Abstract
A food-grade system for the delivery of desired genes to Lactococcus lactis, their inducible expression, and their transfer to related strains was established. Based on the thermosensitive pG+host replicon, two types of plasmid vectors were constructed which contained sections of either the chromosomal leu operon of L. lactis or the tel operon from the lactococcal sex factor. Genes cloned into the leu or tel sequences of these vectors were delivered to the homologous regions of the chromosome or the sex factor through two single crossovers, leading to integration of the recombinant plasmids and subsequent excision of the vector portions. Inducible transcription of integrated genes was achieved by using the nisin-controlled expression (NICE) system. To establish the signal transduction genes nisRK in L. lactis, the vectors pLNG1363 (targeted to the chromosome) and pUK500 (targeted to the sex factor) were constructed. Fusions of six different peptidase genes (pep) from Lactobacillus delbrueckii with the nisin-inducible promoter PnisA were delivered to the sex factor with derivatives of the vector pUK300. Food-grade recombinants of L. lactis were constructed which had the nisRK genes and individual PnisA::pep fusions integrated either separately into the chromosome and the sex factor or simultaneously into the sex factor. With both types of recombinants, expression of PnisA::pep fusions after induction with nisin was demonstrated. Depending on the loci used for integration of nisRK, variable induction rates were observed. Furthermore, an engineered sex factor carrying a PnisA::pepI fusion was transfered by conjugation between two strains of L. lactis at a frequency of 4 × 10−4.
Lactic acid bacteria have long been recognized as essential constituents of the microflora present in fermented milk and other food products, and they are widely used as starters in industrial fermentations. Due to their economic importance, the genetics and physiology of lactic acid bacteria have been studied extensively. This applies especially to Lactococcus lactis, which now is one of the best-characterized bacteria. Molecular approaches to lactic acid bacteria required the development of new genetic tools, including plasmid vectors, selection markers, and tranformation protocols. However, in many cases the use of these tools does not conform to the food-grade criterion, which implies that all genetic elements used to construct recombinants should be derived exclusively from strains which have traditionally been used in food fermentations and therefore are “generally recognized as safe” (GRAS).
The development of reliable systems for the food-grade genetic modification of lactic acid bacteria is of considerable interest. Food-grade recombinants may be used as starters in food fermentations and for the safe production of metabolites used as food adjuncts. Our present knowledge about the relationships of distinct genetic traits of L. lactis to relevant fermentation parameters and organoleptic properties of the fermentation products opens new prospects for the deliberate construction of genetically modified strains with desired phenotypes.
The use of plasmids for food-grade introduction of new or modified genes into L. lactis often seems unfavorable because the presence of antibiotic resistance genes in the final constructs is not permitted and the range of alternative selection markers is limited. Several food-grade vectors have been developed by using sugar utilization (25) or the suppression of auxotrophic markers (9) for positive selection of transformants. Selection for these vectors, however, requires special genotypes of the respective host strains. Further drawbacks associated with the use of recombinant plasmids are structural instabilities and variable copy numbers, which often affect the maintenance and expression of cloned genes.
We therefore considered integration into the bacterial genome to be a more reliable approach to stabilizing and maintaining the desired genetic features. Several food-grade integration systems for Lactococcus have been described. They include vectors which either do not replicate at all in the Lactococcus host (e.g., ColE1 derivatives) or are conditionally nonreplicative (repA-deficient or thermosensitive) variants of the L. lactis Wg2 plasmid pWV01 (22, 23). Gene delivery by nonreplicative vectors requires efficient transformation of the recipients, whereas repA-deficient and thermosensitive vectors allow the temporal separation of the transformation and integration steps and therefore can also be used with poorly transformable strains. As an advantage of thermosensitive vectors, they are independent of host genotypes, whereas cloning in repA-deficient vectors is limited to special hosts.
Here we report on the use of a thermosensitive vector to design a food-grade gene delivery system for L. lactis which is also suited for regulated overexpression of desirable functions. Only a few tightly controllable gene expression systems for lactococci have been described which fulfill the demands for food safety and technological applicability of the induction signal (for reviews, see references 8 and 10). One of the most convenient systems is based on the use of the lantibiotic nisin to induce fusions of desired genes to the nisA promoter via a membrane-associated histidine kinase (NisK) and a transcriptional regulator (NisR) (6). As a major advantage, this system is dynamically inducible by subinhibitory concentrations of nisin in a range of up to several hundred fold (5).
We present a set of plasmid vectors that are suitable to stably establish the nisin signal transduction pathway as well as nisin-inducible gene fusions in any strain of L. lactis. Selected peptidase genes from Lactobacillus delbrueckii subsp. lactis (20) were efficiently expressed in this system.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. Escherichia coli was grown at 37°C in Luria-Bertani medium (26), and L. lactis was cultivated at 30°C in M17 medium (Difco) supplemented with 0.5% (wt/vol) glucose (GM17). When appropriate, ampicillin (200 μg/ml for E. coli), chloramphenicol (15 μg/ml for E. coli, 10 μg/ml for L. lactis), erythromycin (100 μg/ml for E. coli, 5 μg/ml for L. lactis), or streptomycin (200 μg/ml for L. lactis) was added to the media.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| TG1 | supE Δhsd thi Δ(lac-proAB) F′[traD36 proAB+lacIqlacZΔM15] | 26 |
| JM109 | recA endA gyrA thi hsdR relA supE Δ(lac-proAB) | 29 |
| L. lactis | ||
| MG1363 | Derivative of NCDO712, plasmid free, prophage cured | 12 |
| CHCC377 | Industrial strain | Chr Hansen A/S |
| NZ9800 | Tn5276 ΔnisA, Str | 21 |
| UKLc10 | pepN::nisRK | 27 |
| UKLc11 | MG1363 leuB::nisRK, pLP712 | This work |
| UKLc11-I | UKLc11, sex factor telB::pepI | This work |
| UKLc11-Q | UKLc11, sex factor telB::pepQ | This work |
| UKLc11-L | UKLc11, sex factor telB::pepL | This work |
| UKLc11-G | UKLc11, sex factor telB::pepG | This work |
| UKLc11-W | UKLc11, sex factor telB::pepW | This work |
| UKLc11-C | UKLc11, sex factor telB::pepC | This work |
| UKLc13(pLP712) | MG1363(pLP712), sex factor telB::nisRK | This work |
| UKLc13-I(pLP712) | UKLc13(pLP712), sex factor telB::nisRK-pepI | This work |
| Plasmids | ||
| pGEM-T | Apr, Plac-lacZα, linearized, with 3′T overhangs, ColE1 replicon | Promega |
| pBluescriptIISK | Apr, Plac-lacZα, ColE1 replicon | Stratagene |
| pUC18E | pUC18 with 1.2-kb ermG (Err)-containing fragment from L. reuteri in SmaI site | U. Wegmann, personal communication |
| pNZ9573 | Cmr Err, pepN::nisRK, p15a replicon | 5 |
| pG+host9 | Err, pWV01 replicon (temperature sensitive) | 23 |
| pLP712 | lac+prt+, 33 MDa | 12 |
| pJKC4 | Kmr, pepC, pSC105 replicon | 18 |
| pUK200 | Cmr, PnisA, terminator of brnQ, pSH71 replicon | 27 |
| pUK200I | pUK200, PnisA::pepI | 27 |
| pUK200Q | pUK200, PnisA::pepQ | 27 |
| pUK200L | pUK200, PnisA::pepL | 27 |
| pUK200G | pUK200, PnisA::pepG | 27 |
| pUK200W | pUK200, PnisA::pepW | 27 |
| pUK200C | pUK200, PnisA::pepC | This work |
| pUK300 | pG+host9 with ′telA-telB-telC′ from lactococcal sex factor interrupted by multiple-cloning site in telB | This work |
| pUK300I | pUK300 with PnisA::pepI-TbrnQ in EcoRV site | This work |
| pUK300Q | pUK300 with PnisA::pepQ-TbrnQ in EcoRV site | This work |
| pUK300L | pUK300 with PnisA::pepL-TbrnQ in EcoRV site | This work |
| pUK300G | pUK300 with PnisA::pepG-TbrnQ in EcoRV site | This work |
| pUK300W | pUK300 with PnisA::pepW-TbrnQ in EcoRV site | This work |
| pUK300C | pUK300 with PnisA::pepC-TbrnQ in EcoRV site | This work |
| pUK500 | pUK300 with nisRK in ClaI site | This work |
| pUK500I | pUK500 with PnisA::pepI-TbrnQ in SmaI site downstream of nisRK | This work |
DNA preparation, cloning, sequencing, and PCR.
Standard techniques were applied to the cloning of DNA fragments (26) and the preparation of plasmid DNA from E. coli (2) and L. lactis (7). To prepare total DNA from L. lactis, cells were pelleted from a 5-ml overnight culture and resuspended in 1 ml of 3 mM MgCl2-25% (wt/vol) sucrose-30 mM Tris-HCl (pH 8.0). After addition of lysozyme (2 mg/ml, final concentration) and incubation for 1 h at 37°C, the suspension was cenrifuged for 5 min at 12,000 × g, the sediment was resuspended in 0.1 ml of 3 mM MgCl2-25% (wt/vol) sucrose-30 mM Tris-HCl (pH 8.0), and 0.4 ml of 50 mM NaCl-0.5% (wt/vol) sodium dodecyl sulfate-50 mM Tris-HCl (pH 8.0) was added. The sample was extracted several times with phenol-CHCl3 (24:1) until a clean interphase was formed, and once with CHCl3. The DNA was then precipitated with ethanol and redissolved in 0.1 ml of 10 mM Tris-HCl (pH 7.6)-1 mM EDTA. Restriction endonucleases and nucleic acid-modifying enzymes (Roche, New England Biolabs) were used as recommended by the suppliers. E. coli and L. lactis were transformed by electroporation (11, 28) with a Gene Pulser (Bio-Rad). Plasmid DNA used for automated sequencing with a LI-COR model 4200 sequencer (MWG-Biotech) was purified on Nucleobond AX columns (Machery-Nagel). Nucleotide sequences were analyzed with the BlastN program (1). PCR amplifications were performed under standard conditions as recommended by the suppliers of the DNA polymerases used. Primers for PCR and nucleotide sequencing were purchased from MWG-Biotech.
Construction of a PnisA::pepC translational fusion.
The pepC gene from L. delbrueckii subsp. lactis was PCR amplified from plasmid pJKC4 by using the primers 5′-atacatgtCAAAAGAAATCAGCTTTGACACGATCGAAGACTTCACC and 5′-atggatccTTAGAAGTCGAAAGCCAAAGCGCCCATTGGG (the nucleotides printed in lowercase letters were added to introduce the underlined AflIII and BamHI sites). After the ends of the PCR product were cut with AflIII and BamHI, it was inserted between the NcoI site at the ATG start codon of nisA and the BamHI site of the vector pUK200. Transformants of strain UKLc10 carrying the resulting plasmid pUK200C were identified by their enhanced ability to cleave the chromogenic PepC substrate Lys-β-naphthylamide (18) in the presence of nisin. The sequence of the pepC gene and its junction with PnisA in pUK200C was verified by nucleotide sequencing.
Construction of delivery vectors.
E. coli strains TG1 and JM109 were used as hosts during cloning of DNA fragments into pGEM-T, pUC18E and pG+host9.
To construct vectors for targeted integration of nisRK into the leuB locus of L. lactis strains MG1363 and CHCC377, a section of the leu operon (′leuABC′) was PCR amplified from chromosomal DNA of both strains by using the primers 5′-CAGACACCGGGCGTTAGTTTCTCC and 5′-CACGTAAGCCCTGAAATGCTTGCG. In the case of strain MG1363, the 5′-A overhangs of the PCR product generated with the Expand High-Fidelity PCR system (Roche) were ligated with the pGEM-T vector to give pL1363. A 2,485-bp ClaI-BspMI fragment, containing nisR, nisK, and the 3′ end of nisP, was excised from plasmid pNZ9573, treated with T4 DNA polymerase to fill in the ends, and inserted in the direction of leuB at the single NruI site of pL1363. The resulting plasmid, pLN1363, was linearized with NotI and ligated with NotI-linearized pG+host9 to give the final construct, pLNG1363 (see Fig. 1). In the case of strain CHCC377, the ′leuABC′ region was PCR amplified with ULTma DNA polymerase (Perkin-Elmer) and inserted into the filled-in HindIII site of the vector pUC18E to give pL377. The nisRK-containing ClaI-BspMI fragment from pNZ9573, after the ends were filled in with T4 DNA polymerase, was inserted at the single NruI site of pL377, in the opposite direction to that of leuB. From the new plasmid pLN377, a leu::nisRK-containing fragment (5,312 bp) was amplified with the Expand High-Fidelity PCR system by using the same primer pair as above and was ligated with the pGEM-T vector. The resulting plasmid, pLN1, was linearized with PstI and ligated with PstI-linearized pG+host9 to give the final construct, pLNE12 (see Fig. 1).
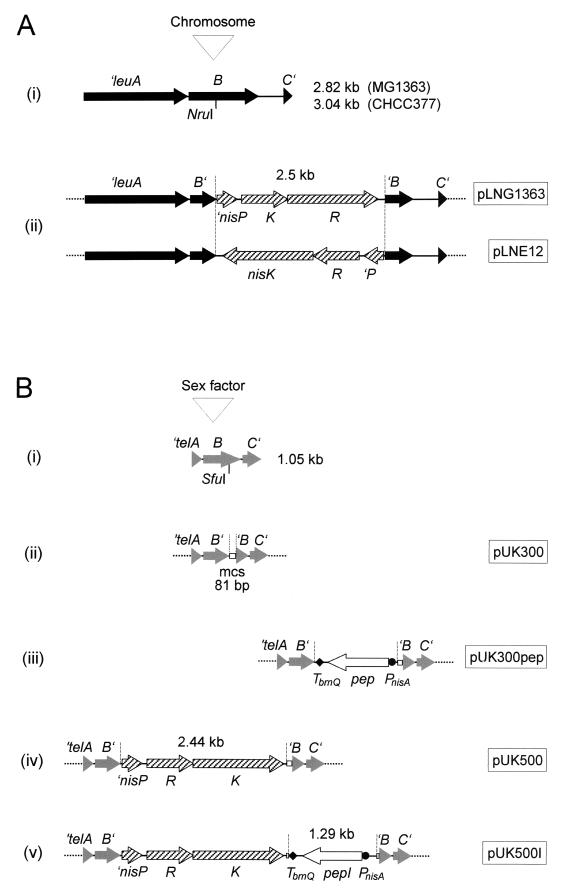
FIG. 1.
Construction of gene delivery vectors for L. lactis. Details of the constructions are outlined in Materials and Methods. Of the resulting vectors (names indicated in boxes), only the sections relevant to integration are shown. (A) Vectors for delivery of genes to the chromosome. DNA fragments subcloned from the chromosomal leu operon of strains MG1363 and CHCC377 (i) were interrupted by the insertion of the nisRK genes isolated from pNZ9573 (ii). (B) Vectors for delivery of genes to the sex factor. A DNA fragment subcloned from the tel operon of the sex factor (i) was interrupted by the insertion of the multiple-cloning site (mcs) from pBluescriptIISK (ii). The multiple-cloning site was used to insert six different PnisA::pep fusions isolated from pUK200 derivatives (iii), the nisRK genes isolated from pNZ9573 (iv), or nisRK together with PnisA::pepI (v). pUK300pep stands for six different derivatives of pUK300 (pUK300I, pUK300Q, pUK300L, pUK300G, pUK300W, and pUK300C) containing fusions of PnisA with pepI, pepQ, pepL, pepG, pepW, and pepC, respectively.
To construct a vector for delivery of genes to the lactococcal sex factor, a 1,721-bp DNA fragment, covering the telABC genes, was PCR amplified from the sex factor with Goldstar Taq DNA polymerase (Eurogentec) by using total DNA from L. lactis MG1363 as the template and the primers 5′-GAACATCCAACCATGGGA and 5′-CTCGATCGGATGTTTCGTCT. The 5′-A overhangs of the PCR product were ligated with the pGEM-T vector, and the resulting plasmid, pGEMtel, after linearization with BglII and treatment with Klenow fragment, was joined with the KpnI-linearized and Klenow fragment-treated vector pG+host9. To eliminate the pGEM-T portion, the new plasmid, pKB02, was digested with AccI, and the largest (4,649 bp) of the resulting four fragments, after its ends were filled in with Klenow fragment, was circularized to give pKB03. This plasmid was linearized at its unique SfuI site within telB, treated with T4 DNA polymerase to fill in the ends, and ligated with an 81-bp HincII-Ecl136II fragment, derived from the polylinker region of the pBluescriptIISK vector. The final construct was designated pUK300 (see Fig. 1).
Derivatives of pUK300, suitable for the integration of peptidase genes from L. delbrueckii subsp. lactis into the lactococcal sex factor, were constructed as follows. The respective translational PnisA::pep fusions, together with the downstream terminator TbrnQ, were excised from the expression plasmids pUK200I, pUK200Q, pUK200L, and pUK200G as BglII-XhoI fragments and from pUK200W and pUK200C as SspI-XhoI fragments. The fragments, after their ends were filled in with T4 DNA polymerase, were inserted into the unique EcoRV site of pUK300. Recombinant plasmids, carrying the respective PnisA::pep fusions opposed to the orientation of the tel genes, were named pUK300I, pUK300Q, pUK300L, pUK300G, pUK300W, and pUK300C, respectively.
To construct a derivative of pUK300, suitable for delivering nisRK to the lactococcal sex factor, a nisRK-containing DNA fragment (2,443 bp) was PCR amplified from pNZ9573 by using the Expand High-Fidelity PCR system and the primers 5′-tatcgatGGAGAATCTTAAAGAGTCTAGGG and 5′-cagaatcgatTCAGAAACAAAAAAAGTAATCC (nucleotides in lowercase letters were added to introduce the underlined ClaI sites). After the ends of the PCR product were cut with ClaI, it was ligated with ClaI-linearized pUK300. A recombinant plasmid, carrying nisRK in the direction of the tel genes, was named pUK500 (Fig. 1).
A derivative of pUK500, suitable for simultaneous integration of pepI from L. delbrueckii subsp. lactis and nisRK into the lactococcal sex factor, was constructed as follows. The PnisA::pepI translational fusion and the downstream terminator TbrnQ were excised from pUK200I with BglII and XhoI. The fragment (1,289 bp), after its ends were treated with T4 DNA polymerase, was inserted into the single SmaI site downstream of nisRK in pUK500. A recombinant plasmid, carrying PnisA::pepI in the opposite orientation to that of the tel and nisRK genes, was named pUK500I (see Fig. 1).
Gene delivery to the chromosome and the sex factor.
Derivatives of the pG+host9 vector, carrying copies of the respective target sequences from the chromosome (leu) or the sex factor (tel) interrupted by desired genetic functions (nisRK and PnisA::pep), were used essentially as described by Biswas et al. (3). To establish an appropriate pG+host9 derivative in L. lactis, transformants were initially selected at the permissive temperature (28°C) in the presence of erythromycin. Overnight cultures, grown under the same conditions, were then diluted and plated at the nonpermissive temperature (37°C) with antibiotic to obtain single-crossover integrants. To calculate the frequency of plasmid integration, the cultures were also plated at 37°C in the absence of antibiotic. Excision of the vector by a second single-crossover event was subsequently stimulated by growing individual integrants in liquid medium at 28°C without antibiotic. When appropriate, this incubation was extended to up to 100 cell generations by repeated redilution of the cultures. At intervals, dilutions of the cultures were plated at 37°C without erythromycin to eliminate the excised vector and in the presence of erythromycin to determine the frequency of plasmid excision (percentage of Ers clones). Ers clones were identified by replica plating a number of single colonies from plates without antibiotic on plates with and without erythromycin. Ers clones were checked for the presence of integrated DNA fragments by PCR with appropriate primer pairs.
Preparation and electrophoresis of cell extracts.
Cultures of UKLc11-C, UKLc11-I, and UKLc11-Q were grown at 30°C, induced with nisin (3 ng/ml, final concentration) (Sigma-Aldrich) at the midexponential growth phase (optical density at 600 nm [OD600], 0.5 U), and further incubated. Cell extracts were prepared from culture aliquots, removed at different times after induction, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described previously (27).
Assay for peptidase I.
To determine the activity of PepI in cell extracts of L. lactis, 1,000-fold dilutions of the samples were incubated with the chromogenic substrate Pro-p-nitroanilide as described previously (19). The specific PepI activity was calculated as nanomoles of nitrophenol released per milligram of protein per minute.
Conjugation.
For conjugational transfer of the PnisA::pepI-containing sex factor from UKLc11-I to NZ9800 (Str), both strains were grown at 30°C in GM17 medium until an OD600 of about 0.45 U was reached. Aliquots (1.5 ml) of both cultures were then mixed in a total volume of 15 ml of GM17 medium and further incubated for 4 h at 30°C. Dilutions of the mixture were plated on GM17 agar containing nisin (at 5 ng/ml) and streptomycin. PepI-expressing transconjugants of NZ9800 were identified by a plate staining procedure adapted from that of Miller and Mackinnon (24) by using the chromogenic compound Pro-β-naphthylamide (19).
RESULTS AND DISCUSSION
We previously used the vector pUK200, derived from the lactococcal pSH71 replicon, to clone several peptidase genes from L. delbrueckii subsp. lactis as translational fusions with the nisA promoter (PnisA). They were induced in a fivefold pep-deficient strain of L. lactis, which carried the nisin signal transduction genes nisR and nisK integrated at the chromosomal pepN locus (27). This system was well suited to study enzymatic activities and physiological effects. Further evaluation of the nisin-inducible constructs under food-grade conditions and in industrial strains of L. lactis, however, required elimination of the chloramphenicol resistance marker and the development of appropriate devices for efficient introduction, genetic stabilization, and reliable expression of nisRK and desired PnisA fusions.
Delivery of nisRK to the chromosomal leu locus.
To achieve stable and constitutive expression of the nisin signal transcuction proteins, we integrated nisRK into the lactococcal chromosome at the site of the leucine biosynthesis genes leuABCD. This locus was chosen because it appears to be present and transcribed in most dairy strains of L. lactis subsp. lactis without being essential for efficient growth in milk. In a previous survey of 17 dairy strains, all of them were found to be auxotrophic for branched-chain amino acids (14).
By using primers derived from the published nucleotide sequence of the leu genes of the leucine-auxotrophic dairy strain L. lactis subsp. lactis IL1403 (14), we were able to amplify specific DNA fragments from the chromosomal leuABC regions of both the laboratory strain, L. lactis subsp. cremoris MG1363, and the industrial strain, L. lactis CHCC377. To verify their identity, the PCR products were completely (MG1363) or partially (CHCC377) sequenced. Compared to the known sequence of the corresponding fragment (3,044 bp) from the leucine-prototrophic nondairy strain NCDO2118 (13), the MG1363 sequence was 220 bp shorter, contained two nonsense mutations in the leuA and leuB frames, and lacked three of four copies of a 72-bp direct repeat between leuB and leuC (GenBank accession no. AJ429069). The overall identity between the NCDO22118 and MG1363 sequences was 82%. The DNA fragments amplified from MG1363 and CHCC377 were cloned into the temperature-sensitive pG+host9 replicon, and the nisRK genes were inserted into the leuB locus of the cloned fragments (Fig. 1). This left more than 900 bp of the leu region on either side of the nisRK insert for homologous recombination of the resulting plasmids with the chromosomal leu locus (3). Of these plasmids, pLNG1363 (containing nisRK in the direction of the leu genes of MG1363) and pLNE12 (containing nisRK opposed to the leu genes of CHCC377) were integrated into the chromosomes of the respective strains at 37°C and plasmid excision was subsequently stimulated at 28°C. The frequencies of the initial integration were as high as 10−2 (per CFU), and plasmid excision was observed in more than 90% of the clones after about 100 generations at 28°C. Of these clones, 80% (MG1363) and 30% (CHCC377) contained the nisRK insert.
To test the function of nisin signal transduction in the nisRK integrants, we used a previously constructed translational fusion of PnisA with the proline iminopeptidase gene (pepI) of L. delbrueckii (27) as a reporter. The fusion, present on plasmid pUK200I, was introduced into the nisRK integrants, and the activity of PepI was assayed after induction with nisin. With integrants of MG1363, which carried nisRK in the orientation of the leu genes, PepI was clearly inducible, whereas no induction was detected with integrants of CHCC377, which carried nisRK in the opposite direction. From this, we suspected that a potential promoter, previously localized between nisP and nisR (5), is too weak to supply the integrants with sufficient amounts of the signal-transducing proteins NisR and NisK. Therefore, the expression of nisRK in the nisin-inducible integrants of MG1363 appeared to be driven mainly by a promoter located upstream of the leu genes in the chromosome. The existence and activity of such a promoter was also conceivable from the previous observation that the leu genes are efficiently transcribed in both leucine prototrophs and auxotrophs (14). Recombinant plasmids (pNZ9521 and pNZ9531), carrying a ′nisP-nisR-nisK fragment similar to that integrated into MG1363, have been reported to confer effective nisin signal transduction to transformants of L. lactis. Also in these cases, transcription of nisRK was probably maintained by readthrough from the promoter of a preceding rep gene (17).
To enable growth of the nisin-responsive integrants of MG1363 in milk, they were equipped with plasmid pLP712, which allows lactose utilization and protease production (12). The resulting strain was designated UKLc11.
Delivery of desired genes to the sex factor.
To achieve food-grade stabilization of new or engineered genetic traits (e.g., nisin-inducible PnisA fusions) in L. lactis, we constructed another conditionally nonreplicative vector. It was designed for delivering genes to the lactococcal sex factor, a 50-kb genetic element originally characterized in the dairy strain L. lactis 712, which can either exist as a free plasmid or form cointegrates with the chromosome or the lactose-protease plasmid pLP712 (15). The sex factor seemed particularly useful as a target for gene delivery because it is stable and can be efficiently exchanged between strains of L. lactis by conjugation (16). This renders the system independent of transformation efficiencies, which are unknown or unsatisfactory for most production strains. Conjugational transfer of the sex factor is confined to members of the species L. lactis and therefore does not contribute to horizontal DNA exchange with other bacterial species in fermented foods, the consumer, or the environment.
We exploited the known sequence of the telB gene as a locus for integration of desirable genes into the sex factor. It is one of three contiguous genes (telABC), encoding very similar proteins with high homology to tellurium resistance determinants (16). Since resistance to tellurium is not relevant for growth of L. lactis in milk, no adverse effects were expected from its inactivation.
The telB gene and parts of the flanking telA and telC genes were amplified from the sex factor, cloned in the pG+host9 vector, and interrupted by insertion of a multiple-cloning site into telB. In the resulting vector, pUK300, more than 480 bp of the tel sequence is left on either side of 12 unique restriction sites, which should be sufficient to promote the delivery of cloned DNA fragments to the sex factor by homologous recombination (3).
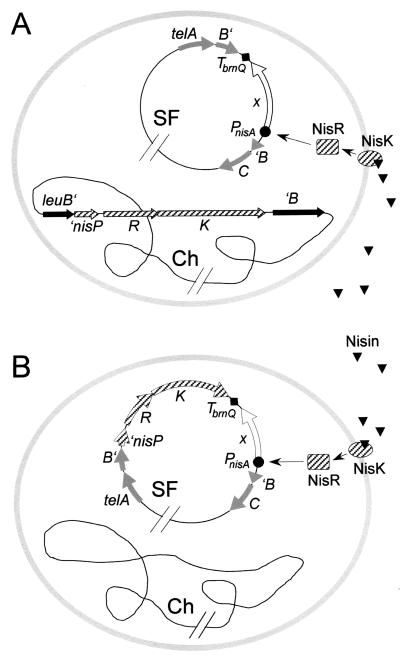
To demonstrate its utility, we used pUK300 to clone translational fusions of PnisA with each of six different peptidase genes (pepI, pepQ, pepL, pepG, pepW, and pepC) previously isolated from L. delbrueckii subsp. lactis (see references 20 and 27 and references therein). Strain UKLc11 was transformed with the resulting pUK300 derivatives, and the tel region of the sex factor (present in UKLc11) was replaced by the PnisA::pep-containing copies of this region present on the respective plasmids through two successive single crossovers, promoted by temperature shifts to 37 and 28°C, respectively. Depending on the individual PnisA::pep fusions, the frequencies of initial plasmid integration ranged between 7 × 10−4 and 1 × 10−2 (per CFU), and subsequent plasmid excision occurred in 17 to 95% of the bacteria in the course of about 100 generations at 28°C. PCR analysis of these clones revealed that in the cases of pepI, pepQ, pepG, pepW, and pepC, 25 to 60% of them carried the respective PnisA::pep fusions integrated into the sex factor whereas no integrants were obtained for pepL. This was probably due to a selective disadvantage of pepL carriage, since pepL integrants could be recovered only after shortening the incubation period at 28°C to about eight generations, which in turn decreased the plasmid excision frequency to 0.5%. The resulting strains, carrying the respective PnisA::pep fusions integrated into telB of the sex factor, were designated UKLc11-I, UKLc11-Q, UKLc11-L, UKLc11-G, UKLc11-W, and UKLc11-C (Fig. 4A).
FIG. 4.
Outline of two available versions of the food-grade expression system. The signal transduction genes nisRK and a PnisA fusion of a desired gene (x) may be integrated either separately into the chromosome (Ch) and the sex factor (SF) (A) or adjacent to each other into the sex factor (B). Arrows indicate signal transduction from the extracellular inducer nisin via the histidine kinase NisK and the response regulator NisR to the promoter PnisA.
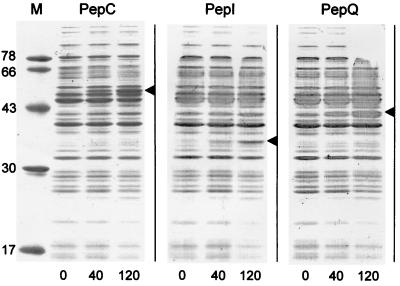
The inducibility of the integrated PnisA::pep fusions was verified by electrophoretic analysis of cell extracts prepared from UKLc11-C, UKLc11-I, and UKLc11-Q (Fig. 2). Protein bands corresponding to the molecular masses of each of the three peptidases PepC, PepI, and PepQ were clearly detectable 40 min after addition of nisin (3 ng/ml), and their intensities increased with time after induction. Cell extracts of UKLc11-I, in addition, were used to determine the specific activity of PepI by assaying the hydrolysis of the chromogenic substrate Pro-p-nitroanilide (Fig. 3B). PepI activity was found to increase with an average rate of 128 U/min during the first hour after inductuion with 1 ng of nisin per ml. Increased activities of PepI, PepQ, PepG, and PepW after nisin induction of the respective UKLc11 integrants have recently also been measured by Courtin et al. (4).
FIG. 2.
Induction of PnisA::pep fusions. Strains UKLc11-C (PepC), UKLc11-I (PepI), and UKLc11-Q (PepQ), carrying the nisRK genes integrated into the chromosomal leuB locus and the respective PnisA::pep fusions integrated into the sex factor, were grown in GM17. At an OD600 of 0.5 U, pepI expression was induced by adding nisin to a final concentration of 3 ng/ml. Cell extracts prepared from culture aliquots at 0, 40, and 120 min after induction were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% polyacrylamide gels. Arrowheads indicate the expected gel positions of individual peptidases. The molecular masses of protein markers (M) (in kilodaltons) are given on the left.
FIG. 3.
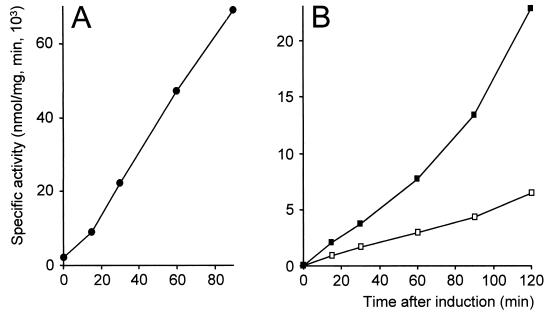
PepI activities of nisRK integrants after induction of a PnisA::pepI fusion. PnisA::pepI was expressed from a cloning vector in strain UKLc13(pUK200I) (sex factor tel::nisRK, pUK200::pepI) (•) (A) and from recombinant sex factors in strains UKLc11-I (leu::nisRK, sex factor tel::pepI) (▪) and UKLc13-I (sex factor tel::nisRK-pepI) (□) (B). Strains were grown in GM17, and at an OD600 of 0.5 U, pepI expression was induced by adding nisin to a final concentration of 1 ng/ml. Specific PepI activities were determined in cell extracts prepared from culture aliquots at various times after induction.
To estimate the value of the new gene delivery system under conditions of milk fermentation, strains UKLc11-I, UKLc11-Q, UKLc11-G, and UKLc11-W have been applied in a cheese-ripening model and in a pilot plant for the productuion of semihard cheeses. In both systems, significant effects on the overal content of free amino acids and the concentrations of distinct amino acids in the resulting fermentation products were detected after induction of PepQ, PepG, and PepW (4; W. Bockelmann, personal communication).
In addition, we demonstrated conjugational transfer of the engineered sex factor to another strain of L. lactis. Strain UKLc11-I, carrying the PnisA::pepI fusion integrated into the sex factor, was mated with L. lactis NZ9800, which, due to the presence of Tn5276, expressed the nisin signal transduction genes nisR and nisK. Transconjugants were detected on nisin-containing agar plates by use of a chromogenic PepI substrate. They occurred at a frequency of 4 × 10−4 per CFU of the streptomycin-resistant recipient. This indicated that the conjugational capability of the lactococcal sex factor was not disturbed by the insertion of foreign genes into telB and that derivatives of the sex factor, engineered at this site, are suited for the transfer of desired functions to other L. lactis strains.
Simultaneous delivery of nisRK and PnisA fusions to the sex factor.
For applications where desired genes, under the transcriptional control of PnisA, should be transferable to and inducible in any strains of L. lactis, we constructed the integration vector pUK500. It is a derivative of pUK300 (described above), with nisRK integrated into the multiple-cloning site in the same orientation as the tel genes. This left 10 unique restriction sites downstream of nisRK for the insertion of the desired PnisA fusions. To ensure exclusive transcription from PnisA, we recommend cloning of the fusions in the direction opposite that of tel and nisRK. In the resulting pUK500 derivatives, nisRK and the respective PnisA fusions are prepared for simultaneous delivery to the lactococcal sex factor by two successive single crossovers through the flanking tel sequences.
The utility of pUK500 as a tool for delivering nisRK in a functional state to the sex factor was demonstrated by transformation of L. lactis MG1363(pLP712) and selection of integrants at 37°C. Integration of pUK500 into the sex factor occurred at a frequency of 5 × 10−2 per CFU, and subsequent plasmid excision was observed in 82% of the clones after 100 generations at 28°C. Of these clones, 30% had the desired nisRK insertion in the sex factor. One of them was named UKLc13(pLP712). In transformants of UKLc13(pLP712) with pUK200I (PnisA::pepI), the activity of PepI increased with a rate of 783 U/min during the first hour after addition of nisin (Fig. 3A). This showed that the nisRK genes were functionally expressed from the modified sex factor, most probably due to transcriptional readthrough from a promoter upstream of the preceding telA gene.
In a further step, the PnisA::pepI fusion was cloned downstream of nisRK in pUK500. After transformation of L. lactis MG1363(pLP712) with the resulting plasmid pUK500I and selection at 37°C, initial plasmid integration was observed at a frequency of 5 × 10−4 (per CFU). Subsequent plasmid excision occurred in 74% of the bacteria in the course of about 100 generations at 28°C. Of these clones, 40% carried the engineered sex factor as expected. One of them was called UKLc13-I(pLP712) (Fig. 4B). Induction of PnisA::pepI transcription in this strain led to a continuous increase of specific PepI activity, demonstrating that the sex factor-based system is suitable for controlled expression of PnisA fusions (Fig. 3B). The induction rate (49 U/min during the first hour after addition of nisin), however, was 2.6-fold lower than with strain UKLc11-I, which carries nisRK integrated into the chromosomal leuB locus. This may be explained by different transcription levels of the nisin signal transduction functions nisR and nisK in the two strains. Since the convergently oriented telB::nisRK and PnisA::pepI elements in UKLc13-I(pLP712) are separated by the efficient transcriptional terminator TbrnQ (Fig. 4B), countertranscription from PnisA most probably does not account for this effect.
Acknowledgments
This work was supported by the STARLAB project of the European Community (contract ERBBIO4CT960016).
We thank Oscar Kuipers and Roland Siezen (NIZO Food Research, Ede, The Netherlands) for strains and plasmids relating to the patented nisin-controlled expression (NICE) system. We are grateful to Holger Kneuper for the preparation and analysis of cell extracts. We thank Wilhelm Bockelmann (Bundesanstalt für Milchforschung, Kiel, Germany) for communicating unpublished fermentation results, Ulrike Klein for providing reliable assistance, and Joachim Schick for valuable discussions.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas, I., A. Gruss, S. D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courtin, P., M. Nardi, U. Wegmann, V. Joutsjoki, J. C. Ogier, J. C. Gripon, A. Palva, B. Henrich, and V. Monnet. 2002. Accelerating cheese proteolysis by enriching Lactococcus lactis proteolytic system with lactobacilli peptidases. Int. Dairy J. 12:447-454. [Google Scholar]
- 5.de Ruyter, P. G., O. P. Kuipers, M. M. Beerthuyzen, I. van Alen-Boerrigter, and W. M. de Vos. 1996. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J. Bacteriol. 178:3434-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vos, W. M., P. Vos, H. de Haard, and I. Boerrigter. 1989. Cloning and expression of the Lactococcus lactis subsp. cremoris SK11 gene encoding an extracellular serine proteinase. Gene 85:169-176. [DOI] [PubMed] [Google Scholar]
- 8.de Vos, W. M. 1999. Gene expression systems for lactic acid bacteria. Curr. Opin. Microbiol. 2:289-295. [DOI] [PubMed] [Google Scholar]
- 9.Dickely, F., D. Nilsson, E. B. Hansen, and E. Johansen. 1995. Isolation of Lactococcus lactis nonsense suppressors and construction of a food-grade cloning vector. Mol. Microbiol. 15:839-847. [DOI] [PubMed] [Google Scholar]
- 10.Djordjevic, G. M., and T. R. Klaenhammer. 1998. Inducible gene expression systems in Lactococcus lactis. Mol. Biotechnol. 9:127-139. [DOI] [PubMed] [Google Scholar]
- 11.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godon, J. J., M. C. Chopin, and S. D. Ehrlich. 1992. Branched-chain amino acid biosynthesis genes in Lactococcus lactis subsp. lactis. J. Bacteriol. 174:6580-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godon, J. J., C. Delorme, J. Bardowski, M. C. Chopin, S. D. Ehrlich, and P. Renault. 1993. Gene inactivation in Lactococcus lactis: branched-chain amino acid biosynthesis. J. Bacteriol. 175:4383-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godon, J. J., C. J. Pillidge, K. Jury, C. A. Shearman, and M. J. Gasson. 1995. Molecular analysis of the Lactococcus lactis sex factor. Dev. Biol. Stand. 85:423-430. [PubMed] [Google Scholar]
- 16.Godon, J. J., C. J. Pillidge, K. Jury, and M. J. Gasson. 1996. Caractérisation d'un élément conjugatif original: le facteur sexuel de Lactococcus lactis 712. Lait 76:41-49. [Google Scholar]
- 17.Kleerebezem, M., M. M. Beerthuyzen, E. E. Vaughan, W. M. de Vos, and O. P. Kuipers. 1997. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl. Environ. Microbiol. 63:4581-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein, J. R., B. Henrich, and R. Plapp. 1994. Cloning and nucleotide sequence analysis of the Lactobacillus delbrueckii ssp. lactis DSM 7290 cysteine aminopeptidase gene pepC. FEMS Microbiol. Lett. 124:291-300. [DOI] [PubMed] [Google Scholar]
- 19.Klein, J. R., U. Schmidt, and R. Plapp. 1994. Cloning, heterologous expression, and sequencing of a novel proline iminopeptidase gene, pepI, from Lactobacillus delbrueckii subsp. lactis DSM 7290. Microbiology 140:1133-1139. [DOI] [PubMed] [Google Scholar]
- 20.Klein, J. R., C. Ulrich, U. Wegmann, E. Meyer-Barton, R. Plapp, and B. Henrich. 1995. Molecular tools for the genetic modification of dairy lactobacilli. Syst. Appl. Microbiol. 18:493-503. [Google Scholar]
- 21.Kuipers, O. P., M. M. Beerthuyzen, R. J. Siezen, and W. M. de Vos. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216:281-291. [DOI] [PubMed] [Google Scholar]
- 22.Leenhouts, K., G. Buist, A. Bolhuis, A. ten Berge, J. Kiel, I. Mierau, M. Dabrowska, G. Venema, and J. Kok. 1996. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol. Gen. Genet. 253:217-224. [DOI] [PubMed] [Google Scholar]
- 23.Maguin, E., H. Prevost, S. D. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, C. G., and K. Mackinnon. 1974. Peptidase mutants of Salmonella typhimurium. J. Bacteriol. 120:355-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platteeuw, C., I. van Alen-Boerrigter, S. van Schalkwijk, and W. M. de Vos. 1996. Food-grade cloning and expression system for Lactococcus lactis. Appl. Environ. Microbiol. 62:1008-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Wegmann, U., J. R. Klein, I. Drumm, O. P. Kuipers, and B. Henrich. 1999. Introduction of peptidase genes from Lactobacillus delbrueckii subsp. lactis into Lactococcus lactis and controlled expression. Appl. Environ. Microbiol. 65:4729-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells, J. M., P. W. Wilson, and R. W. Le Page. 1993. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Bacteriol. 74:629-636. [DOI] [PubMed] [Google Scholar]
- 29.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]