Abstract
The protein kinase CK1 phosphorylates serine residues that are located close to another phosphoserine in the consensus pSer-Xaa-Xaa-Ser. This specificity generates regions in its target proteins containing two or more neighbouring phosphoserine residues, termed here multisite phosphorylation domains (MPDs). In this paper, we demonstrate that D4476 is a potent and rather selective inhibitor of CK1 in vitro and in cells. In H4IIE hepatoma cells, D4476 specifically inhibits the phosphorylation of endogenous forkhead box transcription factor O1a (FOXO1a) on Ser322 and Ser325 within its MPD, without affecting the phosphorylation of other sites. Our results indicate that these residues are targeted by CK1 in vivo and that the CK1-mediated phosphorylation of the MPD is required for accelerated nuclear exclusion of FOXO1a in response to IGF-1 and insulin. D4476 is much more potent and specific than IC261 or CKI-7, and is therefore the most useful CK1 inhibitor currently available for identifying physiological substrates of CK1.
Introduction
CK1 (formerly known as casein kinase 1) comprises a family of constitutively active serine/threonine protein kinases (Gross & Anderson, 1998). An interesting property of CK1, which it shares with glycogen synthase kinase-3 (GSK3), is its requirement for a ‘priming' phosphate in many of its substrates, which is introduced by a ‘priming' kinase. Thus, CK1 phosphorylates the serine residue (underlined) in the motif pSer-Xaa-Xaa-Ser (Flotow et al, 1990), where pSer is phosphoserine, while GSK3 recognizes the motif Ser/Thr-Xaa-Xaa-Xaa-pSer (Fiol et al, 1987). Consequently, phosphorylation catalysed by CK1 or GSK3 creates domains containing two or more neighbouring phosphorylated residues, termed here multisite phosphorylation domains (MPDs). The first MPDs involving CK1 and GSK3 were identified in critical regions required for the inactivation of glycogen synthase (Poulter et al, 1988; Nakielny et al, 1991).
Recently, MPDs have been found in other mammalian proteins, and common roles are beginning to emerge. For example, the MPDs on β-catenin and inhibitor of κB (IκB) bind an E3 ubiquitin ligase that promotes ubiquitylation and degradation of the protein (Karin & Ben-Neriah, 2000). In contrast, nuclear exclusion, a common mechanism for transcription factor inactivation, is regulated by MPDs in forkhead box transcription factor O1a (FOXO1a; formerly FKHR) (Rena et al, 2002), PER isoforms (Vielhaber et al, 2000), NF-AT isoforms (Beals et al, 1997) and SMADs (Kretzschmar et al, 1997), a function that is conserved by the MPD of STAT-A (signal transducer and activator of transcription-A) in Dictyostelium discoideum (Ginger et al, 2000).
We have been studying the MPD on FOXO1a (Rena et al, 2002; Woods & Rena, 2002), a member of the FOXO subfamily of transcription factors that also include FOXO3a (formerly FKHRL1) (Brunet et al, 1999) and FOXO4 (formerly AFX) (Kops et al, 1999). The MPD in FOXO1a contains at least four closely spaced phosphorylated residues: Ser319, Ser322, Ser325 and Ser329 (Woods et al, 2001; Rena et al, 2002). Ser329, which is phosphorylated specifically in vitro by the dual-specificity tyrosine phosphorylated and regulated kinase (DYRK), is highly phosphorylated in unstimulated cells and phosphorylation is unaffected by stimulation with any agonists tested so far (Woods et al, 2001). In contrast, phosphorylation of the rest of the MPD requires the initial phosphorylation of Ser319, triggered by acute stimulation with agonists such as insulin-like growth factor-1 (IGF-1) and insulin. Phosphorylation of Ser319 in vivo requires the activity of phosphatidylinositol 3-kinase and 3-phosphoinositide-dependent protein kinase-1, and occurs by a protein kinase B (PKB) and/or serum and glucocorticoid induced kinase (SGK)-catalysed mechanism (Rena et al, 1999, 2002; Brunet et al, 2001). The phosphorylation of Ser319 is followed by the phosphorylation of Ser322 and then Ser325, accelerating the rate of nuclear exclusion in live cells (Rena et al, 2002). In vitro, the phosphorylation of Ser319 by PKB allows CK1 to phosphorylate Ser322 and Ser325 sequentially, but whether CK1 phosphorylates Ser322 and Ser325 in cells has not yet been established.
