Abstract
The seasonal abundance of γ-subclass Proteobacteria, Vibrio-Photobacterium, Vibrio cholerae-Vibrio mimicus, Vibrio cincinnatiensis, and Vibrio vulnificus in the Choptank River of Chesapeake Bay associated with zooplankton was monitored from April to December 1996. Large (>202-μm) and small (64- to 202-μm) size classes of zooplankton were collected, and the bacteria associated with each of the zooplankton size classes were enumerated by fluorescent oligonucleotide direct count. Large populations of bacteria were found to be associated with both the large and small size classes of zooplankton. Also, the species of bacteria associated with the zooplankton showed seasonal abundance, with the largest numbers occurring in the early spring and again in the summer, when zooplankton total numbers were correspondingly large. Approximately 0.01 to 40.0% of the total water column bacteria were associated with zooplankton, with the percentage of the total water column bacteria population associated with zooplankton varying by season. A taxonomically diverse group of bacteria was associated with zooplankton, and a larger proportion was found in and on zooplankton during the cooler months of the year, with selected taxa comprising a larger percent of the Bacteria in the summer. V. cholerae-V. mimicus and V. vulnificus comprised the bulk of the large and small zooplankton-associated Vibrio-Photobacterium species. In contrast, V. cincinnatiensis accounted for less than 0.1 to 3%. It is concluded that water column and zooplankton bacterial populations vary independently with respect to species composition since no correlation was observed between taxa occurring with highest frequency in the water column and those in association with zooplankton.
Vibrio spp. comprise a significant portion of the natural bacterial flora of zooplankton, especially zooplankton with a chitinous exoskeleton, e.g., copepods (8, 10, 24, 38, 40, 42, 43, 45). In general, larger numbers of vibrios are associated with zooplankton than are found in the surrounding water column (24, 26, 43), and this finding has led to the conclusion that Vibrio spp. have a competitive advantage in the chitinous exoskeleton microenvironment of zooplankton.
A great deal of attention in marine microbial ecology has been focused on microorganisms associated with particles, especially marine snow (9, 14, 29, 30). However, pelagic particle-associated bacterial populations are different, in both metabolic activity and taxonomic diversity, from free-living oceanic bacteria (9, 14, 30). The differences arise, at least in part, because marine particles provide stable, localized microhabitats for colonization by bacteria (2, 14, 30). For example, particle-associated bacteria are generally larger and more active metabolically and occur in higher population densities than free-living oceanic bacteria. In the open ocean, with respect to total bacterial abundance (or total bacterial carbon), free-living bacteria often comprise the dominant communities, primarily because fewer particles are present in the water column (14, 41, 44). In freshwater, coastal, and estuarine systems, where particles are more abundant, particle-associated microorganisms are present in higher (per volume) concentrations than in the open ocean (7, 16, 29).
The taxonomic compositions of free-living and particle-associated bacteria differ (7, 14). In the open ocean, Cytophaga, Planktomyces, and the γ-Proteobacteria are dominant in the particle-associated Bacteria populations, while the α-Proteobacteria comprise the majority of the free-living Bacteria (14). However, this is a more complex relationship (1, 13, 36). It is understood that marine snow provides a very different microecosystem from the exoskeleton surfaces of zooplankton, but the data to date suggest that the taxonomic compositions of Bacteria associated with zooplankton and those living free in the water column may be significantly different.
Zooplankton-associated Vibrio populations play an important role in the mineralization of chitin by binding to the chitin (6, 24, 27, 43, 45, 46) and utilizing it as a sole source of both carbon and nitrogen (5, 48). Several Vibrio spp. are also pathogenic for aquatic animals, including Vibrio vulnificus biotype 2 and Vibrio alginolyticus, both of which cause significant losses of commercially important finfish and shellfish (4, 15, 33).
The Vibrio-zooplankton association may be important in the occurrence of Vibrio spp. during colder months of the year, since Vibrio cholerae can persist at 0°C in the environment, especially when associated with chitin. The cryoprotective property of chitin has been postulated to maintain V. cholerae in the environment during the winter (3, 39).
Culture methods have routinely been employed for detection and enumeration of Vibrio spp. in the gut or on the surfaces of zooplankton, but these methods do not always succeed in enumerating attached bacterial populations (46). Part of the difficulty is that many bacteria, including Vibrio spp., enter a viable but nonculturable (VBNC) state under environmental conditions adverse to active cell metabolism and division (37). When a large portion of Vibrio spp. are VBNC, culture methods significantly underestimate bacterial populations present in a sample (47). Therefore, in this study, both in situ and direct bacterial detection methods were used to study the bacteria associated with zooplankton (25, 26).
The objectives of this study were to determine whether Bacteria, γ-Proteobacteria, including Vibrio-Photobacterium, V. cholerae-Vibrio mimicus, Vibrio cincinnatiensis, and V. vulnificus are associated with zooplankton in the environment, whether the composition of zooplankton-associated bacterial communities is the same as or different from that of the water column, whether water column and zooplankton-attached bacterial populations are related, whether bacterial communities found on small (64- to 202-μm) and large (>202-μm) zooplankton are the same or differ, and, finally, what percentage of these taxa in the total samples is zooplankton associated.
MATERIALS AND METHODS
Sample collection.
