Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) is a chloride channel that is expressed in many epithelia and in the heart. Phosphorylation of CFTR by protein kinases is thought to be an absolute prerequisite for the opening of CFTR channels. In addition, nucleoside triphosphates were shown to regulate the opening of phosphorylated CFTR. Here, we report that phosphatidylinositol 4,5-bisphosphate (PIP2) activates human CFTR, resulting in ATP responsiveness of PIP2-treated CFTR. PIP2 alone is not sufficient to open CFTR, but ATP opens nonphosphorylated CFTR after application of PIP2. The effect of PIP2 is independent of protein kinases, as PIP2 activates CFTR in the complete absence of Mg. Phosphatidylinositol and phosphatidylinositol monophosphate activate CFTR less efficiently than PIP2. PIP2 application to phosphorylated CFTR may inhibit the CFTR chloride current. We suggest that regulation of CFTR by PIP2 is a previously unrecognized, alternative mechanism to control chloride conductance.
Introduction
The cystic fibrosis transmembrane conductance regulator (CFTR), the gene defective in the autosomal recessive disease cystic fibrosis (CF), was first cloned and sequenced in 1989 (Riordan et al, 1989). CFTR is expressed in the heart (Nagel et al, 1992) and in different exocrine glands, for example, the pancreas, airways, gastrointestinal tract and sweat glands (Riordan, 1993; Quinton, 1999; Sheppard & Welsh, 1999). Therefore, abnormalities in CFTR interfere with vital exocrine and absorptive functions in CF patients (Quinton, 1999; Sheppard & Welsh, 1999). Although its amino-acid sequence placed it into the family of ABC transporters (Riordan et al, 1989; Hyde et al, 1990), most of which mediate active transport across membranes, CFTR functions as an anion channel enabling the passage of chloride or other anions along their electrochemical gradient. CFTR is subject to complex regulation that requires interaction with nucleoside triphosphates and phosphorylation by protein kinases (Anderson et al, 1991; Nagel et al, 1992; Riordan, 1993; Hwang et al, 1994; Gadsby et al, 1995; Gadsby & Nairn, 1999; Nagel, 1999; Sheppard & Welsh, 1999).
CFTR contains multiple sites of phosphorylation by cyclic AMP (cAMP)-dependent protein kinase (PKA) and protein kinase C (PKC) (Riordan et al, 1989; Riordan, 1993; Gadsby & Nairn, 1999). It was also shown that the membrane-associated cGMP-dependent protein kinase isoform II (French et al, 1995; Szellas & Nagel, 2003) and tyrosine kinases (reviewed by Gadsby & Nairn, 1999) are able to phosphorylate CFTR.
Recently it was reported that in human sweat gland ducts, endogenous CFTR is activated by heterotrimeric G proteins via a cAMP-independent pathway (Reddy & Quinton, 2001). This G- protein-mediated activation of CFTR is dependent on hydrolysable nucleoside triphosphates and Mg, indicating that phosphorylation is involved in the activating process although it is independent of the cAMP cascade.
The question arose whether alternative pathways exist to activate CFTR, apart from protein phosphorylation. In this study, we tested the effect of phosphatidylinositol 4,5-bisphosphate (PIP2) on excised inside-out plasma membrane patches of Xenopus laevis oocytes, heterologously expressing human CFTR. Previously it was shown that PIP2 plays an important role in the regulation of ion channels and transporters (Hilgemann et al, 2001; Hardie, 2003). For example, Na+/Ca+ exchangers (Hilgemann & Ball, 1996), all inwardly rectifying potassium channels (Huang et al, 1998) or the epithelial sodium channel (ENaC) (Yue et al, 2002), are reported to be activated by direct interaction with PIP2. Unpublished experiments indicated that CFTR chloride channels were insensitive to PIP2 (Hilgemann et al, 2001). Here we report a robust activation of nonphosphorylated CFTR by PIP2, whereas phosphorylated CFTR may be inhibited by PIP2.