Here we identify D4476 (4-[4-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-5-pyridin-2-yl-1H-imidazol-2-yl]benzamide) as a novel and relatively specific inhibitor of CK1 in vitro, which inhibits the phosphorylation of FOXO1a at Ser322 and Ser325 in cells, but not the phosphorylation of Thr24, Ser256, Ser319 or Ser329. D4476 also blocks accelerated nuclear exclusion of FOXO1a, consistent with our previous data (Rena et al, 2002). Together, our results indicate that CK1 phosphorylates the MPD of FOXO1a at Ser322 and Ser325 in cells, one function of which is to accelerate the nuclear exclusion of FOXO1a. D4476 is the most useful CK1 inhibitor available and should facilitate the identification of its physiological substrates.
Results and Discussion
Structure and specificity of D4476
D4476 (Fig 1A) is a member of a compound class originally identified as inhibitors of activin receptor-like kinase (ALK) 5, a member of the family of type-I TGFβ receptors (Callahan et al, 2002). We identified D4476 as a CK1 inhibitor during routine profiling of this compound against a large panel of protein kinases. At a concentration of 10 μM, D4476 inhibited CK1δ by more than 90%, but had almost no effect on the other protein kinases tested, apart from SAPK2a/p38 and ALK5 (Table 1). CK1δ assayed at 0.1 mM ATP using a phosphorylated peptide TFRPRTSpSNASTIS (where pS is phosphoserine) corresponding to residues 312–325 of FOXO1a was inhibited with an IC50 value of 0.3 μM (Fig 1B). A similar value was obtained with the standard synthetic peptide substrate for CK1 (Bain et al, 2003). CK1 from Schizosaccharomyces pombe was inhibited with an IC50 of 0.2 μM (data not shown). However, further work will be required to ascertain whether D4476 inhibits other CK1 isoforms similarly. The IC50 value for CK1δ decreased progressively as the concentration of ATP was lowered (data not shown), indicating that D4476 is an ATP-competitive inhibitor of CK1. ALK5 was inhibited with a slightly higher IC50 value than CK1 (0.5 μM at 0.1 mM ATP), while the IC50 for p38α MAP kinase was much higher (12 μM). Importantly, from the standpoint of studying the phosphorylation of FOXO1a, D4476 did not inhibit PKB or SGK (Table 1).
Figure 1.
Structures of CK1 inhibitors and their effects on CK1 activity in vitro. (A) Structures of D4476 and IC261 (Mashhoon et al, 2000). (B) Purified CK1δ was assayed with phosphorylated peptide TFRPRTSpSNASTIS (30 μM), corresponding to the sequence surrounding residues 312–325 of FOXO1a, at an ATP concentration of 0.1 mM and varying concentrations of D4476 (filled circles), IC261 (open squares) and CKI-7 (open circles). The results are the average of at least four separate determinations, each performed in duplicate.
Table 1.