Water and zooplankton samples were collected 15 cm below the surface off the dock in the Choptank River at the University of Maryland Horn Point Laboratory, Cambridge, Md. Samples were collected within 1 h of high tide on the rising tide, so that they were collected when tidal mixing at the site was greatest, reducing the chance of bacteria entering the collection site with flow from the tidal marsh. Zooplankton samples were collected using a Hormelite model AP-125 water pump with approximately 1,000 liters of water pumped through a 64-μm plankton net at each sampling. The water intake hose was placed inside a wire enclosure (15 by 15 by 15 cm, 1-cm2 mesh) to exclude gelatinous zooplankton during sample collection. To reduce physical damage to the zooplankton, the water was pumped through a net suspended in a bucket of water (D. Nemazie, personal communication). The zooplankton samples were transported immediately after collection (<15 min) to the laboratory and processed.
In the laboratory, zooplankton samples were split, using a Folsom plankton splitter (Aquatic Research Instruments, Lemhi, Idaho). Each zooplankton sample was further divided into 64- to 202-μm and >202-μm size fractions by filtration through Nytex screens. One sample of each size class was used to enumerate zooplankton, and the other was used to enumerate zooplankton-associated bacteria. Samples for zooplankton enumeration were stored in 5% formalin. Zooplankton-associated bacterial samples were processed immediately.
Enumeration of zooplankton.
Zooplankton samples (both 64- to 202-μm and >202-μm size classes) were randomly subsampled using a 5-ml Stemple pipette and enumerated by counting at least 100 individuals of the most abundant zooplankter. In the summer months, when >202-μm zooplankton were less abundant, the entire sample was counted. Plankton were identified to order for copepods, barnacle nauplii, copepod nauplii, polychaetes, tintinids, rotifers, eciliates, or mollusks and measured, i.e., copepod cepholothorax length and midbody width, other plankton total body length and midbody width, using a version 4.5 Scan Array 2 image analysis system (Galai Corp., Gosford, Australia). External surface area was estimated by modeling zooplankters as right-angle cylinders (surface area = π r2 + L2). Zooplankton abundance was converted to number of individuals per cubic meter of Choptank River water (CRW) and per square millimeter of external surface area.
Analysis of zooplankton-associated bacteria.
Zooplankton were concentrated from each sample by filtration through Nytex screens (202 or 64 μm) and suspended in 10 ml of filter-sterilized (FS) phosphate-buffered saline (PBS). Zooplankton-associated bacteria were removed and fixed for fluorescent oligonucleotide direct count (FODC) by adding 30 ml of fresh FS 4% (wt/vol in phosphate-buffered saline) paraformaldehyde and 10 μg of Tween 80 ml−1. The sample was incubated on ice with gentle shaking for 4 h and then sonicated (10 W) for 30 s. Large particles, which could obstruct the flow cytometer, were removed by filtration through a 25-μm Nytex filter. The Nytex filter was washed twice with approximately 25 ml of FS CRW. The paraformaldehyde and Tween 80 were removed by centrifugation, and the sample was resuspended in 1 ml of FODC hybridization buffer containing 50% ethanol. All samples were stored at −20°C until enumerated.
Enumeration of probe-positive bacterial cells.
Total Bacteria (probe EUB 338), γ-Proteobacteria (probe GAM), Vibrio-Photobacterium (probe Vib/Pho), V. cholerae-V. mimicus (probe Vcho/mim), V. cincinnatiensis (probe Vcinc), and V. vulnificus (probe Vvul3) in each sample were enumerated by the FODC method (17, 18). The probes were generated for the study and are described in detail elsewhere (18, 19). Bacterial abundance was estimated using a Epics II flow cytometer (Coulter Corp., Miami, Fla.), and bacterial cells per cubic meter of CRW and per square millimeter of zooplankton external surface area were calculated.
Data analysis.
SigmaStat (version 3.0; Jandel Scientific, Corte Madera, Calif.) was used for statistical analysis of the data. Pearson product moment correlation was used to determine relationships between zooplankton-associated and water column bacterial abundance. To equalize the variance, bacterial and zooplankton abundance were natural-logarithm transformed. Normality of transformed data was tested by the Komogorov-Smirnov test. Also, analysis of variance and regression analysis (linear and stepwise forward regression, model II) were used to determine the relationships among bacterial abundance and physical parameters.
RESULTS
Large zooplankton (>202 μm).
Total number of large zooplankton ranged from 5 × 102 to 1.3 × 105 zooplankters per m3 of CRW. Zooplankton populations were numerically largest during the spring and, later, during the early winter (Fig. 1). Calanoid copepods, the dominant copepod taxonomic group, were most abundant in the spring (Acartia spp.) and later in the year during early winter (Eurytemora spp.). Cyclopoid copepods (Oithona spp.) and unidentified harpacticoid copepods were present intermittently in lesser numbers throughout the year. Copepod nauplii were most abundant during the spring and late summer, barnacle nauplii were most abundant in the early summer and fall, and polychaetes were most abundant during the fall and early winter (Fig. 1).
FIG. 1.
Composition of >202-μm zooplankton. (A) Number of zooplankton per cubic meter of CRW; (B) surface area of zooplankton per cubic meter of CRW.
The large-zooplankton external surface area data showed a pattern similar to that of total zooplankton abundance (Fig. 1). Zooplankton surface area ranged from 2.5 × 101 to 2.8 × 104 mm2 external surface area per m3 of CRW, with a larger zooplankton total surface area observed during spring and later in the year in the early winter. Copepods accounted for the majority of the total external surface area during the spring and contributed significantly (along with polychaetes) to the total external surface area in the fall and early winter (Fig. 1).
