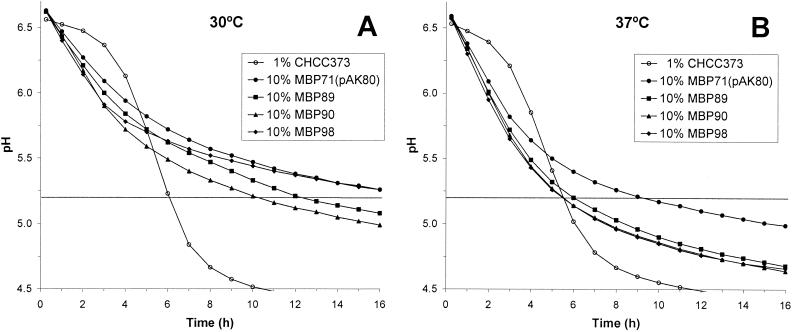
Since faster acidification by the nonreplicating and phage-resistant cells would be beneficial from an application perspective, it was relevant to study the effect of ATPase activity in nonreplicating MBP71 in milk. MBP71(pAK80), MBP89, MBP90, and MBP98 were inoculated into RSM, and the pH was monitored over time (Fig. 5). Cultures were grown at 30 and 37°C. For comparison, the normally growing mother strain of MBP71, CHCC373, was also incubated at both temperatures by using a 1% inoculum, which is around the amount normally used in a dairy fermentation (24).
FIG. 5.
Acidification curves in milk at 30 and 37°C for MBP71 and derivatives with ATPase activity. Stationary-phase cultures of MBP71, MBP89 (low activity), MBP90 (medium activity), and MBP98 (high activity) were washed and inoculated (10%) into 200 ml of cold RSM. MBP71 was also inoculated (1%) into RSM containing thymidine. Parallel cultures were incubated at 30 and 37°C, and the pH was monitored automatically over time.
The target pH, pH 5.2, should be reached in 6 h (23), and this is what was found for CHCC373 at both temperatures. For MBP71 at 30°C, pH 5.2 was reached in 18.5 h (data not shown). For MBP89 (low activity) and MBP90 (medium activity) acidification was substantially faster, and pH 5.2 was reached after 12.5 and 10.5 h, respectively (Fig. 5A). MBP98 (high activity) had the fastest acidification for the first 2 to 3 h, but then acidification slowed down, presumably due to a lack of biomass formation, as shown in Fig. 4A.
At 37°C, acidification was generally faster, and for MBP71 pH 5.2 was reached in 8.5 h. However, for the three strains with ATPase activity this target pH was reached in around 5.5 h. Thus, at both 30 and 37°C, the acidification time was approximately 40% less when ATPase activity was present. A somewhat surprising result obtained at 37°C was that acidification by MBP98 was comparable to acidification by MBP89 and MBP90 during the entire fermentation, unlike the results obtained at 30°C. This could have been due to altered promoter strengths at the elevated temperature.
Cells grown to the stationary phase are normally used in dairies, so stationary-phase cells were also studied here. As shown previously for MBP71 (24), the exact ages of overnight stationary-phase cultures of derivatives with ATPase activity had only a marginal effect on acidification (data not shown).
Changing the sugar source for MBP98 did not increase the lactic acid flux.
It is shown above that the growth of MBP98 (high activity) was hampered by the ATPase activity, in contrast to the growth of MBP89 (low activity) and MBP90 (medium activity). To investigate whether sugar uptake is likely to be limiting for MBP98, the lactic acid fluxes were determined for eight different sugars (Table 2). For comparison MBP71 and MBP89 (low activity) were grown on lactose, and the flux for MBP71 at 3 h was defined as 100%. For MBP89, the flux was found to be 146%, which is similar to the value shown in Fig. 4D (about 137%). The fluxes for MBP98 grown on lactose, fructose, glucose, and mannose were 131 to 169% (Table 2). The increase in biomass was substantially slower for mannose and even slower for fructose than it was for lactose and glucose (data not shown).
TABLE 2.
Nonreplicating lactic acid fluxes for MBP98 and specific growth rates for MBP71 on various sugar sourcesa
| Sugar | Relative lactic acid flux for nonreplicating growth (%)
|
Specific growth rate (h−1) for exponential growth MBP71 | ||
|---|---|---|---|---|
| MBP71 | MBP89 (low activity) | MBP98 (high activity) | ||
| Lactose | 100 | (146) | 169 | 1.01 |
| Fructose | 152 | 0.97 | ||
| Glucose | 141 | 1.09 | ||
| Mannose | 131 | 1.03 | ||
| Galactose | 105 | 0.85 | ||
| Maltose | 85 | 0.86 | ||
| Trehalose | 78 | 0.81 | ||
| Cellobiose | 66 | 0.84 | ||
Stationary-phase cultures of MBP98 in M17 containing thymidine and erythromycin supplemented with 1% sugar were washed and inoculated into M17 supplemented with 1% sugar. The OD600 and pH were determined every hour. From 2.5 to 5.5 h the rates of acid production were practically constant, and they were divided by the cell density at 3 h to obtain the lactic acid flux. The fluxes were expressed relative to the value for an MBP71 culture grown in LM17. The value for MBP89 grown in LM17 was obtained in another experiment. The specific growth rates for MBP71 on the various sugars are indicated. MBP71 was grown in M17 containing thymidine supplemented with 1% sugar. The rates were determined from exponential fits of four or five values (R2 > 0.998). The value in parentheses is from another experiment.
The relative nonreplicating fluxes for MBP98 on the sugars galactose, maltose, trehalose, and cellobiose were 66 to 105% of the fluxes for MBP71. This division into two groups based on the glycolytic flux in MBP98 matched the division based on the specific growth rates of MBP71 on the various sugars, which were found to be 0.97 to 1.09 and 0.81 to 0.86 h−1 (Table 2).
Mutations that reduce the ATPase activity are likely to occur.
Exponentially growing strains of L. lactis with ATPase activity have very reduced growth rates (18). When the organisms were plated on agar plates, the sizes of the colonies decreased as the ATPase activity increased. However, substantially larger colonies were observed among the small colonies, and these colonies could be mutants in which the catabolic limitation had been overcome. Mutants of MBP89 (low activity), MBP90 (medium activity), MBP98 (high activity), MBP111 (very high activity), and MBP115 (very high activity) were screened. There were various numbers of larger colonies on the MBP111 plates (three of three plates) and MBP115 plates (four of five plates) but not on the plates containing the other strains. The stationary-phase OD600 for 10 of these mutants were found to vary between 2.5 and 3.1 AU. These values were higher than the values observed for the MBP111 and MBP115 cultures (1.8 to 2.0 AU) (Fig. 3B) and indicated that some ATPase activity had been lost. However, the fact that an OD600 was lower than the OD600 for MBP71 (3.4 AU) indicated that some ATPase activity was still present.
For 4 of the 10 mutants the exponential lactic acid flux was determined relative to that of MBP71. The mutants had growth rates which were higher than those of the mother strains (i.e., MBP111 and MBP115) but lower than that of MBP71. The lactic acid fluxes were within ±10% of the MBP71 value (data not shown).
In another experiment the lactic acid fluxes of the four mutants were determined in the nonreplicating state. It was found that the fluxes of three of the mutants were close to the flux of MBP89 (data not shown). The stationary-phase OD600 of these mutants were around 2.8 AU, a level which fits well with the OD600 of MBP89 (Fig. 3B), and it appeared that the ATPase activity in these mutants was reduced.
DISCUSSION
We found that incorporation of ATPase activity into nonreplicating MBP71 ΔthyA increased the lactic acid flux from 60% of the flux of exponentially growing cells to 100%, but not above this point. Furthermore, we found that in milk incorporation of ATPase activity reduced the time necessary to reach pH 5.2 by 40%. Thus, the amount of inoculum required to reach pH 5.2 in 6 h (23) was reduced from 17% for nonreplicating MBP71 (24) to 10% when ATPase activity was incorporated. However, this value is still 10-fold higher than the amount normally employed in dairy fermentations (24), although of course the exact amount depends on the desired final pH of the dairy product and the fermentation time. Since the final cell number, the size of the cells, and the physiology of the cells are different in a nonreplicating culture than in a normally growing culture, the organoleptic properties of the product could be affected, although maturation of certain cheeses may be improved due to increased lysis (24).
The chromosomes are limiting for growth of nonreplicating MBP71.
The optical density increased linearly for nonreplicating cells, showing that one or more components limit growth. The limiting factors could be the RNA polymerases or the ribosomes. However, these components may be synthesized in the nonreplicating state, and it is therefore more likely that it is the chromosomes, or the actual gene dosage, which controls biomass formation. Therefore, the rate of acidification and the maximum optical density are proportional to the amount of inoculum used.
The optical densities of nonreplicating strains with ATPase activity reached higher maximum values than the optical density of MBP71. A lower energy state could alter the cell wall composition, but this finding could also be due the higher glycolytic flux, which could alter the pools of the glycolytic intermediates and make some of them more or less readily available for cell wall synthesis.
Macromolecule synthesis is important for acidification by nonreplicating MBP71 cultures.
For nonreplicating cultures of MBP71, the lactic acid flux decreased after addition of chloramphenicol, showing that protein synthesis is important for acidification. The most likely explanation for this is that the actual energy requirement (i.e., the ATP required by enzymes synthesis) decreases. In the culture in which protein synthesis could in theory continue throughout the experiment, the optical density stopped increasing at 1.0 AU after a 3.3-fold increase. Either this was due to termination of macromolecule synthesis or it was because biomass formation equaled cell lysis.
Previously, it has been shown that in exponentially growing L. lactis blocked in protein synthesis acidification continues at a constant rate, while the optical density remains unchanged (4). Therefore, acidification seems to be at least partially uncoupled from growth, indicating that a relatively large amount of energy is utilized for maintenance, such as sustaining the proton gradient.
It was found that nonreplicating MBP71 had a lactic acid flux which was 60% that of exponentially growing MBP71. This seems to be in agreement with results obtained with a thyA mutant of E. coli starved for thymine, in which the levels of RNA synthesis and protein synthesis were 50 and 40%, respectively, of the levels of RNA synthesis and protein synthesis of exponentially growing cells (7). In all, the ATP demand in the nonreplicating cells is lower. The reason for the lower demand could be blockage of DNA replication and a reduced need for DNA replication and cell division enzymes. However, the lower demand may be also caused by a regulatory mechanism (i.e., a slowdown of metabolism if the cells sense that they are not able to divide).
One or more catabolic enzymes becomes limiting in nonreplicating cells when the ATPase activity is high.
ATPase activity increased the lactic acid flux of nonreplicating MBP71 to the level of exponentially growing MBP71, but not above this point. These results establish that ATPase activity is a functional means of increasing the glycolytic flux for cells with reduced flux (i.e., nonreplicating or slowly growing cells).
However, when the ATPase activity was relatively high, biomass formation was hampered, presumably due to a shortage of ATP. This indicates that catabolism cannot supply sufficient ATP to sustain biomass formation at the maximal rate. Therefore, one or more enzymes of catabolism (i.e., sugar uptake or glycolysis) may be limiting, although the limitation could also be regulatory or related to a disturbance in the cofactor balance.
It may be glycolysis which becomes limiting when high ATPase activity is introduced.
We found that the lactic acid fluxes for nonreplicating MBP98 (high activity) grown on the sugars lactose, fructose, glucose, and mannose were 131 to 169% of the flux for nonreplicating MBP71. These four sugars are taken up by PTSLac, PTSFru, and PTSMan (both mannose and glucose) (2, 20, 21, 26), and at least lactose and glucose may also be taken up by a permease (6, 21). Since it is unlikely that the capacity of three different uptake systems would be limited to almost the same extent through evolution, one could argue that it is probably not the sugar uptake which is limiting. One of the systems could have substantial overcapacity, such as that found for lactate dehydrogenase in L. lactis subsp. cremoris MG1363 by lowering expression of ldh (1) or through enzyme assays for the glycolytic enzymes of L. lactis subsp. lactis IL1403 (10). Furthermore, the data indicate that each of the sugar uptake systems should be close to limiting in exponentially growing cells. Altogether, these results indicate that for nonreplicating derivatives of MBP71 with high ATPase activity sugar uptake does not exert the majority of the control on the glycolytic flux. Instead, the control may lie with glycolysis itself, and thus it would be interesting to determine the pools of the glycolytic intermediates, since a buildup of a substrate could indicate a relatively high level of control by the enzyme that utilizes the substrate (10). However, it should be noted that the control may also lie with the two nonspecific sugar cytoplasmic phospho carriers of the phosphotransferase system (i.e., HPr and EI encoded by ptsH and ptsI, respectively) (21). Specifically, the fact that HPr alone constitutes 5% of total visualized protein on a two-dimensional gel should be considered (27). Also, it is noteworthy that for lactose only one-half of the enzymatic capacity would be required compared to the capacity required for fructose, glucose, and mannose, since two hexose moieties are taken up per phospho transfer.
For nonreplicating MBP98 it was found that the increase in biomass was substantially slower for mannose and even slower for fructose than it was for lactose and glucose. At the same time, the lactic acid fluxes were similar, and this indicates that anabolism is limited but catabolism is not. The anabolic limitation may arise because mannose and fructose feed into glycolysis at the level of fructose 6-phosphate and fructose 1,6-bisphosphate, respectively, instead of at the level of glucose at the top of glycolysis for glucose and the glucose moiety of lactose. Therefore, glucose 6-phosphate utilized for synthesis of nucleotides via ribose 5-phosphate may be less readily available.
In this study we assumed that the cultures remained homolactic; obviously, if there were even a partial switch to mixed acid fermentation, the acidification results would be distorted, since some formate, acetate, and ethanol would be produced instead of lactic acid (11). However, it was shown previously that nonreplicating MBP71 remained homolactic (24) and that exponentially growing L. lactis MG1363 with ATPase activity was homolactic (18). Also, sugars supporting relatively high growth rates generally result in homolactic fermentation (5), and therefore at least the cultures grown on lactose, fructose, glucose, and mannose should be homolactic.
Mutations that reduce the ATPase activity are more likely to occur than mutations that increase the lactic acid flux.
We showed how mutants without catabolic limitations (i.e., mutants with a mutation in a repressor or in the promoter of a gene encoding an enzyme with high flux control over catabolism) could not easily be isolated from strains with high ATPase activity. Instead, the ATPase activity was reduced, which led to increased growth rates but not increased fluxes. One obvious explanation for this result is that a multitude of mutations in the 4.0-kb atpAGD genes decrease the ATPase activity. This could result in inactivation of atpA encoding the α subunit, and therefore only the γ and β subunits would be present; although the β subunit has been shown to possess ATPase activity (13), it is reasonable to assume that the amount of this enzyme would be reduced compared to the amount of the complete F1 unit. Similarly, the αβ complex without the γ subunit has been shown to exhibit reduced ATPase activity (9, 17). These highly likely mutations are in contrast to specific up-mutations in the promoter of the glycolytic gene encoding the enzyme with the greatest control over the flux.
We are currently working with a screening strategy to obtain faster acidifiers in which this and other potential complications should be eliminated or greatly reduced. Although screening for mutants that acidify faster will be carried out for dividing cells, it is likely that such a mutant would also acidify faster in the nonreplicating state.
Acknowledgments
This work was supported by a grant from The Danish Academy of Technical Sciences (ATV).
We thank Ida Jørring and Regina Schürmann for their help with various tasks and Fergal Patrick Rattray for reading the manuscript.
REFERENCES
- 1.Andersen, H. W., M. B. Pedersen, K. Hammer, and P. R. Jensen. 2001. Lactate dehydrogenase has no control on lactate production but has strong negative control on formate production in Lactococcus lactis. Eur. J. Biochem. 268:6379-6389. [DOI] [PubMed] [Google Scholar]
- 2.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond, D. R., B. M. Tsai, and J. B. Russell. 1998. The diversion of lactose carbon through the tagatose pathway reduces the intracellular fructose 1,6-bisphosphate and growth rate of Streptococcus bovis. Appl. Microbiol. Biotechnol. 49:600-605. [DOI] [PubMed] [Google Scholar]
- 4.Breheny, S., M. Kanasaki, A. J. Hillier, and G. R. Jago. 1975. Effect of temperature on the growth and acid production of lactic acid bacteria, the uncoupling of acid production from growth. Aust. J. Dairy Technol. 30:145-148. [Google Scholar]
- 5.Cocaign-Bousquet, M., C. Garrigues, P. Loubiere, and N. D. Lindley. 1996. Physiology of pyruvate metabolism in Lactococcus lactis. Antonie Leeuwenhoek 70:253-267. [DOI] [PubMed] [Google Scholar]
- 6.Crow, V. L., G. P. Davey, L. E. Pearce, and T. D. Thomas. 1983. Plasmid linkage of the d-tagatose-6 phosphate pathway in Streptococcus lactis: effect on lactose and galactose metabolism. J. Bacteriol. 153:28-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deutch, C. E., and C. Pauling. 1971. Survival and macromolecular synthesis during incubation of Escherichia coli in limiting thymine. J. Bacteriol. 106:197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du, Z., and Z. Gromet-Elhanan. 1999. Refolding of recombinant α and β subunits of the Rhodospirillum rubrum F0F1 ATP synthase into functional monomers that reconstitute an active α1β1-dimer. Eur. J. Biochem. 263:430-437. [DOI] [PubMed] [Google Scholar]
- 9.Dunn, S. D., and L. A. Heppel. 1981. Properties and functions of the subunits of the Escherichia coli coupling factor ATPase. Arch. Biochem. Biophys. 210:421-436. [DOI] [PubMed] [Google Scholar]
- 10.Even, S., N. D. Lindley, and M. Cocaign-Bousquet. 2001. Molecular physiology of sugar catabolism in Lactococcus lactis IL1403. J. Bacteriol. 183:3817-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fordyce, A. M., V. L. Crow, and T. D. Thomas. 1984. Regulation of product formation during glucose or lactose limitation in nongrowing cells of Streptococcus lactis. Appl. Environ. Microbiol. 48:332-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris, D. A., J. Boork, and M. Balscheffsky. 1985. Hydrolysis of adenosine 5′ triphosphate by the isolated catalytic subunit of the coupling ATPase from Rhodospirillum rubrum. Biochemistry 24:3876-3883. [Google Scholar]
- 14.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israelsen, H., S. M. Madsen, A. Vrang, E. B. Hansen, and E. Johansen. 1995. Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector, pAK80. Appl. Environ. Microbiol. 61:2540-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen, P. R., and K. Hammer. 1998. The sequence of spacers between the consensus sequence modulates the strength of prokaryotic promoters. Appl. Environ. Microbiol. 64:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kagawa, Y., S. Ohta, and Y. Otawara-Hamamoto. 1989. α3β3 complex of thermophilic ATP synthase. Catalysis without the γ-subunit. FEBS Lett. 22:67-69. [DOI] [PubMed] [Google Scholar]
- 18.Koebmann, B. J., C. Solem, M. B. Pedersen, D. Nilsson, and P. R. Jensen. 2002. Expression of genes encoding F1-ATPase results in uncoupling of glycolysis from biomass production in Lactococcus lactis. Appl. Environ. Microbiol. 68:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koebmann, B. J., H. V. Westerhoff, J. L. Snoep, D. Nilsson, and P. R. Jensen. 2002. The glycolytic flux in Escherichia coli is controlled by the demand for ATP. J. Bacteriol. 184:3909-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberman, E. S., and A. S. Bleiweis. 1984. Transport of glucose and mannose by a common phosphoenolpyruvate-dependent phosphotransferase system in Streptococcus mutans GS5. Infect. Immun. 43:1106-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luesink, E. J., C. M. Beumer, O. P. Kuipers, and W. M. de Vos. 1999. Molecular characterization of the Lactococcus lactis ptsHI operon and analysis of the regulatory role of HPr. J. Bacteriol. 181:764-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Pearce, L. E. 1980. Description of the activity test for cheese starters. Bull. Int. Dairy Found. 129:11. [Google Scholar]
- 24.Pedersen, M. B., P. R. Jensen, T. Janzen, and D. Nilsson. 2002. Bacteriophage resistance of a ΔthyA mutant of Lactococcus lactis blocked in DNA replication. Appl. Environ. Microbiol. 68:3010-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Environ. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson, J., and B. M. Chassy. 1985. Intracellular phosphorylation of glucose analogs via the phosphoenolpyruvate:mannose-phosphotransferase system in Streptococcus lactis. J. Bacteriol. 162:224-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wouters, J. A., H. H. Kamphuis, J. Hugenholtz, O. P. Kuipers, W. M. de Vos, and T. Abee. 2000. Changes in glycolytic activity of Lactococcus lactis induced by low temperature. Appl. Environ. Microbiol. 66:3686-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]