Specificity of D4476
| D4476 (10 μM) | IC261 (25 μM) | |
|---|---|---|
| Activity (% of control) | Activity (% of control) | |
| MKK1 |
99±5 |
100±1 |
| ERK2 |
107±5 |
104±2 |
| JNK1a1 |
106±3 |
93±6 |
| p38α MAPK |
56±13 |
86±6 |
| p38β2 MAPK |
75±0 |
95±6 |
| p38γ MAPK |
102±0 |
101±4 |
| p38δ MAPK |
96±7 |
99±1 |
| RSK1 |
100±0 |
n.d. |
| MAPKAP-K2 |
101±2 |
71±10 |
| MSK1 |
97±10 |
82±4 |
| PRAK |
98±8 |
91±4 |
| PKA |
108±11 |
93±8 |
| PKCα |
74±4 |
101±1 |
| PDK1 |
102±0 |
96±8 |
| PKBα |
84±9 |
85±8 |
| SGK |
104±11 |
90±4 |
| S6K1 |
112±2 |
104±0 |
| GSK3β |
91±1 |
114±3 |
| ROCK-II |
96±6 |
96±1 |
| AMPK |
100±3 |
98±13 |
| CHK1 |
107±6 |
95±4 |
| CK2 |
91±4 |
108±3 |
| PHK |
97±1 |
105±4 |
| LCK |
94±1 |
106±12 |
| CSK |
106±0 |
125±5 |
| CDK2/cyclin A |
92±2 |
91±1 |
| CK1 |
7±3 |
23±4 |
| DYRK 1A |
87±7 |
97±3 |
| ALK5 | 22±1 | n.d. |
Protein kinase activities are presented as a percentage of control incubations (mean of duplicate determinations). The expression and purification of the protein kinases used have been described (Davies et al, 2000), apart from ALK5, where the C-terminal 356 residues were expressed in Escherichia coli as a glutathione S-transferase fusion protein and purified on glutathione sepharose. Assays were carried out at 30°C as described (Davies et al, 2000; Bain et al, 2003). All protein kinases were of human origin, apart from MKK1 and PHK (rabbit), ERK2 (mouse), PKA (cow), and AMPK, CK1δ, DYRK1a, ROCK-II and RSK1 (rat). ATP was present at 0.1 mM in all assays.
In summary, D4476 is relatively specific for CK1 and ALK5 with a >20-fold selectivity over SAPK2a/p38 and a much greater selectivity over all the other protein kinases tested. Since ALK5 is inactive in cells in the absence of TGFβ in the culture medium and p38 is inactive in cells that have not been exposed to cellular stresses or proinflammatory cytokines, they should not interfere with the use of D4476 as a CK1 inhibitor in most cell-based assays.
We compared D4476 with IC261, another compound reported to inhibit CK1 without inhibiting the cyclin-dependent protein kinase cdc2, the tyrosine kinase fyn and PKA (Mashhoon et al, 2000). We found that, at 0.1 mM ATP, IC261 was also a specific inhibitor of CK1 (Table 1), but the IC50 (2.5 μM) was nearly an order of magnitude higher than for D4476. This may explain the lack of effect of this compound in cell-based assays, as mentioned later. IC261 was previously reported to inhibit several CK1 isoforms, including CK1δ, with an IC50 of 1 μM. This lower value is explained by the much lower concentration of ATP (0.01 mM) used in these studies. In our assay, the Km of CK1δ for ATP is 25 μM.
We also compared D4476 with CKI-7, which is reported to inhibit CK1 (Chijiwa et al, 1989). At 0.1 mM ATP, we found that CKI-7 inhibits SGK as potently as CK1, and several other kinases, such as ribosomal S6 kinase-1 (S6K1) and mitogen- and stress-activated protein kinase-1 (MSK1), are inhibited almost as strongly (data not shown). The IC50 of 6 μM for CKI-7 was 20-fold higher than for D4476.
D4476 inhibits the phosphorylation of FOXO1a by CK1
We showed previously that CK1 phosphorylates FOXO1a at Ser322 and Ser325 in vitro, an effect that is dependent on the PKB-mediated ‘priming' phosphorylation at Ser319. The phosphorylation of Ser322 occurs initially and primes the phosphorylation of Ser325 (Rena et al, 2002). In the present study, we first incubated FOXO1a for 30 min with PKB and MgATP to phosphorylate Thr24, Ser256 and Ser319, and then examined the phosphorylation of Ser322 and Ser325 after a further 10 min of phosphorylation with CK1 in the presence of different concentrations of D4476. D4476 effectively inhibited the phosphorylation of FOXO1a at Ser322 and Ser325, but not the PKB-catalysed phosphorylation of Thr24 (Fig 2A), Ser256 and Ser319 (data not shown). The pSer325 antibody was consistently more sensitive to D4476 than the pSer322 antibody, with inhibition occurring at 0.2 and 2 μM, respectively. This difference may be explained, at least in part, by the dependence of Ser325 phosphorylation on the prior phosphorylation of Ser322. Thus, the sensitivity of Ser325 phosphorylation to D4476 will be amplified by the inhibitory effect of D4476 on the phosphorylation of Ser322, as well as at Ser325.