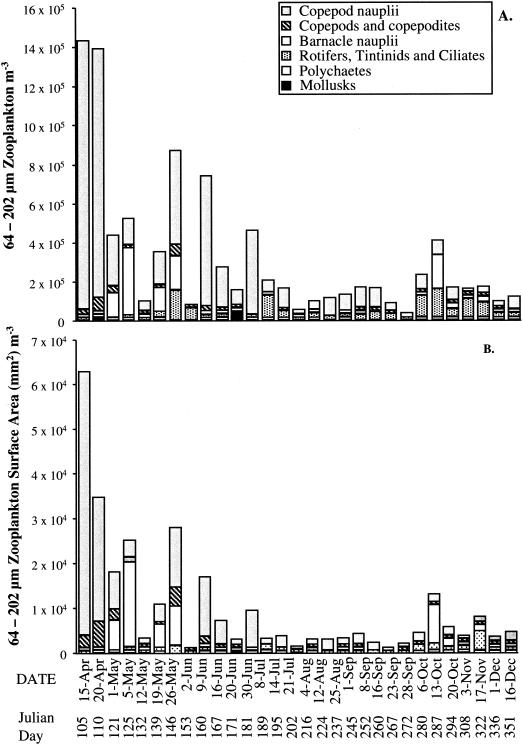
Small zooplankton.
The total number of small zooplankton (64 to 202 μm) ranged from 2.6 × 104 to 1.4 × 106 zooplankters per m3 of CRW, with the greatest abundance observed in samples collected during the spring (Fig. 2). Copepod nauplii were dominant among the small-zooplankton populations and were most abundant during the spring. Barnacle nauplii were most frequently observed in the early summer and fall, ciliates were most frequently observed during the fall, mollusks were most frequently observed during the summer, and tintinids were most frequently observed during late spring and early fall. Adult copepods and copepod nauplii comprised a portion of the population throughout the year but were not a dominant part of the 64- to 202-μm size class (Fig. 2).
FIG. 2.
Composition of 64- to 202-μm zooplankton. (A) Number of zooplankton per cubic meter of CRW; (B) surface area of zooplankton per cubic meter of CRW.
The pattern of the small-zooplankton surface area distribution was similar to that of the total small-zooplankton abundance (Fig. 2). The external surface area of the small zooplankton ranged from 6.4 × 102 to 6.2 × 104 mm2 per m3 of CRW, with greater total surface area during the spring. Copepod nauplii dominated the total surface area, i.e., 1.4 × 101 to 5.9 × 104 mm2 (external surface area) per m3 of CRW (Fig. 2).
Acridine orange direct counts of CRW samples.
The total number of bacteria, estimated by AODC, in centrifuge-concentrated CRW samples collected at the dock site between 15 April and 16 December 1996 ranged from 2.0 × 109 to 24 × 109 cells liter−1. The highest bacterial concentrations in the CRW samples were obtained during the warm summer months, and the lowest concentrations were obtained during the winter and early spring.
Bacterioplankton abundance, enumerated using centrifuge-concentrated water samples, ranged from 64 to 93% of the total cell abundance measured by direct counts of unconcentrated water samples. However, no more than 36% of the total number of bacteria was lost during concentration, and it was observed that larger percentages of cells were lost when early-spring and winter water samples were analyzed than when summer water samples were analyzed. The effect of water temperature on centrifuge concentration efficiency may be a result of cell size, since large cells are more efficiently concentrated by centrifugation, and warm water may be associated with larger cell sizes (19). Measurement of the biovolume of the cells was not an objective of this study.
Bacterial populations associated with zooplankton.
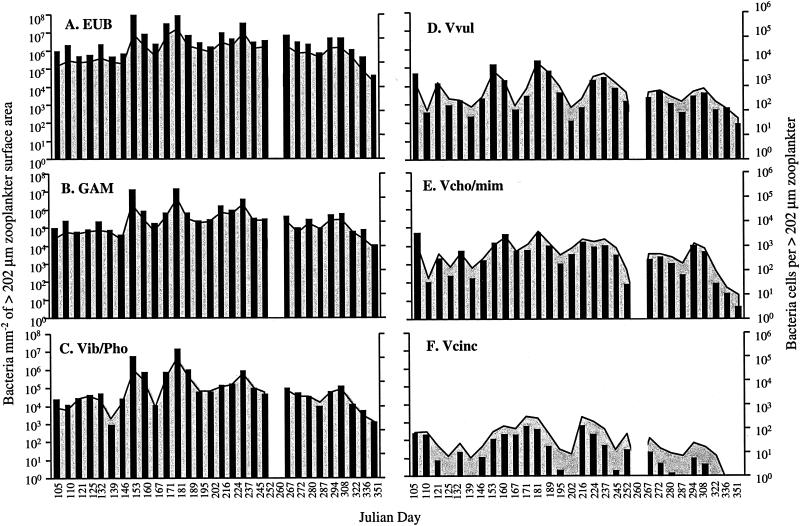
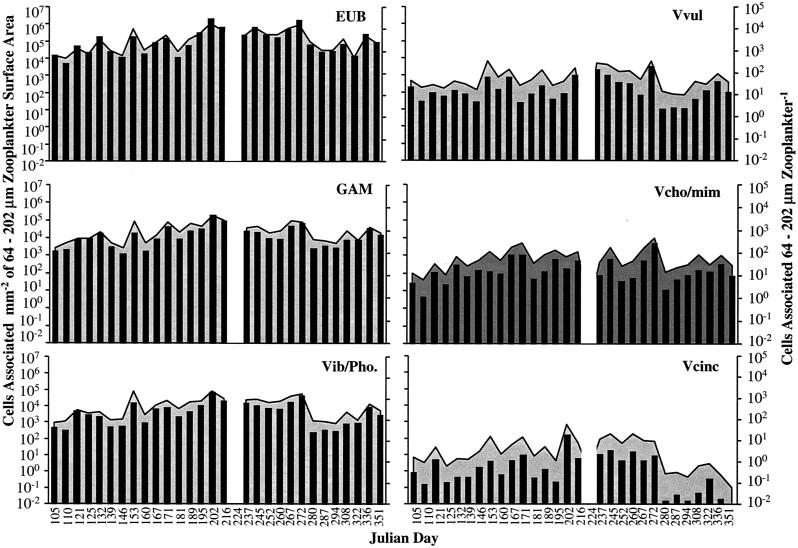
The number of Bacteria associated with the large zooplankton ranged from 1.0 × 108 to 3.8 × 109 cells per m3 of CRW, the total number of γ-Proteobacteria was 1.9 × 107 to 7.0 × 108 cells m−3, the total number of Vibrio-Photobacterium 1.9 × 106 to 2.4 × 108 cells m−3, the total number of V. vulnificus was 2.4 × 105 to 1.3 × 107 cells m−3, the total number of V. cholerae-V. mimicus was 1.3 × 105 to 6.4 × 107 cells m−3, and the total number of V. cincinnatiensis was 1.9 × 103 to 7.9 × 106 cells m−3 (Fig. 3). The number of Bacteria associated with the large zooplankton was largest in the early spring and fall, whereas γ-Proteobacteria, Vibrio-Photobacterium, and V. vulnificus populations were largest in the early spring and late summer. V. cholerae-V. mimicus was most abundant in the early spring and summer, and V. cincinnatiensis was most abundant in the early spring.