Results
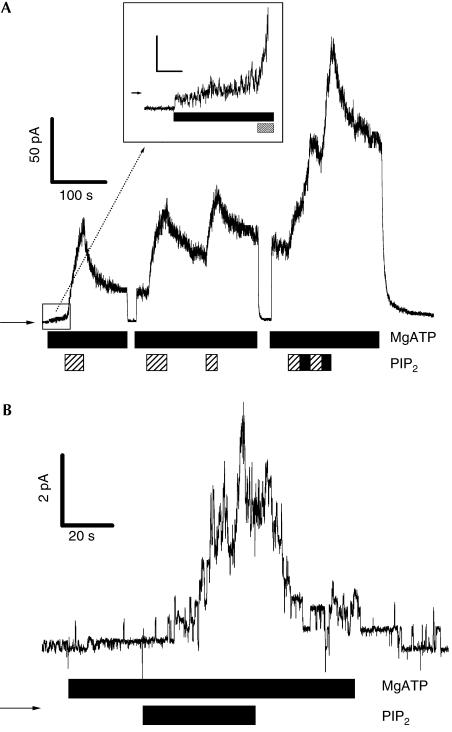
Basal cAMP concentration, and therefore PKA activity in Xenopus oocytes, is normally low so that CFTR response to MgATP is marginal when excised from an oocyte (Weinreich et al, 1997). Figure 1A shows a very small effect of MgATP on membrane current in an excised inside-out giant membrane patch from an oocyte expressing human CFTR. This small CFTR activity might be due to the effects of basal [cAMP] and the resulting low kinase activity of the native intact oocyte before excising the membrane patch (Chan et al, 2000), but see alternative explanation below. Subsequent application of 1 μM PIP2, however, activates a large chloride current (Fig 1A). On the basis of voltage pulses, it was proved that the reversal potential (−85±4 mV, n=4) of the PIP2-induced current is that of a chloride current as it is comparable to the value calculated for the employed [Cl−] gradient. This PIP2-induced chloride current is not observed in control oocytes that are not expressing CFTR (n=4; data not shown). A further argument for the CFTR specificity of the PIP2 effect is the CFTR-typical conductance (5.3±0.7 pS with [Cl−] gradient at 20–80 mV, n=8, for PIP2-activated channels, compared to 5.2±0.3 pS, n=9, for PKA-activated channels) and the ATP dependence of PIP2-activated single channels (Fig 1B). Therefore, the PIP2-induced chloride conductance is attributed to activation of CFTR, but not of other Cl− channels, for example, the oocyte-endogenous Ca2+-activated chloride channel (Takahashi et al, 1987). Our repeated observation that prolonged ATP application to nonphosphorylated and not PIP2-treated CFTR may lead to an increase of the small CFTR current, as shown in the inset of Fig 1A, may be explained by an ATP-induced increase of PIP2. Such a generation of PIP2 from endogenous precursors via membrane-bound lipid kinases was previously described for excised membrane patches from heart cells (Hilgemann & Ball, 1996). The CFTR current observed before PIP2 application (ATP-induced current increase in Fig 1A), which may be ascribed to basal oocyte-endogenous PKA activity, may therefore alternatively be caused, at least partially, by an endogenous small concentration of PIP2 in the oocyte membrane.
Figure 1.
PIP2 activates chloride current by unphosphorylated CFTR. (A) Giant patch experiment. Several PIP2 additions activate CFTR, followed by the decrease of the signal in consequence of PIP2 washout. Bars indicate changes of the bath solution. PIP2=1 μM (hatched bar) or 10 μM (black bar), MgATP=500 μM, membrane potential (Vm)=0 mV. The arrow identifies zero current. Inset: Magnification of the beginning of the experiment showing increase of chloride current by prolonged MgATP application. Scale bars: 5 pA/10 s. 20 Hz filtered. (B) Single-channel experiment demonstrating activation of 9–11 channels by PIP2 addition, followed by decay after PIP2 washout and complete closing after ATP removal. PIP2=1 μM, MgATP=500 μM, Vm=50 mV.
The observed increase of the chloride current on addition of PIP2 to CFTR-containing membrane patches depends on PIP2 concentration. At lower concentrations (1 μM, n=7) in the bath solution, PIP2 addition exclusively led to a current increase during application (Fig 1); however, at 10 μM PIP2 (n=15) an exclusively increasing chloride current was observed in 73% of patches, and in the residual 27% a chloride current increase was followed by a decrease during PIP2 application. This antagonistic effect of PIP2 on CFTR is noticeably different from the purely stimulating effect of PKA on CFTR or from the effect of PIP2 on KATP channels (Baukrowitz et al, 1998), where it was shown that PIP2 application slowly antagonized the inhibition of KATP channels by ATP.
In accordance with the membrane solubility of PIP2, its effect on CFTR was not completely reversible (Fig 1). The effect of removing PIP2 from the bath solution was variable and was dependent on PIP2 concentration. When removing 10 μM PIP2 we observed in 6 of 15 experiments a still continuing increase of chloride channel activity as long as ATP was present. Alternatively, removal of 10 μM PIP2 from the bath solution could also lead to a decrease (40%) or no change in the chloride current (20%).
In contrast, on removing 1 μM PIP2 from the bath, a decay of the chloride current was observed in 100% of patches (Fig 1). This ‘rundown' after PIP2 application appeared similar to the rundown after PKA withdrawal, but is probably caused by decreasing membrane concentration of PIP2, for example, by dilution or because of enzymatic digestion.
As expected from the known ATP dependence (Weinreich et al, 1999), MgATP withdrawal from phosphorylated CFTR chloride channels yielded a current decay with time constants of about τ1=2.9±0.2 s and τ2=24±2.5 s (n=6). The time constants of ATP withdrawal-induced channel closing were faster (τ1=0.8±0.3 s, τ2=9±1.7 s, n=2) for CFTR activated by 1 μM PIP2 than for CFTR activated by 10 μM PIP2 in the bath solution (τ1=2.3±0.4 s, τ2=20±1.9 s, n=7), similar to the previously described dependence of the closing rates on CFTR phosphorylation and rundown (Weinreich et al, 1999).
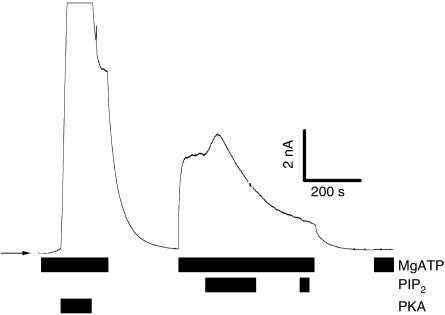
Next we investigated the effect of 10 μM PIP2 on phosphorylated CFTR. Compared to unphosphorylated CFTR, where application of 10 μM PIP2 led to activation of the chloride current in 100% of the patches, the addition of 10 μM PIP2 to PKA-activated CFTR resulted in variable effects. A further activation of the chloride current was observed in only 18% of the experiments (n=17). The stimulating effect was relatively small (maximally +23%), due to already strong activation by PKA. In 47% of the patches an inhibition of PKA-activated current (Fig 2) and in 35% no apparent change of the PKA-activated chloride current (data not shown) was observed.
Figure 2.
Inhibitory effect of PIP2 on phosphorylated CFTR. Bars indicate the bath solution. PIP2=10 μM, MgATP=500 μM. PKA (100 U/ml) addition activates a large CFTR chloride current. This current is first increased by application of PIP2, directly followed by a strong decrease.
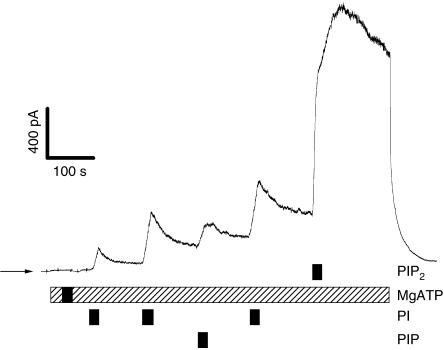
To test the influence of the number of free phosphate groups at the inositol ring, we added to the patch either phosphatidylinositol (PI), which has no phosphate residue, or phosphatidylinositol monophosphate (PIP) with one phosphate group at position 4, or PIP2 with two phosphate groups at positions 4 and 5 of the inositol ring. Fig 3 shows that already PI had a clear stimulating effect on unphosphorylated CFTR, PIP was similar to PI, and PIP2 yielded the strongest stimulation of CFTR chloride current.
Figure 3.
Comparison of the stimulating effects of PI, PIP and PIP2 on unphosphorylated CFTR. Bars indicate bath solution. MgATP=500 μM (hatched bar) or 5 mM (black bar).
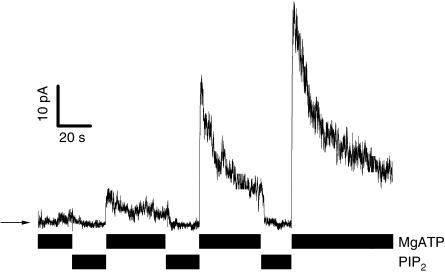
An obvious question is whether PIP2 alone can open unphosphorylated CFTR, that is, in the absence of nucleoside triphosphates. Application of 1 or 10 μM PIP2 alone, however, resulted in no change of electrical current (Fig 4). If ATP was applied after the application of 1 μM PIP2, CFTR opened very fast (Fig 4). Channel activity, which increased 9.2 (±2)-fold, was then followed by a decrease of about 75±4% (n=6) (Fig 4), as described above for PIP2 removal from the bath. Similar to CFTR activated by protein kinases, CFTR activated by PIP2 also needs ATP to open. An initial application of PIP2 in the absence of ATP, however, is sufficient to allow ATP to open CFTR chloride channels rapidly (Fig 4). This result already suggests that no protein phosphorylation is involved in PIP2-mediated activation of CFTR, but it does not yet unambiguously rule out this possibility. We addressed this question by applying PIP2 in the complete absence of Mg2+ or other divalent cations, that is, conditions that are known to abolish any kinase activity. Alternatively, we tested the effect of PIP2 on CFTR in the absence of ATP by replacing it with the nonhydrolysable ATP analogue adenylyl imido diphosphate (AMP-PNP).
Figure 4.
ATP is needed to open PIP2-treated CFTR channels. Bars indicate changes of the bath solution. PIP2=1 μM, MgATP =500 μM.
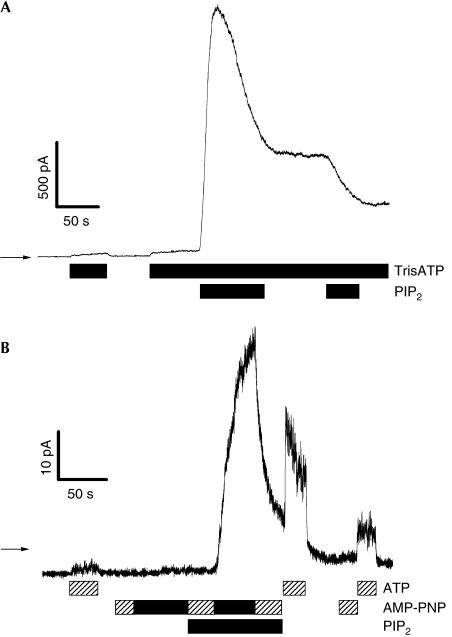
Although Mg-free conditions sometimes cause problems with the baseline stability of the patch current, we could unambiguously demonstrate that PIP2 application in the presence of ATP (0 Mg) always leads to an increase of CFTR chloride current (n=11), with both 1 and 10 μM PIP2 (Fig 5A). In most cases, the increase of CFTR-mediated chloride current is directly followed by a decrease. This antagonistic effect of PIP2 on CFTR is demonstrated in Fig 5A by a second treatment of the patch with PIP2. The activation of CFTR by PIP2 in the absence of Mg strongly argues against a PIP2-mediated protein phosphorylation of CFTR. Although we previously concluded, from experiments with 500 μM AMP-PNP, that phosphorylated CFTR may not be opened by application of the nonhydrolysable ATP analogue AMP-PNP (Nagel et al, 1992; Weinreich et al, 1999), it was recently demonstrated that AMP-PNP may open phosphorylated CFTR when used at high concentrations (5 mM) (Aleksandrov et al, 2000). We recently confirmed this observation for PKA-activated human CFTR in giant patches of oocyte membrane (data not shown). Fig 5B shows that CFTR in PIP2-treated membrane patches does not open on application of 500 μM AMP-PNP but rapidly opens on application of 5 mM AMP-PNP, in the complete absence of ATP, whereas 5 mM AMP-PNP has no effect on CFTR before PIP2 application. This result is further proof that no protein phosphorylation takes place during PIP2 activation of CFTR.
Figure 5.
PIP2 activates CFTR without protein phosphorylation. (A) CFTR activation in the absence of Mg. Bars indicate bath solution. TrisATP=5 mM. (B) PIP2 activates CFTR in the absence of ATP. Bars indicate bath solution. PIP2=1 μM, MgATP=500 μM, AMP-PNP=500 μM (hatched bar) or 5 mM (black bar). Before PIP2 application, neither ATP nor AMP-PNP is able to open CFTR. AMP-PNP at 5 mM, but not 500 μM, opens PIP2-activated unphosphorylated CFTR.
Discussion
Interaction between PIP2 and ion channels or transporters was described previously in several reports and reviews (Hilgemann et al, 2001). With this report, we add another ion channel to the growing list of membrane proteins regulated by phosphoinositides. For several ion-translocating membrane proteins, a direct interaction with PIP2 was demonstrated. All inward rectifying K+ channels, the Na+/Ca2+ exchanger and the epithelial sodium channel directly interact with PIP2. In Kir6.2 channels, a conserved carboxy-terminal region was identified as interacting with cell membranes. From systematic mutagenesis, it was proposed that a lipid interaction domain could be identified that shows structurally significant features of phospholipid interacting pleckstrin homology (PH) domains (Cukras et al, 2002b). Positively charged amino acids in the amino terminus (N-terminus) of Kir6.2 were also shown to affect regulation by ATP and PIP2 (Cukras et al, 2002a). A conserved region with some homology to PH domains is also present within the N-terminus of CFTR. Interestingly, Kirk and co-workers found that the N-terminal tail and especially four conserved acidic amino acids play an important role in the positive regulation of CFTR activity by directly binding to the R-domain (Naren et al, 1999) or with NBD2 (Fu et al, 2001).
Before trying to pinpoint the region on CFTR that is interacting with PIP2, it is necessary to exclude the possibility that PIP2 mediates its effect indirectly via a protein kinase. Two lines of evidence support a direct CFTR–PIP2 interaction: treatment of membrane patches with PIP2 in the complete absence of ATP did not open CFTR but resulted in an activation of CFTR as subsequently demonstrated by fast ATP-induced opening of CFTR. As the time constants were at least as fast as those measured for ATP-induced opening of phosphorylated CFTR (Weinreich et al, 1999), it seems unlikely that ATP addition first triggered a PIP2-mediated phosphorylation and then the opening of CFTR. Even more compelling are the results with PIP2 and ATP in the complete absence of divalent cations. Because hydrolytic enzymes require Mg2+ or other divalent cations, phosphorylation by kinases is abolished in their absence (Schultz et al, 1996). For CFTR, we show stimulating effects of PIP2 even under Mg2+-free conditions, indicating that activation of CFTR by PIP2 cannot be attributed to protein phosphorylation, for example, by a membrane-bound PIP2-dependent kinase. The fact that PIP2 alone has no effect on the patch current, but needs application of ATP to induce a chloride current, is additional evidence for the CFTR specificity of the PIP2 effect. This argument is strengthened by the CFTR-typical gating and single-channel conductance observed in PIP2-treated CFTR-expressing membrane patches.
Until now, CFTR channel activity under physiological conditions was thought to have an absolute requirement for protein phosphorylation and nucleoside triphosphates. However, a recent report showing dATP-induced activation of carefully controlled, nonphosphorylated CFTR already indicated that protein phosphorylation may not be an absolute requirement to open CFTR chloride channels (Aleksandrov et al, 2002). Even more surprising is the recent demonstration that human CFTR, endogenously expressed in the sweat duct, may conduct chloride ions simply by the addition of cytoplasmic glutamate, in a phosphorylation- and ATP-independent manner (Reddy & Quinton, 2003).
Our studies indicate that also under virtually physiological conditions CFTR may be activated by a pathway that does not involve protein phosphorylation. The addition of PIP2 to the ATP-containing bath solution activated a strong CFTR chloride conductance, smaller but still similar to activation by PKA (18% of PKA-activated conductance with 10 μM PIP2, n=6, and 7% of PKA-activated conductance with 1 μM PIP2, n=8; data not shown). In contrast to activation of CFTR by glutamate (Reddy & Quinton, 2003), activation of CFTR by PIP2 still needs ATP to open the CFTR chloride channel. As we found no effect of glutamate on CFTR in excised membrane patches, with or without ATP (n=6; data not shown), we conclude that glutamate does not activate CFTR directly. We suggest a possible involvement of phosphatidylinositols in transmitting the signal from glutamate receptors to CFTR in the sweat duct, even if this seems in contrast to the reported ATP independence of activation by glutamate (Reddy & Quinton, 2003).
Already the reported cAMP-independent activation of CFTR by G proteins in the sweat duct (Reddy & Quinton, 2001) provided evidence for the involvement of alternative kinase phosphorylation in CFTR activation. Although this elegant study could not pinpoint which kinase led to activation of CFTR, it seemed most probable to presume a protein kinase. From our results we suggest the possibility that activation of G proteins in the sweat duct stimulates one or several lipid kinase(s). A kinase-induced increase of PIP2 in the membrane should then activate CFTR. Alternatively, our results would also predict activation of CFTR if PIP2 content of the membrane increases by inhibition of PIP2 degradation.
We suggest that there is a previously unrecognized alternative way to regulate CFTR by membrane components, such as phosphatidylinositides. We speculate that the recently reported cAMP-independent activation of CFTR in sweat gland ducts (Reddy & Quinton, 2001) is due to G-protein-mediated production of PIP2 via lipid kinase(s) and binding of PIP2 to unphosphorylated CFTR.
Methods
Materials. Egtazic acid (EGTA), ethylenediaminetetraacetic acid (EDTA), MgCl2, D,L-aspartic acid, MgATP, TrisATP, L-glutamic acid, PI, PIP, Tricain and collagenase IA were obtained from Sigma (Deisenhofen, Germany). N-methyl-D-glucamine, HCl and CaCl2 were obtained from Merck (Darmstadt, Germany). HEPES was purchased from AppliChem (Darmstadt, Germany). AMP-PNP and PIP2 were obtained from Sigma (Deisenhofen, Germany) or Roche (Mannheim, Germany). PKA catalytic subunit was obtained from Promega (Wisconsin, USA). Oocytes and human CFTR cRNA were prepared as described before (Weinreich et al, 1997). The oocytes were incubated for 3–4 days at 16–18°C in modified Ringer's solution containing 100 μg/ml gentamycin.
Solutions. A holding potential of 0 mV (50 mV for single-channel experiments) and a [Cl−] gradient of 4/156 mM (bath/pipette) was applied as previously reported by Nagel et al (1992). The Mg2+-containing bath solution was composed of 5 mM EGTA, 10 mM HEPES, 2 mM MgCl2 and 160 mM NMG, and was titrated to pH 7.4 with D,L-aspartic acid. The Mg2+-free bath solution was composed of 2 mM EDTA, 5 mM EGTA, 4 mM HCl, 10 mM HEPES and 160 mM NMG, and was titrated to pH 7.4 with D,L-aspartic acid. The pipette solution was composed of 10 mM HEPES, 150 mM NMG and 2 mM MgCl2, and was adjusted to pH 7.4 with HCl. PI was obtained as a solution (10 mg/ml in chloroform); PIP or PIP2 was dissolved in a mixture containing chloroform, methanol, water and 1 N HCl in a ratio of 20:10:1:1 or 20:9:1:0.1, respectively, to a final concentration of 10 mM. The phosphoinositides were dried under argon or nitrogen, and then sonicated or vortexed in bath solution directly before use.
Patch clamp experiments. Giant patch experiments were performed as described (Hilgemann & Lu, 1998; Weinreich et al, 1999). All the experiments were performed at room temperature (20–22°C) with a standard holding potential of 0 mV for giant patch and 50 mV for single-channel experiments. Short test pulses in the range of −90 to +30 mV, or +20 to +80 mV for single-channel experiments, were performed to obtain the I–V relationship. The electrical measurements were performed with an Axopatch 200B (Axon instruments) or a Patch Clamp L/M EPC7 amplifier (List-Medical, Darmstadt, Germany). Clampfit 8.0 and Microcal Origin 6.1 were used to perform data analysis. To reduce data points for the analysis of giant patch experiments, only every tenth point was used for the figures.
Acknowledgments
We thank D. Ollig for expert technical assistance and E. Bamberg for continuous support. We are grateful to J.R. Riordan and A. Aleksandrov for helpful discussions. This work was supported by the German Research Foundation (DFG) and the Max-Planck-Society.
References
- Aleksandrov AA, Chang X, Aleksandrov L, Riordan JR (2000) The non-hydrolytic pathway of cystic fibrosis transmembrane conductance regulator ion channel gating. J Physiol 528: 259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrov AA, Aleksandrov L, Riordan JR (2002) Nucleoside triphosphate pentose ring impact on CFTR gating and hydrolysis. FEBS Lett 518: 183–188 [DOI] [PubMed] [Google Scholar]
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ (1991) Nucleoside triphosphates are required to open the CFTR chloride channel. Cell 67: 775–784 [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B (1998) PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science 282: 1141–1144 [DOI] [PubMed] [Google Scholar]
- Chan KW, Csanady L, Seto-Young D, Nairn AC, Gadsby DC (2000) Severed molecules functionally define the boundaries of the cystic fibrosis transmembrane conductance regulator's NH(2)-terminal nucleotide binding domain. J Gen Physiol 116: 163–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukras CA, Jeliazkova I, Nichols CG (2002a) The role of NH2-terminal positive charges in the activity of inward rectifier KATP channels. J Gen Physiol 120: 437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukras CA, Jeliazkova I, Nichols CG (2002b) Structural and functional determinants of conserved lipid interaction domains of inward rectifying Kir6.2 channels. J Gen Physiol 119: 581–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French PJ, Bijman J, Edixhoven M, Vaandrager AB, Scholte BJ, Lohmann SM, Nairn AC, de Jonge HR (1995) Isotype-specific activation of cystic fibrosis transmembrane conductance regulator-chloride channels by cGMP-dependent protein kinase II. J Biol Chem 270: 26626–26631 [DOI] [PubMed] [Google Scholar]
- Fu J, Ji HL, Naren AP, Kirk KL (2001) A cluster of negative charges at the amino terminal tail of CFTR regulates ATP-dependent channel gating. J Physiol 536: 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby DC, Nairn AC (1999) Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev 79: S77–S107 [DOI] [PubMed] [Google Scholar]
- Gadsby DC, Nagel G, Hwang TC (1995) The CFTR chloride channel of mammalian heart. Annu Rev Physiol 57: 387–416 [DOI] [PubMed] [Google Scholar]
- Hardie RC (2003) Regulation of trp channels via lipid second messengers. Annu Rev Physiol 65: 735–759 [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Ball R (1996) Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science 273: 956–959 [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Lu CC (1998) Giant membrane patches: improvements and applications. Methods Enzymol 293: 267–280 [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C (2001) The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE 2001: RE19. [DOI] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW (1998) Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature 391: 803–806 [DOI] [PubMed] [Google Scholar]
- Hwang TC, Nagel G, Nairn AC, Gadsby DC (1994) Regulation of the gating of cystic fibrosis transmembrane conductance regulator C1 channels by phosphorylation and ATP hydrolysis. Proc Natl Acad Sci USA 91: 4698–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, Gallagher MP, Gill DR, Hubbard RE, Higgins CF (1990) Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature 346: 362–365 [DOI] [PubMed] [Google Scholar]
- Nagel G (1999) Differential function of the two nucleotide binding domains on cystic fibrosis transmembrane conductance regulator. Biochim Biophys Acta 1461: 263–274 [DOI] [PubMed] [Google Scholar]
- Nagel G, Hwang TC, Nastiuk KL, Nairn AC, Gadsby DC (1992) The protein kinase A-regulated cardiac Cl− channel resembles the cystic fibrosis transmembrane conductance regulator. Nature 360: 81–84 [DOI] [PubMed] [Google Scholar]
- Naren AP, Cormet-Boyaka E, Fu J, Villain M, Blalock JE, Quick MW, Kirk KL (1999) CFTR chloride channel regulation by an interdomain interaction. Science 286: 544–548 [DOI] [PubMed] [Google Scholar]
- Quinton PM (1999) Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev 79: S3–S22 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM (2001) cAMP-independent phosphorylation activation of CFTR by G proteins in native human sweat duct. Am J Physiol Cell Physiol 280: C604–C613 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM (2003) Control of dynamic CFTR selectivity by glutamate and ATP in epithelial cells. Nature 423: 756–760 [DOI] [PubMed] [Google Scholar]
- Riordan JR (1993) The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol 55: 609–630 [DOI] [PubMed] [Google Scholar]
- Riordan JR et al. (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073 [DOI] [PubMed] [Google Scholar]
- Schultz BD, Bridges RJ, Frizzell RA (1996) Lack of conventional ATPase properties in CFTR chloride channel gating. J Membr Biol 151: 63–75 [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ (1999) Structure and function of the CFTR chloride channel. Physiol Rev 79: S23–S45 [DOI] [PubMed] [Google Scholar]
- Szellas T, Nagel G (2003) Apparent affinity of CFTR for ATP is increased by continuous kinase activity. FEBS Lett 535: 141–146 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Neher E, Sakmann B (1987) Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci USA 84: 5063–5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich F, Wood PG, Riordan JR, Nagel G (1997) Direct action of genistein on CFTR. Pflugers Arch 434: 484–491 [DOI] [PubMed] [Google Scholar]
- Weinreich F, Riordan JR, Nagel G (1999) Dual effects of ADP and adenylylimidodiphosphate on CFTR channel kinetics show binding to two different nucleotide binding sites. J Gen Physiol 114: 55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue G, Malik B, Eaton DC (2002) Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969 [DOI] [PubMed] [Google Scholar]