Figure 2.
D4476 inhibits the phosphorylation of FOXO1a at Ser322 and Ser325, but not at Thr24 in vitro. Bacterially expressed GST-FOXO1a (1 μM) was left unphosphorylated (−) or maximally phosphorylated for 30 min at 30°C with 1 U ml−1 PKB, 10 mM magnesium acetate and 0.1 mM ATP, followed by phosphorylation for 10 min with 30 mU ml−1 CK1 (+), in the presence of the indicated concentrations of D4476. Aliquots of the reaction were spotted onto nitrocellulose and immunoblotted with phospho-specific antibodies recognizing phosphorylated Thr24 (pThr24), Ser322 (pSer322) and Ser325 (pSer325) and an antibody that recognizes the phosphorylated and unphosphorylated forms of FOXO1a equally well (FOXO1a). Similar results were obtained in several independent experiments.
Effect of D4476 on phosphorylation of FOXO1a in cells
We examined the phosphorylation of endogenous FOXO1a in the presence and absence of D4476 after immunoprecipitating the protein and probing with appropriate phospho-specific antibodies. In H4IIE cells, Ser329 is phosphorylated constitutively, while insulin triggers the phosphorylation of residues Thr24, Ser256, Ser319, Ser322 and Ser325 (Fig 3), as reported previously in 293 cells (Rena et al, 2002). In the presence of D4476, phosphorylation of both Ser322 and Ser325 is drastically reduced, but not the phosphorylation of Thr24, Ser256, Ser319 or Ser329 (Fig 3). As observed in vitro, the phospho-Ser325 antibody was consistently more sensitive to D4476 than the phospho-Ser322 antibody, with potent inhibition detected at 50 and 125 μM, respectively. The higher concentrations of D4476 required to inhibit the phosphorylation of Ser322/Ser325 in cells compared to the concentrations needed for inhibition in vitro is presumably explained by the much higher concentration of ATP in cells. Similar observations have been made with many other ATP-competitive inhibitors of protein kinases. Similar results were obtained with overexpressed FOXO1a–GFP in IGF-1-stimulated 293 cells (data not shown). In summary, these results implicate CK1 as a protein kinase that targets Ser322 and Ser325 in vivo.
Figure 3.
D4476 specifically inhibits the phosphorylation at Ser322 and Ser325 specifically in H4IIE cells. Cells were serum starved for 4 h, and then stimulated for 30 min without (−) or with (+) 20 nM insulin. Aliquots of the cell lysates (2.5 mg protein) were then immunoprecipitated with 20 μg of anti-FOXO1a antibody raised against the whole protein. After washing the immunoprecipitates and denaturation in SDS, aliquots of the solubilized material were subjected to SDS–polyacrylamide gel electrophoresis. Following transfer to nitrocellulose, the membranes were subjected to immunoblotting using the antibodies used in Fig 1, as well as with a phospho-specific antibody that recognizes Ser329 (pSer329). Where indicated, the cells were incubated for 60 min with various concentrations of D4476 prior to stimulation with insulin. Similar results were obtained in at least six independent experiments.
In contrast to D4476, IC261 and CKI-7 had no effect on the phosphorylation of either Ser322 or Ser325 in H4IIE hepatoma cells, even at concentrations as high as 0.3 mM. Since these compounds, like D4476, are ATP-competitive inhibitors of CK1, this lack of effect in cell-based assays is consistent with their 10- to 20-fold higher IC50 values for inhibition of CK1 compared to D4476.
Since D4476 inhibits p38α MAP kinase with an IC50 of 12 μM in vitro, we also examined whether D4476 was able to suppress the phosphorylation of MAPKAP-K2, one of its physiological substrates. Incubation of H4IIE cells with 50 μM D4476 did not affect the anisomycin-induced phosphorylation of MAPKAP-K2 at Thr334, a concentration that largely suppressed the phosphorylation of FKHR at Ser325. However, increasing the concentration of D4476 to 150 μM decreased the anisomycin-induced phosphorylation of MAPKAP-K2 (data not shown).
D4476 inhibits nuclear exclusion of FOXO1a
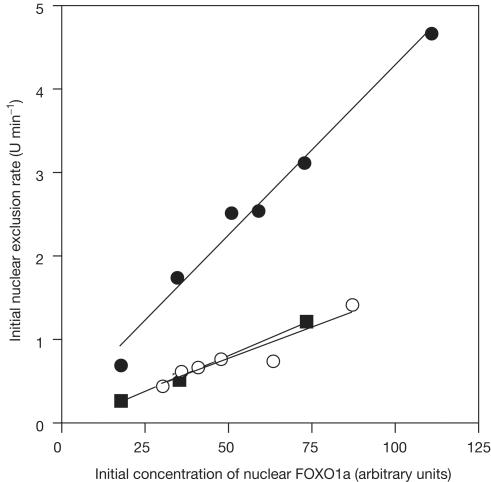
We have shown previously that the mutation of either Ser319 or Ser322 to Ala, to prevent phosphorylation of the MPD, reduces the initial rate of nuclear exclusion and, to a lesser extent, the nuclear import of FOXO1a. These results indicated that although phosphorylation of Ser319 is required to accelerate nuclear exclusion, it is not sufficient. Rather, our data suggested that the phosphorylation of Ser319 is required for accelerated nuclear exclusion because it is the trigger for MPD phosphorylation. However, it could be argued that these mutations change the conformation of FOXO1a in a way that is unrelated to phosphorylation of the wild-type protein. To test this hypothesis further, we therefore examined the effect of D4476-mediated inhibition of MPD phosphorylation on the IGF-1 and serum-induced nuclear exclusion of FOXO1a. Prior treatment of the cells with 150 μM D4476 reduces the initial rate of nuclear exclusion about threefold (Fig 4), in excellent agreement with our previous studies with MPD mutants (Rena et al, 2002). This was confirmed for the Ser319Ala mutant in a parallel experiment (Fig 4). Together, these results indicate that phosphorylation by PKB is necessary but insufficient for accelerated nuclear exclusion, which also requires the CK1-mediated phosphorylation of the MPD. While this paper was being revised, another laboratory described elegant ‘domain-swap' experiments providing further evidence that the FOXO1a MPD is a potent signal for hormone-sensitive nuclear exit (Jacobs et al, 2003).
Figure 4.
D4476 inhibits IGF-1 and serum-stimulated nuclear exclusion of FOXO1a in living cells. HEK293 cells were transfected with wild-type FOXO1a–GFP or mutant (Ser319Ala) FOXO1a–GFP. At 10 h post-transfection, the cells were serum starved for 12 h, and then stimulated in the presence of 50 ng ml−1 IGF-1 and 10% fetal calf serum with or without 150 μM D4476 pretreatment (10 min). Initial rates of nuclear exclusion were determined as described previously (Rena et al, 2002) by time-lapse imaging of live cells using confocal microscopy on a heated stage. Closed circles indicate the initial nuclear exclusion rate of FOXO1a in the absence of D4476. Open circles indicate the initial nuclear exclusion rate of FOXO1a in the presence of D4476. Squares indicate the initial nuclear exclusion rate of Ser319Ala FOXO1a–GFP. Each point is the average of seven cells.
D4476 could retard nuclear exclusion by mechanisms that lead to slower nuclear export and/or faster nuclear import. We therefore measured nuclear import alone by inhibiting nuclear export with leptomycin B. We found that 150 μM D4476 actually causes a small decrease in nuclear import (average rate 0.48 (±0.01) U min−1 in the presence of D4476 compared to 0.63 (±0.05) U min−1 in its absence). Thus, D4476 is likely to retard nuclear exclusion through accelerated nuclear export.
In summary, we have identified a potent and relatively specific inhibitor of CK1, which inhibits the CK1-mediated multisite phosphorylation and nuclear exclusion of FOXO1a. D4476 is likely to inhibit every isoform of CK1 similarly, in view of their high degree of sequence identity and the similar effects of D4476 on CK1 from mammalian cells and S. pombe. Importantly, these studies provide further evidence that MPDs encode functional outputs that are distinguishable from those encoded by phosphorylation of a single residue in the MPD motif. It is likely that acute inhibition of MPD phosphorylation using potent inhibitors of protein kinases, reported here for CK1 and previously for GSK3 (Coghlan et al, 2000), will help to unravel the functions of these interesting signalling modules.
Methods
Materials An antibody was raised against the FOXO1a protein as described previously (Woods et al, 2001) and affinity purified by Dr Jane Leitch and Dr Moustapha Aoubala (Division of Signal Transduction Therapy, University of Dundee). Fugene 6 was from Roche (Lewes, UK), IC261 from Calbiochem (Nottingham, UK) and insulin from Novo Nordisk (Crawley, UK). CKI-7 was obtained from Angus Nairn and Seikagaku (New York, USA). CK1 from S. pombe was from Upstate Inc. (Lake Placid, USA). Details of phospho-specific antibodies, mammalian expression vectors and other materials are given elsewhere (Rena et al, 1999, 2001, 2002) apart from the antibody which recognizes MAPKAP-K2 phosphorylated at Thr334, which was purchased from Cell Signalling Technologies (Hitchin, UK).
Cell culture and use of D4476. 293 cells were cultured and lysed as described previously (Rena et al, 2001). H4IIE hepatoma cells were cultured and lysed as for 293 cells, except that 15 cm dishes were used and maintained in DMEM containing 1 mg ml−1 glucose and 5% fetal calf serum. CKI-7, IC261 and D4476 were dissolved in dimethyl sulphoxide (DMSO) at a concentration of 100 mM. D4476 is only sparingly soluble when diluted directly into the aqueous cell culture medium (data not shown). In order to promote solubility, we first diluted 1 μl of 100 mM D4476 in a mixture of 6 μl serum-free medium and 3 μl Fugene 6 at 21°C before adding this solution dropwise with swirling to the cultured cells. Cellular responses to D4476 are unaffected by at least three freeze and thaw cycles of the compound. In control experiments, D4476 was replaced by DMSO.
Immunoprecipitation and SDS–PAGE. For immunoprecipitation experiments, 20 μg of FOXO1a antibody was used to immunoprecipitate the endogenous protein from 2.5 mg of lysate protein. Immunoprecipitates were washed twice with 1 ml of Buffer A plus 500 mM NaCl, followed by two washes in Buffer A alone. After denaturation in SDS, 10% of the solubilized material was run in each gel lane. Electrophoresis was performed on 4–12% SDS–polyacrylamide gradient gels, and transferred to nitrocellulose membranes for immunoblotting using the ECL system (Amersham, Buckinghamshire, UK).
Transient transfection. Transient transfection of 293 cells was performed in 6 cm dishes. A transfection mix was made up using Fugene 6 as instructed in the manual provided by Roche. Briefly, the solution was made up of 308 μl serum-free medium, 6 μl Fugene 6 and 2 μg FOXO1a–GFP DNA per dish. After 15 min incubation at 21°C, the mixture was applied dropwise to each dish. Cells were incubated in the transfection medium, and then starved of serum overnight.
Confocal microscopy and determination of the rate of nuclear exclusion Experiments with FOXO1a–GFP constructs were carried out as described previously (Rena et al, 2001, 2002), except that nuclear import experiments were carried out in the presence of 10% serum.
Acknowledgments
We thank Angus Nairn (Yale University) for a gift of CKI-7, and Carl-Henrik Heldin (Ludwig Cancer Institute, Uppsala, Sweden) for the clone expressing ALK5. This study was supported by the UK Medical Research Council and The Royal Society.
References
- Bain J, McLauchlan H, Elliott M, Cohen P (2003) The specificities of protein kinase inhibitors: an update. Biochem J 371: 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR (1997) Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275: 1930–1933 [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96: 857–868 [DOI] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME (2001) Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21: 952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan JF et al. (2002) Identification of novel inhibitors of the transforming growth factor-1 (TGF-1) type 1 receptor (ALK5). J Med Chem 45: 999–1001 [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Hagiwara M, Hidaka H (1989) A newly synthesised selective casein kinase I inhibitor N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide and affinity purification of casein kinase I from bovine testis. J Biol Chem 264: 4924–4927 [PubMed] [Google Scholar]
- Coghlan MP et al. (2000) Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol 7: 793–803 [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P (2000) The specificities of some commonly used protein kinase inhibitors and the mechanism of action of U0126 and PD 184352. Biochem J 351: 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiol CJ, Mahrenholz AM, Wang Y, Roeske RW, Roach PJ (1987) Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem 262: 14042–14048 [PubMed] [Google Scholar]
- Flotow H, Graves PR, Wang AQ, Fiol CJ, Roeske RW, Roach PJ (1990) Phosphate groups as substrate determinants for casein kinase I action. J Biol Chem 265: 14264–14269 [PubMed] [Google Scholar]
- Ginger RS, Dalton EC, Ryves WJ, Fukuzawa M, Williams JG, Harwood AJ (2000) Glycogen synthase kinase-3 enhances nuclear export of a Dictyostelium STAT protein. EMBO J 19: 5483–5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SD, Anderson RA (1998) Casein kinase I: spatial organization and positioning of a multifunctional protein kinase family. Cell Signal 10: 699–711 [DOI] [PubMed] [Google Scholar]
- Jacobs FMJ, van der Heide LP, Wijchers PJEC, Burbach JPH, Hoekman MFM, Smidt MP (2003) J Biol Chem 278: 35959–35967 [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-kB activity. Annu Rev Immunol 18: 621–633 [DOI] [PubMed] [Google Scholar]
- Kops GJPL, de Ruiter ND, De Vriets-Smits AMM, Powell DR, Bos JL, Burgering BMT (1999) Direct control of the forkhead transcription factor AFX by protein kinase B. Nature 398: 630–634 [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J (1997) Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature 389: 618–622 [DOI] [PubMed] [Google Scholar]
- Mashhoon N, DeMaggio AJ, Tereshko V, Bergmeier SC, Egli M, Hoekstra MF, Kuret J (2000) Crystal structure of a conformation-selective casein kinase-1 inhibitor. J Biol Chem 275: 20052–20060 [DOI] [PubMed] [Google Scholar]
- Nakielny S, Campbell DG, Cohen P (1991) The molecular mechanism by which adrenaline inhibits glycogen-synthesis. Eur J Biochem 199: 713–722 [DOI] [PubMed] [Google Scholar]
- Poulter L, Ang S-G, Gibson BW, WIlliams DH, Holmes CFB, Caudwell FB, Pitcher J, Cohen P (1988) Analysis of the in vivo phosphorylation state of rabbit skeletal muscle glycogen synthase by fast-atom-bombardment mass spectrometry. Eur J Biochem 175: 497–510 [DOI] [PubMed] [Google Scholar]
- Rena G, Guo SD, Cichy SC, Unterman TG, Cohen P (1999) Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem 274: 17179–17183 [DOI] [PubMed] [Google Scholar]
- Rena G, Prescott AR, Guo SD, Cohen P, Unterman TG (2001) Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem J 354: 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Woods YL, Prescott AR, Peggie M, Unterman TG, Williams MR, Cohen P (2002) Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. EMBO J 21: 2263–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielhaber E, Eide E, Rivers A, Gao ZH, Virshup DM (2000) Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I epsilon. Mol Cell Biol 20: 4888–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods YL, Rena G (2002) Effect of multiple phosphorylation events on the transcription factors FKHR, FKHRL1 and AFX. Biochem Soc Trans 30: 391–398 [DOI] [PubMed] [Google Scholar]
- Woods YL, Rena G, Morrice N, Barthel A, Becker W, Guo S, Unterman TG, Cohen P (2001) The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem J 355: 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]