FIG. 3.
Number of bacteria associated with >202-μm zooplankton per cubic meter of CRW. EUB, Bacteria; GAM, γ-Proteobacteria; Vib/Pho, Vibrio-Photobacterium; Vvul, V. vulnificus; Vcho/mim, V. cholerae-V. mimicus; Vcinc, V. cincinnatiensis. Shaded areas represent the number of cells per square millimeter of surface area; bars represent the number of cells per zooplankter.
The number of Bacteria per individual zooplankter of the >202-μm size class ranged from 2.8 × 103 to 9.6 × 105, that of γ-Proteobacteria ranged from 1.0 × 103 to 2.3 × 105, that of Vibrio-Photobacterium ranged from 1.6 × 102 to 1.2 × 105, that of V. vulnificus ranged from 3.6 × 101 to 1.0 × 104, that of V. cholerae-V. mimicus ranged from 3.0 × 100 to 3.3 × 103, and that of V. cincinnatiensis ranged from 9.3 × 10−2 to 1.2 × 102. Total populations of Bacteria, γ-Proteobacteria, and Vibrio-Photobacterium associated with the large zooplankton demonstrated a clear seasonality, with the largest numbers of these taxa per zooplankter occurring during the summer. The Vibrio spp. varied in total number but demonstrated seasonality in annual distribution, with a trend toward larger populations in the summer.
The number of Bacteria attached to the >202-μm zooplankton ranged from 1.8 × 104 to 1.7 × 107 cells per mm2 of zooplankton external surface area, the number of γ-Proteobacteria ranged from 6.6 × 103 to 4.4 × 106, the number of Vibrio-Photobacterium ranged from 1.3 × 103 to 4.3 × 106, the number of V. vulnificus ranged from 1.7 × 102 to 1.9 × 105, the number of V. cholerae-V. mimicus ranged from 1.9 × 101 to 6.3 × 104, and the number of V. cincinnatiensis ranged from 1.1 × 10−1 to 2.1 × 103. The largest populations of all groups were associated with >202-μm zooplankton surface area, mm−2, during the summer months (Fig. 4).
FIG. 4.
Percentage of total bacteria associated with >202-μm zooplankton. Results are given as number of cells per zooplankter. EUB, Bacteria; GAM, γ-Proteobacteria; Vib/Pho, Vibrio-Photobacterium; Vvul, V. vulnificus; Vcho/mim, V. cholerae-V. mimicus; Vcinc, V. cincinnatiensis.
Approximately 6 to 37% of the total large-zooplankton-associated Bacteria were γ-Proteobacteria, and the Vibrio-Photobacterium spp. comprised 1 to 26%. The three other species included in this study comprised 0 to 7% of the total Bacteria. Seasonally, the percent composition of Bacteria of the γ-Proteobacteria attached to large zooplankton was essentially constant throughout the year. However, Vibrio-Photobacterium spp. comprised a larger percentage of the Bacteria during the spring and summer, with a pronounced seasonal trend.
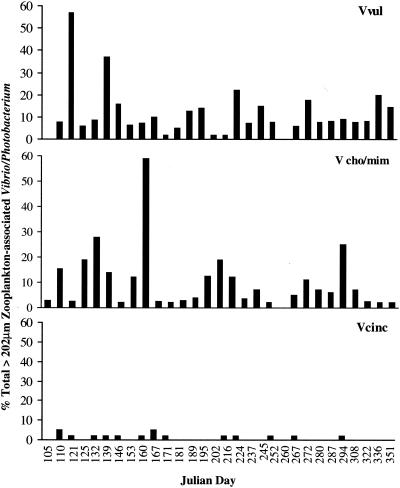
Overall, V. vulnificus comprised 1 to 57% of the total Vibrio-Photobacterium population, V. cholerae-V. mimicus comprised 1 to 60%, and V. cincinnatiensis comprised 0.01 to 5%, with significant variation in species composition observed from week to week (Fig. 5).
FIG. 5.
Percentage of total Vibrio-Photobacterium associated with >202-μm zooplankton; Vvul, V. vulnificus; Vcho/mim, V. cholerae-V. mimicus; Vcinc, V. cincinnatiensis.
Taxa associated with the small size class (64 to 202 μm) of zooplankton did not show the same variation in abundance as did taxa associated with the large zooplankton. The total number of Bacteria was 8.6 × 107 to 1.1 × 109 cells m−3, the total number of γ-Proteobacteria was 2.2 × 107 to 2.1 × 108, the total number of Vibrio-Photobacterium was 4.7 × 106 to 1 × 108, the total number of V. vulnificus was 3 × 105 to 2.9 × 107, the total number of V. cholerae-V. mimicus was 4.2 × 105 to 1.8 × 107, and the total number of V. cincinnatiensis was 3.2 × 102 to 5 × 105. Taxa associated with the smaller zooplankton showed a distinct seasonality, with larger numbers detected in the spring and summer months (Fig. 6).
FIG. 6.
Bacterial cells associated with 64- to 202-μm zooplankton per cubic meter of CRW. EUB, Bacteria; GAM, γ-Proteobacteria; Vib/Pho, Vibrio-Photobacterium; Vvul, V. vulnificus; Vcho/mim, V. cholerae-V. mimicus; Vcinc, V. cincinnatiensis. Shaded areas represent the number of cells per square millimeter of surface area; bars represent the number of cells per zooplankter.
The number of Bacteria associated with an individual small-size-class (64- to 202-μm) zooplankter was 260 to 28,606 cells, the number of γ-Proteobacteria was 88 to 4,690, the number of Vibrio-Photobacterium was 25 to 1,980, the number of V. vulnificus was 2 to 188, the number of V. cholerae-V. mimicus was 1 to 265, and the number of V. cincinnatiensis was 0 to 18. Bacteria associated with the smaller zooplankton, namely, γ-Proteobacteria and Vibrio-Photobacterium, showed a clear seasonal trend, with greater populations observed to occur in the summer months.
The number of Bacteria associated with the small-size-class (64- to 202-μm) zooplankton ranged from 1 × 104 to 1.2 × 106 cells per mm2 of zooplankton surface area, the number of γ-Proteobacteria ranged from 2.7 × 103 to 1.9 × 105, the number of Vibrio-Photobacterium ranged from 8.7 × 102 to 8 × 104, the number of V. vulnificus ranged from 7.2 × 101 to 7 × 103, the number of V. cholerae-V. mimicus ranged from 4.3 × 101 to 9.9 × 103, and the number of V. cincinnatiensis ranged from 9.7 × 10−2 to 7.3 × 102. All of the taxonomic groups analyzed in this study were present in larger numbers on the smaller zooplankton, especially during the summer months. However, total numbers per square millimeter of surace area were significantly smaller than those of the large zooplankton (Fig. 6).
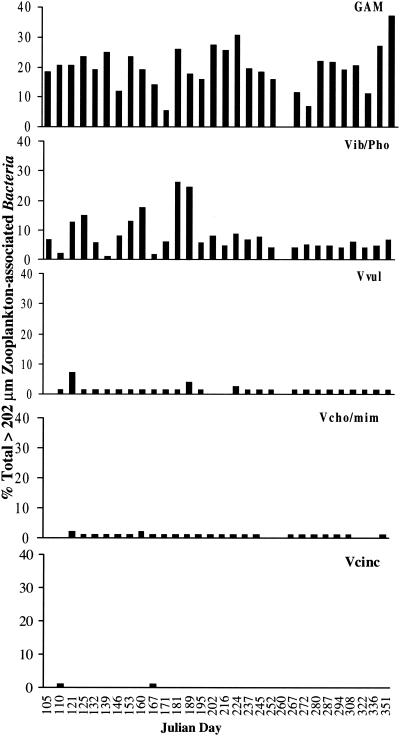
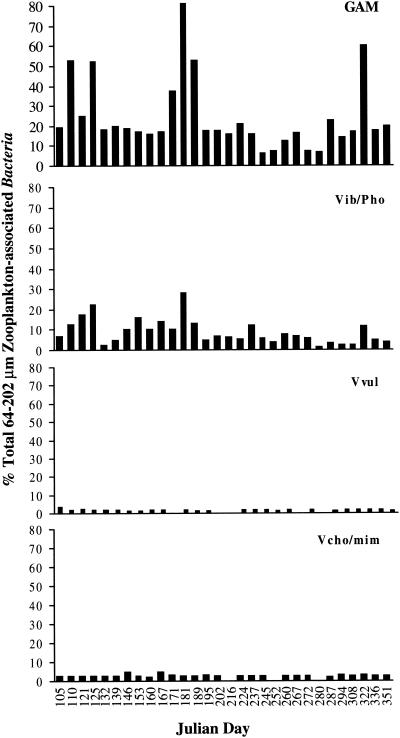
Bacteria associated with the smaller zooplankton included 7 to 80% γ-Proteobacteria, 1.3 to 27% Vibrio-Photobacterium, 0.04 to 3.3% V. vulnificus, 0.07 to 3.5% V. cholerae-V. mimicus, and 0 to 0.11% V. cincinnatiensis. Seasonally, the percent composition of all of these taxonomic groups was higher during the summer months (Fig. 7).
FIG. 7.
Percentage of total bacterial cells associated with 64- to 202-μm zooplankton. GAM, γ-Proteobacteria; Vib/Pho, Vibrio-Photobacterium; Vvul, V. vulnificus; Vcho/mim, V. cholerae-V. mimicus.
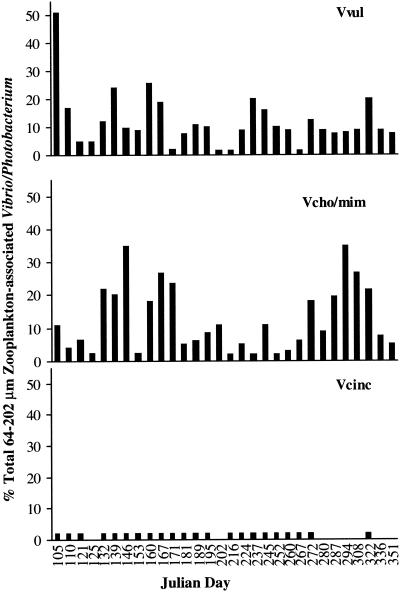
V. vulnificus comprised 0.5 to 51% of the total Vibrio-Photobacterium population, V. cholerae-V. mimicus comprised 1 to 36%, and V. cincinnatiensis comprised 0.002 to 1.1% (Fig. 8).
FIG. 8.
Percentage of total Vibrio-Photobacterium associated with 64- to 202-μm zooplankton. Vvul, vulnificus; Vcho/mim, V. cholerae-V. mimicus; Vcinc, V. cincinnatiensis.
DISCUSSION
The association of V. cholerae with zooplankton is of interest for several reasons, the most important being public health, since this ecological relationship can have significant implications for initiation and spread of cholera epidemics, notably in developing countries (11, 21, 22). The role of zooplankton in the initiation of cholera epidemics can be deduced from the correlations observed between copepod abundance and onset of epidemics. In Bangladesh, cholera epidemics occur biannually, during the spring and fall, and the seasonal cycle of cholera is closely correlated with copepod abundance. Furthermore, in years when copepod populations were small, fewer cholera cases were recorded (20, 21). Specifically, zooplankton appear to amplify V. cholerae populations at the time of zooplankton blooms, thereby either initiating or exacerbating cholera epidemics, or both (24, 26, 43). Chitin, part of the exoskeletal structure of zooplankton, can protect V. cholerae from stomach acid if the bacteria are ingested by humans along with plankton present in untreated water used for drinking (31, 35). It has been shown that by removing zooplankton from drinking water, nearly all of the pathogenic Vibrio spp. are removed (23).
Bacteria, γ-Proteobacteria, Vibrio-Photobacterium, V. cholerae-V. mimicus, V. cincinnatiensis, and V. vulnificus were associated with both the large and small zooplankton size classes in the Choptank River of the Chesapeake Bay, confirming the earlier work of other investigators (11, 28).
There are two important factors to consider in analyzing microbial populations associated with zooplankton, namely, the patchy distribution of zooplankton (32) and the limits of zooplankton surface area estimates. Thus, accurate estimates of populations of bacteria associated with zooplankton per volume of water or with a single zooplankter are difficult to obtain. During sampling, if zooplankton abundance is high (or low) locally, the sampling protocol will overestimate (or underestimate) the number of zooplankton per cubic meter of water and, consequently, the zooplankton-associated bacterial populations per cubic meter water. Thus, comparisons between total-water-column bacterial abundance and zooplankton-associated bacterial abundance are, at best, estimates. In this study, potential error was reduced by collecting samples for both water column and zooplankton-associated bacterial populations simultaneously.
Based on this study, zooplankton-associated Bacteria comprised only a fraction of the total-water-column Bacteria in the Choptank river ecosystem. Only 1:1,000 to 1:10,000 of the total Bacteria per cubic millimeter of CRW were concluded to be attached to, or associated with, zooplankton. In the Choptank River, this relatively low percentage of total Bacteria associated with zooplankton may be a consequence of the total-water-column bacterial populations being relatively large. In ecosystems where nutrient availability limits bacterial abundance and total bacterial abundance is low, selection for specific bacterial taxa would be expected in the zooplankton exoskeleton microecosystem, since bacteria attached to copepods will have chitin available as a substrate. The largest percentage of bacterial populations associated with zooplankton were Vibrio-Photobacterium, V. cholerae-V. mimicus, and V. vulnificus. All, incidentally, are chitin digesters.
In microcosms inoculated with V. cholerae, the number of V. cholerae cells attaching to a single calanoid copepod is large enough to serve as an infectious dose (12, 21, 22, 24). In the present study, the number of V. cholerae-V. mimicus cells per single copepod was not determined directly but was estimated by dividing the number of V. cholerae-V. mimicus per square millimeter of zooplankton surface area by the surface area of the calanoid copepod population in the sample. From this estimation, it was calculated that 2.5 to 4,000 (and a high of 7,100 on 30 June 1996) V. cholerae-V. mimicus organisms were associated with the exoskeleton of a calanoid copepod. However, because the total copepod surface area is an approximation and because of a possible preference of V. cholerae-V. mimicus for specific zooplankton species as well as the copepod gut (24), this number can be considered a very conservative estimate. Even within these limitations, it is clear that relatively large numbers of V. cholerae-V. mimicus are associated with the zooplankton of the Choptank River in Chesapeake Bay.
All bacterial taxa examined in this study were zooplankton associated but to different degrees. Very large populations of Bacteria, γ-Proteobacteria, Vibrio-Photobacterium, V. cholerae-V. mimicus, and V. vulnificus were associated with both the large and small size classes of zooplankton, especially during the summer. In contrast, V. cincinnatiensis comprised only a minor portion of the zooplankton-associated bacterial community. V. cincinnatiensis either is not able to compete with other bacterial species comprising this community, is present in small numbers consistently, or may be preferentially associated with other components of plankton, e.g., phytoplankton, in the water column.
There were large week-to-week variations in the number of bacteria associated with zooplankton, both per square millimeter of zooplankton surface area and per cubic meter of CRW (Fig. 3). These short-term temporal abundance variations create problems with respect to accuracy of monitoring the environment for public health purposes (20, 34). Any monitoring scheme involving infrequent environmental sampling may fail to detect an increase in bacterial abundance associated with a bloom in zooplankton. Based on the large temporal variation in abundance, there can be short periods during the year when zooplankton-associated bacterial pathogen populations are large enough to cause disease, as is the case in Bangladesh, where cholera outbreaks typically occur in April and May and again in August and September (23).
The contribution of each of the bacterial taxa included in this study to zooplankton-associated and water column populations of Bacteria was compared to determine whether each of the zooplankton exoskeleton and water column microhabitats harbored different bacterial populations. There was a 2- to 10-fold increase in the percentage of zooplankton-associated Bacteria represented by the taxonomic groups included in this study, compared to the proportion they comprised in water column Bacteria composition, indicating that the bacterial taxa associated with the zooplankton exoskeleton microhabitat differ from those in the water column, with the possibility of a coevolutionary history in the case of the bacterium-zooplankton association.
Zooplankton-associated Vibrio-Photobacterium showed a seasonal trend in percent composition opposite to that in the water column; i.e., Vibrio-Photobacterium made up a larger percentage of zooplankton-associated Bacteria during the summer but accounted for a larger percentage of total-water-column Bacteria during the spring and early winter, most probably a result of shedding from declining blooms of zooplankton. V. vulnificus, V. cholerae-V. mimicus, and V. cincinnatiensis showed seasonal trends similar to that of the Vibrio-Photobacterium genera. These species comprised a greater percentage of zooplankton-associated Bacteria during the summer months, whereas they were more abundant in the water column in the spring and early winter.
V. vulnificus, V. cholerae-V. mimicus, and V. cincinnatiensis, in total, comprised a greater relative portion of zooplankton-associated Vibrio-Photobacterium than of total-water-column Vibrio-Photobacterium populations. These three species, combined, comprised 10 to 25% of the zooplankton-associated Vibrio-Photobacterium populations but comprised up to 70% of the bacterial populations attached to the large zooplankton. V. cholerae-V. mimicus and V. vulnificus were present in significantly greater numbers as members of the zooplankton-associated Vibrio-Photobacterium, whereas V. cincinnatiensis was only a minor portion (0.0 to 5%) of this population.
A significant correlation between zooplankton and water column bacterial populations was observed only for V. cincinnatiensis. The lack of such correlation for other taxa included in this study leads to the conclusion that zooplankton-associated and water column bacterial populations are independent. If bacterial species moved frequently between these environments, strong correlations would be expected. The data do, furthermore, support the hypothesis that zooplankton provide an overwintering site for the bacteria, in accordance with the diapause exhibited by many species of zooplankton.
In conclusion, large populations of Bacteria, γ-Proteobacteria, Vibrio-Photobacterium, V. vulnificus, and V. cholerae-V. mimicus were found associated with both large and small size classes of zooplankton in the Choptank River of the Chesapeake Bay. In comparison, these zooplankton-associated bacterial populations comprised only a small fraction of the total-water-column bacterial populations. Furthermore, when the genera and species of bacteria associated with zooplankton were identified, the association was found to be significant. Zooplankton-associated bacterial populations showed seasonal trends, with larger populations associated with zooplankton being present in the environment during the spring and fall months of the year. Few differences were noted among taxa of bacterial populations associated with either large or small zooplankton size classes.
V. cholerae-V. mimicus and V. vulnificus comprised a significant portion of the total Vibrio-Photobacterium species found on both large and small class sizes of zooplankton. Also, these species comprised a greater portion of the numbers of Vibrio-Photobacterium in this microhabitat than in the water column, suggesting that these two species either selectively attach to, or are commensal and/or symbiotic with, zooplankton. Since a statistically significant correlation was not observed between either abundance or taxon distribution of the water column and zooplankton-associated bacterial populations, these populations are concluded to be independent and specific to their microhabitat.
Acknowledgments
We gratefully acknowledge support of NIH grants 1RO1A139129-01 and RO1 NRO4527-01A1. We also thank the University of Maryland Center for Environmental Science Horn Point Laboratory for generous use of laboratory facilities.
Jennifer Purcell provided field equipment and microscope equipment for zooplankton collection and analysis.
REFERENCES
- 1.Acinas, S. G., A. Josefa, and F. Rodríguez-Valera. 1999. Diversity of free-living and attached bacteria in offshore western Mediterranean waters as depicted by analysis of genes encoding 16S rRNA. Appl. Environ. Microbiol. 65:514-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alldredge, A. L., and M. W. Silver. 1988. Characteristics, dynamics, and significance of marine snow. Prog. Oceanogr. 20:41-82. [Google Scholar]
- 3.Amako, K., S. Shimodori, T. Imoto, S. Miake, and A. Umeda. 1987. Effects of chitin and its soluble derivatives on survival of Vibrio cholerae O1 at low temperature. Appl. Environ. Microbiol. 53:603-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin, B., and D. A. Austin. 1993. Bacterial fish pathogens: disease in farmed and wild fish, 2nd ed., p. 265-307. Ellis Horwood Ltd, Chichester, England.
- 5.Bassler, B. L., C. Yu, Y. C. Lee and S. Roseman. 1991. Chitin utilization by marine bacteria, degradation and catabolism of chitin oligosaccharides by Vibrio furnissi. J. Biol. Chem. 266:24276-24286. [PubMed] [Google Scholar]
- 6.Belas, M. R., and R. R. Colwell. 1982. Adsorption kinetics of laterally and polarly flagellated Vibrio. J. Bacteriol. 151:1568-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bidle, K. D., and M. Flecher. 1995. Comparison of free-living and particle-associated bacterial communities in the Chesapeake Bay by stable low-molecular-weight RNA analysis. Appl. Environ. Microbiol. 61:944-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carli, A., L. Pane, L. Casareto, S. Bertone, and C. Pruzzo. 1993. Occurrence of Vibrio alginolyticus in Ligurian coast rock pools (Tyrrhenian Sea, Italy) and its association with the copepod Tigriopus fulvus (Fisher 1860). Appl. Environ. Microbiol. 59:1960-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caron, D. A., P. G. Davis, L. P. Madin, and J. M. Sieburth. 1982. Heterotrophic bacteria and bactivorous protozoa in oceanic macroaggregrates. Science 218:795-797. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury, M. A. R., H. Yamanaka, S. Miyoshi, K. M. Aziz, and S. Shinoda. 1989. Ecology of Vibrio mimicus in aquatic environments. Appl. Environ. Microbiol. 55:2073-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colwell, R. R. 1996. Global climate and infectious disease: the cholera paradigm. Science 274:2025-2031. [DOI] [PubMed] [Google Scholar]
- 12.Colwell, R. R., and A. Huq. 1994. Vibrios in the environment: viable but nonculturable Vibrio cholerae, p. 117-133. In I. K. Wachsmuth, O. Olsvik, and P. A. Blake (ed.), Vibrio cholerae and cholera: molecular to global perspectives. American Society for Microbiology, Washington, D.C.
- 13.Crump, B. C., E. V. Armbrust, and J. A. Baross. 1999. Phylogenetic analysis of particle-attached and free-living bacterial communities in the Columbia River, its estuary, and the adjacent coastal ocean. Appl. Environ. Microbiol. 65:3192-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLong, E. F., D. G. Franks, and A. L. Alldredge. 1993. Phylogenetic diversity of aggregate-attached vs. free-living marine bacterial assemblages. Limnol. Oceanogr. 38:924-934. [Google Scholar]
- 15.Egidius, E. 1987. Vibriosis: pathogenicity and pathology. A review. Aquaculture 67:15-28. [Google Scholar]
- 16.Griffith, P. C., D. J. Douglas, and S. C. Wainright. 1990. Metabolic activity of size fractionated microbial plankton in estuarine, nearshore, and continental shelf water. Mar. Ecol. Prog. Ser. 59:263-270. [Google Scholar]
- 17.Heidelberg, J. F. 1997. Seasonal abundance of bacterioplankton populations of Bacteria, gamma proteobacteria, Vibrio/Photobacterium, Vibrio vulnificus, Vibrio cholerae/Vibrio mimicus, and Vibrio cincinnatiensis associated with zooplankton in the Choptank River, Maryland. Ph.D. dissertation. University of Maryland, College Park.
- 18.Heidelberg, J. F., K. R. O'Neill, D. Jacobs, and R. R. Colwell. 1993. Enumeration of Vibrio vulnificus on membrane filters with a fluorescently labeled oligonucleotide probe specific for Kingdom-level 16S rRNA sequences. Appl. Environ. Microbiol. 59:3474-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Seasonality of Chesapeake Bay Bacterioplankton species. Appl. Environ. Microbiol. 68:5488-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huq, A., and R. R. Colwell. 1995. Vibrios in the marine and estuarine environments. J. Mar. Biotechnol. 3:60-63. [Google Scholar]
- 21.Huq, A., and R. R. Colwell. 1996. Environmental factors associated with emergence of disease with special reference to cholera. Eastern Mediterranean Health J. 2:37-45. [Google Scholar]
- 22.Huq, A., and R. R. Colwell. 1996. A microbiological paradox: viable but nonculturable bacteria with special reference to Vibrio cholerae. J. Food Prot. 59:96-101. [DOI] [PubMed] [Google Scholar]
- 23.Huq, A., B. Xu, M. A. R. Chowdhury, M. S. Islam, R. Montilla, and R. R. Colwell. 1996. A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl. Environ. Microbiol. 62:2508-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huq, A., E. B. Small, P. A. West, M. I. Huq, R. Rahman, and R. R. Colwell. 1983. Ecological relationship between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 45:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huq, A., R. R. Colwell, M. A. R. Chowdhury, B. Xu, S. M. Moniruzzaman, M. S. Islam, M. Yunus, and M. J. Albert. 1995. Coexistence of Vibrio cholerae O1 and O139 Bengal in plankton in Bangladesh. Lancet 345:1249. [DOI] [PubMed] [Google Scholar]
- 26.Huq, A., R. R. Colwell, R. Rahman, A. Ali, M. A. R. Chowdhury, S. Parveen, D. A. Sack, and E. Russek-Cohen. 1990. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl. Environ. Microbiol. 56:2370-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneko, T., and R. Colwell. 1975. Adsorption of Vibrio parahaemolyticus onto chitin and copepods. Appl. Microbiol. 29:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaper, J., H. