Abstract
The food-borne pathogen Listeria monocytogenes grows actively under high-salt conditions by accumulating compatible solutes such as glycine betaine and carnitine from the medium. We report here that the dominant transport system for glycine betaine uptake, the Gbu porter, may act as a secondary uptake system for carnitine, with a Km of 4 mM for carnitine uptake and measurable uptake at carnitine concentrations as low as 10 μM. This porter has a Km for glycine betaine uptake of about 6 μM. The dedicated carnitine porter, OpuC, has a Km for carnitine uptake of 1 to 3 μM and a Vmax of approximately 15 nmol/min/mg of protein. Mutants lacking either opuC or gbu were used to study the effects of four carnitine analogs on growth and uptake of osmolytes. In strain DP-L1044, which had OpuC and the two glycine betaine porters Gbu and BetL, triethylglycine was most effective in inhibiting growth in the presence of glycine betaine, but trigonelline was best at inhibiting growth in the presence of carnitine. Carnitine uptake through OpuC was inhibited by γ-butyrobetaine. Dimethylglycine inhibited both glycine betaine and carnitine uptake through the Gbu porter. Carnitine uptake through the Gbu porter was inhibited by triethylglycine. Glycine betaine uptake through the BetL porter was strongly inhibited by trigonelline and triethylglycine. These results suggest that it is possible to reduce the growth of L. monocytogenes under osmotically stressful conditions by inhibiting glycine betaine and carnitine uptake but that to do so, multiple uptake systems must be affected.
Listeria monocytogenes is a food-borne pathogen responsible for a variety of infective syndromes in humans, including meningitis, encephalitis, septicemia, and spontaneous abortion (2). Listeriosis can be life threatening in individuals who have an underlying condition leading to the suppression of T-cell-mediated immunity. Fatality rates among susceptible individuals can range as high as 25% (15).
Conditions of osmotic stress and low temperature favor the growth of L. monocytogenes over that of many other bacteria which under less stressful conditions would dominate in a mixed culture. The ability of L. monocytogenes to grow under conditions of high osmolarity and low temperature has been well documented (1, 7). A primary means by which eubacteria adapt to osmotic stress is the accumulation of compatible solutes or osmoprotectants: substances that have comparatively minimal deleterious effects on cell biochemistry at high concentrations (1).
The two primary osmoprotectants which permit L. monocytogenes to survive extreme salt stress are glycine betaine and carnitine. Unlike other bacterial species such as Bacillus subtilis (6), L. monocytogenes cannot synthesize these compounds de novo but must accumulate them from the environment (7). Glycine betaine is taken up via two transport systems: the betaine-Na+ symporter BetL (4) and the ATP-dependent Gbu porter (8). The gbu operon, encoding the ATP-dependent transporter, is homologous to the opuA operon from B. subtilis and the proU operon from Escherichia coli. The Gbu porter is responsible for most of the salt- and cold-activated glycine betaine uptake in L. monocytogenes (8). The BetL porter has been studied extensively in membrane vesicles. This symporter required both Na+ and a proton or sodium gradient for activity and was stimulated by osmotic stress but not chill stress in vesicles (4). In more-recent work on whole cells, we found detectable but very low glycine betaine uptake through the BetL porter in response to chill stress (9).
Carnitine, the second important osmoprotectant, has previously been reported as taken up through a third, ATP-dependent specific transporter (14). The gene for this transporter, opuC, has recently been described as belonging to the ATP binding cassette (ABC) substrate binding protein-dependent transporter superfamily (3).
In this paper, we present evidence for the previously unreported uptake of carnitine via the Gbu porter and compare it with the kinetics of carnitine uptake through the OpuC porter. We also examine the effects of several analogs of glycine betaine and carnitine on the uptake of osmoprotectants through all three transport systems and discuss the potential impact of such transport inhibitors on food safety.
MATERIALS AND METHODS
Bacterial strains and reagents.
Three L. monocytogenes strains were used in this work, all derivatives of the wild-type isolate 10403S (13). Strain DP-L1044 (hly::Tn917-LTV3) is nonpathogenic but is unaltered with respect to the accumulation of osmoprotectants (13). Strain LTG59 (gbu::Tn917-LTV3) lacks the Gbu porter (8).
Strain LTS4A is a carnitine transport-deficient mutant that was isolated from a pool of transposon insertional mutants of L. monocytogenes 10403S containing Tn917-LTV3, using the procedure of Ko and Smith (8). Strain LTS4A exhibited reduced osmotic tolerance and a reduced rate of carnitine transport. The interrupted gene was identified as opuC, which codes for carnitine transport (3), by direct sequencing of the genomic DNA (L. Angelides, L. Hoffman, and G. Smith, unpublished data).
All cultures were maintained on solid brain heart infusion (BHI) medium containing 10 mg of chloramphenicol/ml. Modified Pine's medium (8) was used as a defined medium. This medium contained 0.5% glucose but no choline.
[methyl-14C]glycine betaine was prepared enzymatically by the oxidation of [methyl-14C]choline (NEN Research Products) (10). [14C]carnitine was purchased from NEN Research Products. Choline oxidase, dimethylglycine, glycine betaine, trigonelline, and carnitine were purchased from Sigma. γ-Butyrobetaine was purchased from Aldrich. Triethylglycine was a generous gift from the laboratory of Gary M. Smith, Department of Food Science and Technology, University of California at Davis.
Measurement of glycine betaine and carnitine uptake rates in salt-stressed bacteria.
L. monocytogenes cultures grown overnight without shaking in 5 ml of BHI broth were centrifuged at 5,000 × g for 10 min, resuspended in 5 ml of modified Pine's medium, and used to inoculate (1%) 5 ml of modified Pine's medium containing NaCl or other additions as specified for individual experiments. These cultures were grown overnight without shaking at 30°C to late log phase, as determined by measuring turbidity with a Klett-Summerson colorimeter with a no. 54 filter. Grown cultures were centrifuged and resuspended without additional washing in an equal volume of assay buffer, which contained 22 mM ACES [N-(2-acetamido)-2-aminoethanesulfonic acid] buffer, 27 mM K2HPO4, 1 mM Na2SO4, 5 mM MgSO4, and 5% glucose at pH 7, in addition to NaCl, glycine betaine, carnitine, or analogs as required for individual experiments. Cells were then diluted fivefold with additional assay buffer. After a 30-min incubation at 30°C, uptake of [14C]glycine betaine (0.02 to 0.05 μCi) was determined by using the method of Ko et al. (7). For most experiments, this required a specific activity of around 200 Ci/mmol. The actual specific activity varied slightly and was determined separately for each batch of [14C]glycine betaine stock solution. Carnitine uptake assays were performed identically, except that [14C]carnitine was used instead of [14C]glycine betaine. Uptake rates were normalized to total cellular protein, as determined by the Pierce BCA protein assay (12). The uptake rates reported here are the averages of three to five replicates, and the standard deviation in most cases was about 5%.
For preloading experiments, glycine betaine or carnitine uptake was measured after the bacteria had been exposed to an analog for various periods of time. Cultures diluted fivefold as described above with assay buffer containing 4% NaCl were distributed into seven assay tubes and incubated for 30 min at the assay temperature, and then 5 mM of glycine betaine or carnitine analog was added to each assay tube. Radioactive glycine betaine or carnitine was then added successively to each of the tubes at 8-min intervals, and the uptake rate was measured.
Growth measurements.
Bacteria were grown in BHI broth overnight and then centrifuged and resuspended in modified Pine's medium without salt. The resuspension was used to inoculate (1%) tubes of modified Pine's medium with additions of NaCl, glycine betaine, carnitine, and analogs as described above. Growth was monitored by measuring turbidity as previously described (7). Mean doubling times of at least three separate cultures are reported, and the standard deviation for a treatment within an experiment was generally less than 5%.
RESULTS
Carnitine uptake via the Gbu porter.
Initial experiments were conducted with L. monocytogenes strain DP-L1044, which possesses both glycine betaine porters and the dedicated carnitine OpuC porter. Results from DP-L1044 were compared with those for strain LTS4A, which lacks the OpuC porter, and strain LTG59, which lacks the Gbu porter. Strains DP-L1044 and LTS4A had similar glycine betaine uptake rates of around 130 nmol/min/mg of protein, while strain LTG59, which lacks the Gbu porter, had a reduced glycine betaine uptake rate of around 25 nmol/min/mg of protein.
Strain LTS4A showed significant carnitine uptake when the assay was performed at a carnitine concentration of 100 μM, despite the absence of the OpuC porter (Fig. 1). Furthermore, strain LTG59 showed reduced carnitine uptake compared with strain DP-L1044. These results suggested that the Gbu porter might have a previously unsuspected role in carnitine uptake.
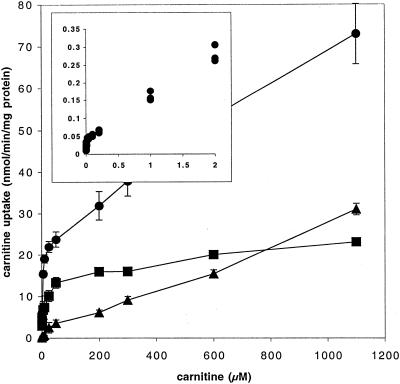
FIG. 1.
Carnitine uptake in three strains of L. monocytogenes. Uptake was measured at a range of carnitine concentrations between 0.5 μM and 1.1 mM in strain DP-L1044 (circles), strain LTG59 (squares), and strain LTS4A (triangles). Error bars reflect the standard deviations. (Inset) Michaelis-Menten plot of carnitine uptake by strain DP-L1044, showing two distinct slopes generated by the two transport systems.
Strains DP-L1044 and LTG59 both showed Kms for carnitine uptake of 1 to 3 μM. This is slightly less than the Kms of 5 to 10 μM reported for a different strain of L. monocytogenes and under different assay conditions by Fraser et al. (3) and Verheul et al. (15). Although the data for DP-L1044 fit Michaelis-Menten kinetics between 0.5 and 50 μM carnitine, the rate increased at very high carnitine concentrations, suggesting that a second, lower affinity transporter was present (Fig. 1, inset). Strain LTG59 had a Vmax of around 15 nmol/min/mg of protein, while that of strain DP-L1044 was higher, at approximately 25 nmol/min/mg of protein. Strain LTS4A showed measurable carnitine uptake at concentrations above 10 μM but did not exhibit saturation over the range of 0.5 to 50 μM used to calculate the Kms of the other two strains. Extending the carnitine concentration range upwards, however, yielded an estimated Km for carnitine uptake via the Gbu porter of 4 mM. We were not able to get a consistent estimate of the Vmax for carnitine uptake via the Gbu porter, but it was clearly much greater than that of the dedicated carnitine porter OpuC.
Promotion of growth in salt-stressed L. monocytogenes by carnitine, glycine betaine, and several analogs.
A primary goal of our work is to discover a means of reducing glycine betaine and carnitine uptake, and thus salt tolerance, in this pathogen. We previously tested a number of glycine betaine and carnitine analogs for their ability to inhibit glycine betaine uptake (9) and carnitine uptake.
The most promising compounds in initial trials were dimethylglycine, γ-butyrobetaine, trigonelline, and triethylglycine. The growths of strains DP-L1044, LTG59, and LTS4A were measured in medium containing a low (100 μM) or high (5 mM) concentration of glycine betaine, carnitine, or an analog. Strains DP-L1044 and LTS4A were grown in medium containing 9% NaCl to show differences between treatments clearly. Strain LTG59 did not grow in 9% NaCl, regardless of the presence or absence of osmoprotectants, and so medium containing only 6% NaCl was used for this strain.
Glycine betaine was the most efficient osmoprotectant studied, even in strain LTG59, which lacks the Gbu porter (Table 1). Carnitine was less efficient at promoting growth under high salt conditions. Carnitine at 100 μM permitted some growth in strain DP-L1044 but not in strain LTS4A, which lacks the OpuC porter. Strain LTS4A grew in medium containing 5 mM carnitine, however. This suggests that the Gbu porter can successfully accumulate carnitine in the absence of the OpuC porter, but only when carnitine is abundant in the growth medium. Strain LTG59, which lacks the Gbu porter, used low concentrations of carnitine almost as efficiently as glycine betaine, with doubling times of 13 and 11 h, respectively.
TABLE 1.
Growth of salt-stressed L. monocytogenes in the presence of glycine betaine, carnitine, and their analogsa
| Added compound | Avg doubling time (h) ± SD for strain:
|
||
|---|---|---|---|
| DP-L1044 | LTG59 | LTS4A | |
| No addition | NG | 44 ± 5 | NG |
| 100 μM GB | 16 ± 0.6 | 11 ± 0.1 | 16 ± 0.2 |
| 100 μM CAR | 94 ± 2 | 13 ± 0.2 | NG |
| 100 μM DMG | NG | 41 ± 1 | NG |
| 100 μM γBB | 99 ± 7 | 13 ± 0.2 | 330 ± 35 |
| 100 μM TRI | NG | 42 ± 1 | NG |
| 100 μM TEG | NG | 47 ± 1 | NG |
| 5 mM CAR | 42 ± 2 | 12 ± 0.6 | 81 ± 3 |
| 5 mM DMG | 18 ± 4 | 22 ± 0.6 | 62 ± 7 |
| 5 mM γBB | 76 ± 9 | 10 ± 0.2 | 64 ± 3 |
| 5 mM TRI | NG | 42 ± 3 | NG |
| 5 mM TEG | NG | 19 ± 0.7 | NG |
Values are the averages of three separate cultures. Strains DP-L1044 and LTS4A were grown in modified Pine's medium containing 9% NaCl in order to show differences clearly; strain LTG59 was grown in the same medium but with only 6% NaCl. Abbreviations: GB, glycine betaine; CAR, carnitine; DMG, dimethylglycine; γBB, γ-butyrobetaine; TRI, trigonelline; TEG, triethylglycine; NG, no growth after 20 days.
Dimethylglycine, γ-butyrobetaine, and triethylglycine were accumulated by L. monocytogenes strains DP-L1044 and LTG59 under conditions of osmotic stress, but trigonelline was not (9). None of the four analogs tested affected the growth of L. monocytogenes at a concentration of 5 mM in the absence of osmotic stress (data not shown).
Dimethylglycine did not affect the growth of any L. monocytogenes strain at a concentration of 100 μM (Table 1). However, at 5 mM, it stimulated growth more effectively than did carnitine in strains DP-L1044 and LTS4A. Carnitine permitted faster growth in strain LTG59, though, suggesting that the Gbu porter may be required for L. monocytogenes to accumulate sufficient dimethylglycine for osmoprotection.
The carnitine analog γ-butyrobetaine was comparable to carnitine at 100 μM in strains DP-L1044 and LTG59, although it permitted only very slow growth in strain LTS4A. This suggests that carnitine and γ-butyrobetaine are transported by similar mechanisms. At a concentration of 5 mM, γ-butyrobetaine was the best osmoprotectant for strain LTG59. This suggests that at high concentrations, this analog can act as an efficient osmoprotectant in the absence of the Gbu porter.
Trigonelline, which was not accumulated by salt-stressed L. monocytogenes, did not permit the growth of strains DP-L1044 and LTS4A in medium containing 9% NaCl in the absence of other osmoprotectants. It also did not affect growth of strain LTG59 in medium containing 6% NaCl.
Triethylglycine, like trigonelline, did not promote growth at any concentration in strains DP-L1044 and LTS4A, although nuclear magnetic resonance data showed that it was accumulated (9). However, at a concentration of 5 mM, this analog permitted strain LTG59 to double its growth rate in medium containing 6% NaCl. This suggests that while triethylglycine can act as an osmoprotectant under these less osmotically stressful conditions, this analog does not provide sufficient protection for the more stressful conditions under which the other two strains were tested.
Effects of four betaine analogs on growth in the presence of glycine betaine.
To test the ability of the analogs to act as growth inhibitors, we examined growth in the presence of glycine betaine and a 20-fold excess of each analog (Table 2). Because our growth conditions stressed the bacteria close to their limit of survival in order to show differences clearly (9% NaCl for strains DP-L1044 and LTS4A and 6% NaCl for strain LTG59), bacterial growth rates proved variable between experiments. For instance, the control rates in the absence of added inhibitor were not the same as those in the experiment whose results are reported in Table 1. Differences between treatments for the same strain in the same experiment were reproducible, even if the absolute growth rates were not. This could be due to differences in the vigor of the initial inoculum, differences between batches of growth medium, or small differences in growth conditions. The variation between the four replicates of each treatment within an experiment was small, and for each strain, all treatments of an individual experiment were grown in the same batch of medium with the same inoculum and concurrently in the same incubator.
TABLE 2.
Effects of glycine betaine analogs on growth of salt-stressed L. monocytogenes in the presence of glycine betainea
| Added compound(s) | Avg doubling time (h) ± SD for strain:
|
||
|---|---|---|---|
| DP-L1044 | LTG59 | LTS4A | |
| GB | 11 ± 0.4 | 6.1 ± 0.1 | 21 ± 0.5 |
| GB + DMG | 12 ± 0.3 | 5.9 ± 0.2 | 19 ± 0.5 |
| GB + γBB | 11 ± 0.4 | 5.1 ± 0.1 | 18 ± 0.5 |
| GB + TRI | 11 ± 0.6 | 6.6 ± 0.2 | 21 ± 0.4 |
| GB + TEG | 13 ± 0.4 | 7.0 ± 0.5 | 18 ± 0.9 |
| GB + TRI + TEG | 14 ± 0.2 | 7.3 ± 0.2 | 19 ± 0.3 |
Values are the averages of three separate cultures. Strains DP-L1044 and LTS4A were grown in modified Pine's medium containing 9% NaCl in order to show differences clearly; strain LTG59 was grown in the same medium but with only 6% NaCl. Abbreviations: GB, glycine betaine; DMG, dimethylglycine; γBB, γ-butyrobetaine; TRI, trigonelline; TEG, triethylglycine. Glycine betaine was added at 100 μM; all other compounds were added at 5 mM.
When glycine betaine was available in the medium at a concentration of 100 μM, the addition of an analog at a concentration of 5 mM had little effect on strain DP-L1044 (Table 2). Of the four, only triethylglycine reduced growth substantially. However, a combination of 5 mM triethylglycine and 5 mM trigonelline did slow growth more than did triethylglycine alone in the presence of 100 μM glycine betaine.
The growth of strain LTG59 was inhibited by both 5 mM trigonelline and 5 mM triethylglycine in the presence of 100 μM glycine betaine (Table 2). In the absence of glycine betaine, trigonelline did not increase growth in this strain, and triethylglycine increased growth only moderately (Table 1). This suggests that in strain LTG59, these two analogs interfere significantly with the protection provided by the superior osmolyte glycine betaine and that this interference is sufficient to decrease growth, despite the osmoprotection provided by one of the analogs, triethylglycine (Table 2). Combining triethylglycine and trigonelline in the culture medium resulted in no greater growth inhibition than that with triethylglycine alone. The other two analogs tested, dimethylglycine and γ-butyrobetaine, did not reduce the growth of strain LTG59.
None of the analogs tested reduced the growth of strain LTS4A in the presence of 100 μM glycine betaine, not even the combination of 5 mM triethylglycine and 5 mM trigonelline, which reduced growth in strain DP-L1044 (Table 2). Strain LTS4A, like strain DP-L1044, possesses both glycine betaine porters.
Effects of four betaine analogs on growth in the presence of carnitine.
Glycine betaine is the superior osmoprotectant in L. monocytogenes, but carnitine accumulation is also important to this bacterium's ability to withstand osmotic stress. The Gbu porter can take up both osmoprotectants, but not with equal efficiency. The OpuC porter displays a much higher affinity for carnitine than does the Gbu porter and could also differ in uptake of analogs. It was therefore of interest to test whether the analogs reduced growth in the presence of carnitine.
All four analogs decreased the growth of strain DP-L1044 in the presence of 100 μM carnitine (Table 3). The degree of inhibition was least for 5 mM γ-butyrobetaine and greatest with 5 mM trigonelline. However, when any two of the analogs were present at a concentration of 5 mM, growth was faster than with 100 μM carnitine alone (Table 3). This suggests that very high concentrations of poor osmoprotectants (in this case, a combined total concentration of 10 mM) can exceed the degree of protection provided by low concentrations of more-efficient osmolytes.
TABLE 3.
Effects of glycine betaine analogs on growth of salt-stressed L. monocytogenes in the presence of carnitinea
| Added compound(s) | Avg doubling time (h) ± SD for strain:
|
||
|---|---|---|---|
| DP-L1044 | LTG59 | LTS4A | |
| CAR | 31 ± 1 | 9.6 ± 0.3 | NG |
| CAR + DMG | 38 ± 3 | 8.4 ± 0.2 | 47 ± 5 |
| CAR + γBB | 34 ± 1 | 8.6 ± 0.2 | 53 ± 2 |
| CAR + TRI | 49 ± 5 | 8.7 ± 0.1 | NG |
| CAR + TEG | 37 ± 1 | 8.5 ± 0.4 | NG |
| CAR + DMG + γBB | 26 ± 1 | 6.8 ± 0.1 | 24 ± 2 |
| CAR + DMG + TRI | 25 ± 1 | 9.0 ± 0.1 | 65 ± 5 |
| CAR + DMG + TEG | 25 ± 1 | 9.8 ± 0.3 | NG |
| CAR + γBB + TRI | 22 ± 1 | 7.7 ± 0.3 | 41 ± 2 |
| CAR + γBB + TEG | 23 ± 1 | 8.0 ± 0.3 | NG |
| CAR + TRI + TEG | 25 ± 1 | 9.0 ± 0.4 | NG |
Values are the averages of three separate experiments. Strains DP-L1044 and LTS4A were grown in modified Pine's medium containing 9% NaCl in order to show differences clearly; strain LTG59 was grown in the same medium but with only 6% NaCl. Abbreviations: CAR, carnitine; DMG, dimethylglycine; γBB, γ-butyrobetaine; TRI, trigonelline; TEG, triethylglycine; NG, no growth observed over 20 days. Carnitine was added at 100 μM; all other compounds were added at 5 mM.
Strain LTG59 accumulates carnitine only through the OpuC porter. When this strain was grown in medium containing 100 μM carnitine and a 5 mM concentration of any analog, the growth rate increased modestly (Table 3). In addition, no combination of analogs reduced the growth of this strain in the presence of 100 μM carnitine (Table 3). This carnitine concentration is well above saturation for the OpuC porter. It is possible that the analogs cannot reduce carnitine uptake through this transporter sufficiently to affect growth under these conditions. If carnitine uptake is unaffected, any additional uptake of analogs through other transport systems might provide additional protection from osmotic stress and increase growth.
The doubling times for strain LTS4A were broadly similar to those seen in the absence of carnitine, as shown in Table 1. Trigonelline did not prevent growth in the presence of either 5 mM dimethylglycine or 5 mM γ-butyrobetaine, while triethylglycine did prevent growth in the presence of these two analogs (Table 3).
Effects of preloading with four betaine analogs on the uptake of glycine betaine and carnitine.
To examine more closely the interactions between the four analogs and the three osmolyte transporters, we studied the ability of each analog to inhibit uptake of carnitine and glycine betaine in the three mutant strains of L. monocytogenes. Initial uptake rates of glycine betaine or carnitine were measured in each strain, after an analog was preloaded for various lengths of time. Thus, the instantaneous effects of adding analogs on the uptake of these osmoprotectants (the initial point in each series) could be determined, as well as any long-term effects of analog accumulation. We felt that this approach gives a more complete picture of the interaction between analogs and osmoprotectants and more accurately reflects the conditions under which osmoprotectant analogs might be used in the food industry. The results are reported in Fig. 2 through 5 as the percent change in initial uptake rate over time for each strain. This allows for direct comparison of the degree of inhibition between strains, even though the strains have different uptake rates for carnitine and glycine betaine.
FIG. 2.
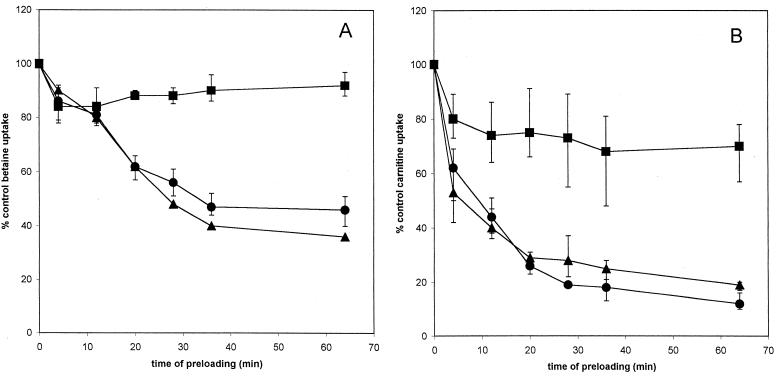
Effects of preloading with dimethylglycine on uptake of glycine betaine and carnitine. Glycine betaine (A) or carnitine (B) uptake in strain DP-L1044 (circles), strain LTG59 (squares), and strain LTS4A (triangles) was measured after preloading for the indicated amount of time with 5 mM dimethylglycine. Error bars reflect the standard deviations.
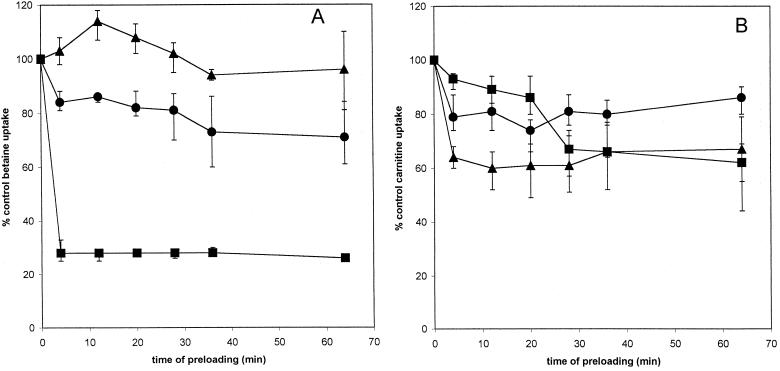
FIG. 5.
Effects of preloading with triethylglycine on uptake of glycine betaine and carnitine. Glycine betaine (A) or carnitine (B) uptake in strain DP-L1044 (circles), strain LTG59 (squares), and strain LTS4A (triangles) was measured after preloading for the indicated amount of time with 5 mM triethylglycine. Error bars reflect the standard deviations.
Dimethylglycine inhibited both carnitine and glycine betaine uptake in strains DP-L1044 and LTS4A (Fig. 2A and B). The time-dependent decrease in initial uptake rates with preloading suggests that this analog act within the cell, reducing carnitine and glycine betaine uptake as it is accumulated.
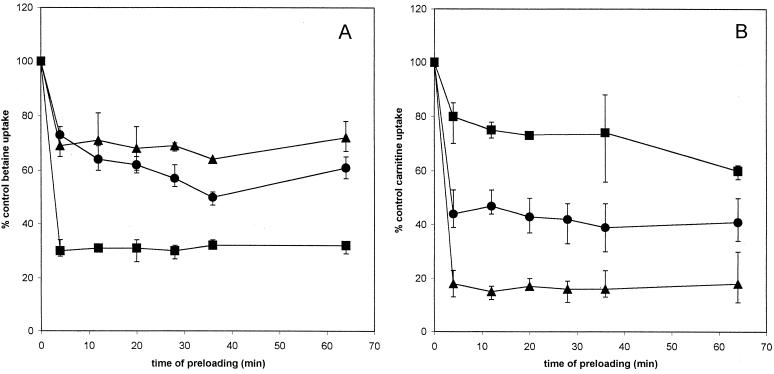
All three of the L. monocytogenes strains showed similar patterns of glycine betaine uptake in the presence of 5 mM γ-butyrobetaine (Fig. 3A). Inhibition was moderate and occurred primarily after 60 min of preloading. In contrast, carnitine uptake showed constant inhibition in the presence of this analog, which was not affected by the time of preloading (Fig. 3B). Strains DP-L1044 and LTG59 showed a 70% decrease in carnitine uptake. Strain LTS4A, however, showed only a 40% decrease in carnitine uptake after preloading with γ-butyrobetaine.
FIG. 3.
Effects of preloading with γ-butyrobetaine on uptake of glycine betaine and carnitine. Glycine betaine (A) or carnitine (B) uptake in strain DP-L1044 (circles), strain LTG59 (squares), and strain LTS4A (triangles) was measured after preloading for the indicated amount of time with 5 mM γ-butyrobetaine. Error bars reflect the standard deviations.
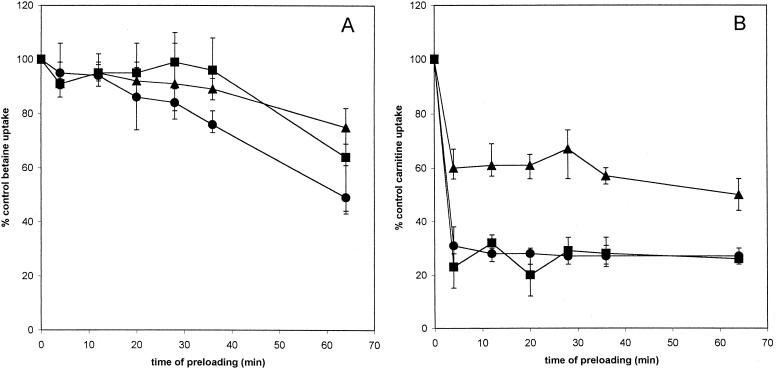
Trigonelline's strongest effect was reduction of glycine betaine uptake in strain LTG59 (Fig. 4A), implying that it primarily affects the BetL porter. Carnitine uptake was inhibited slightly by this analog in all strains, however (Fig. 4B), suggesting an additional effect of this compound on the ability of L. monocytogenes to adapt to osmotic stress.
FIG. 4.
Effects of preloading with trigonelline on uptake of glycine betaine and carnitine. Glycine betaine (A) or carnitine (B) uptake in strain DP-L1044 (circles), strain LTG59 (squares), and strain LTS4A (triangles) was measured after preloading for the indicated amount of time with 5 mM trigonelline. Error bars reflect the standard deviations.
Preloading with triethylglycine strongly decreased glycine betaine uptake in strain LTG59 (Fig. 5A). Glycine betaine uptake was reduced by about 30% in strains DP-L1044 and LTS4A, which possess both uptake systems for this osmolyte. In contrast, preloading with triethylglycine inhibited carnitine uptake in strain LTS4A by over 80% (Fig. 5B). Triethylglycine also inhibited carnitine uptake in strains LTG59 and DP-L1044, although to a much lesser degree.
DISCUSSION
Since the Gbu porter has not previously been described as a transporter of carnitine, we expected that strains DP-L1044 and LTG59 would behave similarly with respect to carnitine uptake. The opuC mutant LTS4A was originally selected for reduced growth on carnitine, and we expected greatly reduced carnitine uptake in this strain. Instead, strains LTG59 and LTS4A both showed reduced but measurable carnitine uptake, suggesting a previously unsuspected role for the Gbu porter as an uptake system for carnitine.
Our finding that L. monocytogenes uses the Gbu porter as an important secondary transporter for carnitine is novel and demonstrates this pathogen's flexibility in accumulating osmolytes. The OpuC porter had a Km for carnitine uptake 3 orders of magnitude lower than that of the Gbu porter. However, OpuC had a maximum carnitine uptake rate of just 15 to 25 nmol/min/mg of protein, while the Gbu porter could accumulate carnitine much more rapidly when the concentration in the medium was sufficiently high. For example, at 1.1 mM carnitine, the rate of uptake in strain LTS4A was 31 nmol/min/mg of protein, but in strain LTG59 it was 23 nmol/min/mg of protein. Thus, when the carnitine level is low, the dedicated carnitine OpuC porter functions as an efficient, specific scavenger of this osmoprotectant. As the carnitine concentration rises, the Gbu porter becomes the primary means of accumulation. At the concentration of 100 μM carnitine used in preloading assays, both transport systems contributed significantly to carnitine uptake.
The 3 orders of magnitude between the Kms for carnitine uptake via the OpuC porter and the Gbu porter is the most likely reason that the Gbu porter has not previously been described as an uptake system for carnitine. It was the use of strain LTG59, which lacks gbu, that led to the discovery that there were two transport systems for carnitine. The comparatively low Vmax of the OpuC porter, however, means that the Gbu porter provides a significant percentage of total carnitine uptake at the carnitine concentrations historically used to study carnitine uptake.
Working with Bacillus subtilis, Kappes and Bremer (5) found that adding 0.4 M NaCl to the growth medium did not affect the Km for carnitine uptake but increased the Vmax from 41 to 71 nmol/min/mg of protein. Glycine betaine uptake by the Gbu porter is dependent on the osmotic potential of the medium in L. monocytogenes, with maximum uptake at about 0.7 M NaCl (9). The salt-induced increase in Vmax reported by Kappes and Bremer could be due to activation of the Gbu porter.
An important goal of this research is to discover ways to decrease salt tolerance in L. monocytogenes by reducing the uptake of osmoprotectants. Dimethylglycine, trigonelline, and triethylglycine are glycine betaine analogs, while the structure of γ-butyrobetaine is similar to carnitine. However, since the structures of carnitine and glycine betaine are themselves similar enough for both compounds to be transported by the Gbu porter, we examined the effects of all four analogs on the transport of both carnitine and glycine betaine.
Competitive inhibition is a common mode of action by which an analog reduces the uptake of a substrate. However, the structural similarity which allows competitive inhibition to take place also gives analogs the potential to act as osmoprotectants in their own right, particularly when they are present in high concentrations. It was therefore of interest to discover if these analogs were accumulated by L. monocytogenes under salt stress conditions, and if so, whether they provided sufficient protection to the bacteria to allow growth.
Strain LTG59 would not grow in medium containing 9% NaCl, even in the presence of glycine betaine, suggesting that the Gbu porter plays a critical role in the ability of L. monocytogenes to survive extreme salt stress. Both the OpuC porter and the BetL porter have a relatively low Vmax for their primary substrates: 15 to 25 nmol/min/mg of protein for carnitine uptake via the OpuC porter, and about 20 nmol/min/mg of protein for glycine betaine uptake via the BetL porter (M. L. Mendum and L. T. Smith, unpublished results). It is possible that these two transporters do not accumulate their respective substrates quickly enough for strain LTG59 to survive the initial shock of inoculation into medium containing 9% NaCl. Alternatively, the Gbu porter may have a critical role in regulating the accumulation of osmoprotectants.
In strains DP-L1044 and LTS4A, glycine betaine was the superior osmoprotectant. Strain LTG59 was able to use carnitine almost as efficiently as glycine betaine, with doubling times of 13 and 11 h, respectively. This was most likely due to the lower salt concentration in the medium, combined with this strain's lack of the Gbu porter. At salt concentrations above 4%, the Gbu porter dominates glycine betaine uptake (9). Carnitine uptake is more evenly shared between the Gbu porter and the OpuC porter. Thus, glycine betaine uptake may be more hindered than carnitine uptake by the loss of the Gbu porter when the growth medium contains 6% NaCl.
Strain LTS4A, which lacks the OpuC porter, could not accumulate osmotically protective amounts of carnitine through the Gbu porter at the relatively low concentration of 100 μM used (Table 3). Thus, the doubling times reported reflected primarily the effect of each analog at a concentration of 5 mM and are broadly similar to the effects reported for the experiment whose results are given in Table 1. Dimethylglycine and γ-butyrobetaine offered sufficient osmoprotection to allow growth, while trigonelline and triethylglycine did not.
An analog's ability to provide osmoprotection does not in itself prevent it from acting as a growth inhibitor in the presence of more-efficient osmoprotectants. If accumulating the analog does not compensate fully for protection lost due to less accumulation of the superior osmoprotectants glycine betaine and carnitine, the bacteria would grow more slowly. While some of the test compounds were able to act as alternative osmolytes in L. monocytogenes, none was as efficient as glycine betaine, and only γ-butyrobetaine was comparable to carnitine in two strains, DP-L1044 and LTG59. Bayles and Wilkinson (1) also reported that γ-butyrobetaine was comparable to carnitine as an osmoprotectant.
In strain DP-L1044, which has all three transporters, triethylglycine slowed growth in the presence of glycine betaine, while trigonelline slowed growth in the presence of carnitine. A mixture of triethylglycine and trigonelline slowed growth in the presence of glycine betaine more than either analog alone, but the same mixture in the presence of carnitine stimulated growth in this strain (data not shown). This demonstrates that caution is necessary when attempting to predict the effects of multiple inhibitors on the growth of L. monocytogenes.
The dramatic differences in growth rates among the three L. monocytogenes strains in the presence of the four analogs suggest that these compounds were interacting in different ways with the BetL porter, the Gbu porter, and the carnitine OpuC porter. This allowed us to draw tentative conclusions about which transporter(s) was most affected by each analog.
Dimethylglycine inhibited both glycine betaine and carnitine uptake in strains DP-L1044 and LTS4A to a similar degree (Fig. 2A and B). However, strain LTG59, which lacks the Gbu porter, showed much less inhibition of glycine betaine and carnitine uptake than did the other two strains. This suggests that dimethylglycine inhibits both carnitine and glycine betaine uptake primarily through the Gbu porter, which transports both osmoprotectants. The growth experiments for which results are shown in Tables 2 and 3 showed that this analog had only a modest effect on the growth of L. monocytogenes when glycine betaine was present but that it slowed the growth of strain DP-L1044 in the presence of carnitine. Strain LTG59, which lacks the Gbu porter, showed an increase in growth, as did strain LTS4A, which was unable to accumulate sufficient carnitine for growth without the OpuC porter.
Our findings contrast with those of Verheul and colleagues (15), who reported that carnitine uptake was not inhibited by dimethylglycine. However, bacteria used in this study had been preexposed to salt stress, while Verheul et al. applied stress only at the start of the actual assay. We found similar rates of glycine betaine uptake in strains DP-L1044 and LTG59 directly after an osmotic upshock, with an increase of glycine betaine uptake in strain DP-L1044 after half an hour of osmotic stress (9). This suggests that the Gbu porter may not have been very active under the assay conditions used by Verheul et al., making it difficult to detect the effect of dimethylglycine on carnitine uptake.
The structure of γ-butyrobetaine is most similar to that of carnitine. This analog had little effect on glycine betaine uptake in any strain, except after an hour of preloading (Fig. 3A). This is consistent with an indirect effect of γ-butyrobetaine, perhaps by reducing the need for additional osmoprotectants as it accumulates. In contrast, γ-butyrobetaine rapidly reduced carnitine uptake by 70% in strains DP-L1044 and LTG59 (Fig. 3B). This suggests that γ-butyrobetaine may inhibit carnitine uptake through the OpuC porter but that it probably does not compete directly with glycine betaine uptake through the BetL and Gbu porters. Strain LTS4A, however, showed a 40% reduction in carnitine uptake in the presence of γ-butyrobetaine, even though this strain does not possess the OpuC porter. This effect cannot be due to an interaction with the Gbu porter, because strains DP-L1044 and LTG59 behave identically. It appears likely, therefore, that γ-butyrobetaine interacts with at least one additional system in L. monocytogenes which is not directly involved with glycine betaine or carnitine uptake but which can indirectly affect the uptake of carnitine through the OpuC porter.
Verheul and colleagues (14) reported that γ-butyrobetaine almost completely inhibited carnitine uptake. At 25 μM carnitine, we observed carnitine uptake rates of around 10 nmol/min/mg of protein in strain LTG59, which lacks the Gbu porter, but only about 2.4 nmol/min/mg of protein in strain LTS4A, which lacks OpuC (Fig. 1). The assay used by Verheul et al. carnitine concentrations of around 20 μM (14). Under these conditions, the Gbu porter may not have taken up much carnitine.
The effect of γ-butyrobetaine on carnitine uptake in B. subtilis has been studied extensively. The OpuC porter in this bacterium differs from that of L. monocytogenes in that it can transport glycine betaine (6). γ-Butyrobetaine was able to inhibit both glycine betaine uptake (6) and carnitine uptake (5) via OpuC. This is consistent with our findings that this analog acts primarily on the OpuC porter of L. monocytogenes and not on the two glycine betaine porters.
Triethylglycine inhibited carnitine uptake but not glycine betaine uptake in strain LTS4A (Fig. 5A and B). The Km for carnitine uptake through the Gbu porter is much greater than that for glycine betaine uptake, so it is not entirely unexpected to find that an analog might inhibit carnitine uptake but not glycine betaine uptake through this transport system. Triethylglycine also inhibited carnitine uptake through the OpuC porter in strain LTG59, but not very strongly. Strain DP-L1044 had an intermediate degree of inhibition of carnitine uptake. The carnitine concentration in our assays was 100 μM. At this concentration, the OpuC porter is saturated and carnitine uptake through the Gbu porter contributes significantly to the total uptake. The intermediate degree of inhibition in strain DP-L1044 thus reflects the relative contributions and degrees of inhibition of the two transport systems.
L. monocytogenes causes outbreaks of food poisoning in humans precisely because it can actively grow in processed meats, dairy products, and other common foods to which salt is added as a preservative. Smith (11) found glycine betaine concentrations ranging from 340 to 480 mM in extracts of processed lunch meats. Carnitine concentrations in the same samples ranged from 230 to 950 mM. Woollard et al. reported finding 120 and 140 μM carnitine in milk (16), which has glycine betaine levels of around 50 μM (L. T. Smith, unpublished results). It is therefore to the advantage of L. monocytogenes to accumulate glycine betaine and carnitine efficiently over a wide range of concentrations.
The incidence and severity of listeriosis in susceptible individuals are affected by the size of the initial inoculum (2). A glycine betaine or carnitine analog that slows growth in the presence of these osmoprotectants, even to a modest degree, has the potential to act as a food preservative. The work reported here shows that it is possible to reduce the growth of L. monocytogenes under osmotically stressful conditions by inhibiting uptake of the effective osmoprotectants glycine betaine and carnitine. However, the existence of multiple uptake systems for both glycine betaine and carnitine in L. monocytogenes complicates the search for a simple counteragent to this pathogen's ability to withstand osmotic stress. Ideally, such a counteragent would have three properties. First, it would reduce uptake of both glycine betaine and carnitine through all three transporters: the glycine betaine porters BetL and Gbu and the OpuC porter. Second, a counteragent would be a poor osmoprotectant. It either would not be accumulated or would not provide protection from osmotic stress if accumulated. Finally, to be useful as a preventive measure against food poisoning outbreaks, a counteragent would have to be nontoxic to humans.
The four analogs we tested inhibited uptake of one or both osmoprotectants through at least one transport system, and all reduced growth in at least some strains of L. monocytogenes. Of the four, triethylglycine came closest to fulfilling the first property of a good counteragent: it inhibited uptake of both carnitine and glycine betaine sufficiently to reduce growth, and it affected multiple transport systems. Triethylglycine acted as an osmoprotectant, but it was generally a poor one. Its toxicity to mammals has not been determined at this time.
Although none of the glycine betaine analogs that we tested met all the criteria for a commercially useful inhibitor, the experiments reported here confirm that it is possible to reduce the salt tolerance of L. monocytogenes by reducing uptake rates of the osmoprotectants glycine betaine and carnitine. Further investigation of analogs, and their effects on the three transport systems responsible for carnitine and glycine betaine uptake, could lead to an inhibitor or combination of inhibitors which are capable of reducing the growth of L. monocytogenes in foods for human consumption.
Acknowledgments
We thank Gary M. Smith and his student Patrick Fitzgerald, Department of Food Science and Technology, University of California, Davis, for the generous gift of triethylglycine.
We acknowledge the support of Dairy Management Inc. (grant 98TSL-01).
REFERENCES
- 1.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 2.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser, K. R., D. Harvie, P. J. Coote, and C. P. O'Byrne. 2000. Identification and characterization of an ATP binding cassette l-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerhardt, P. N. M., L. T. Smith, and G. M. Smith. 1996. Sodium-driven, osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicles. J. Bacteriol. 178:6105-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kappes, R. M., and E. Bremer. 1998. Response of Bacillus subtilis to high osmolarity: uptake of carnitine, cronobetaine and γ-butyrobetaine via the ABC transport system OpuC. Microbiology 144:83-90. [DOI] [PubMed] [Google Scholar]
- 6.Kappes, R. M., B. Kempf, S. Knelp, J. Boch, J. Gade, J Meier-Wagner, and E. Bremer. 1999. Two evolutionarily closely related ABC transporters mediate the uptake of choline for synthesis of the osmoprotectant glycine betaine in Bacillus subtilis. Mol. Microbiol. 32:203-216. [DOI] [PubMed] [Google Scholar]
- 7.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko, R., and L. T. Smith. 1999. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl. Environ. Microbiol. 65:4040-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendum, M. L., and L. T. Smith. 2002. Characterization of glycine betaine porter I from Listeria monocytogenes and its roles in salt and chill tolerance. Appl. Environ. Microbiol. 68:813-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perroud, B., and D. Le Redulier. 1985. Glycine betaine transport in Escherichia coli: osmotic modulation. J. Bacteriol. 161:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith, L. T. 1996. Role of osmolytes in adaptation of osmotically stressed and chill-stressed Listeria monocytogenes grown in liquid media and on processed meat surfaces. Appl. Environ. Microbiol. 62:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 13.Sun, A. N., A. Camilli, and D. A. Portnoy. 1990. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 58:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verheul, A., F. M. Rombouts, R. R. Beumer, and T. Abee. 1995. An ATP-dependent l-carnitine transporter in Listeria monocytogenes Scott A is involved in osmoprotection. J. Bacteriol. 177:3205-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verheul, A., E. Glaasker, B. Poolman, and T. Abee. 1997. Betaine and l-carnitine transport by Listeria monocytogenes Scott A in response to osmotic signals. J. Bacteriol. 179:6979-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woollard, D. C., H. E. Indyk, and G. A. Wollard. 1999. Carnitine in milk: a survey of content, distribution and temporal variation. Food Chem. 66:121-127. [Google Scholar]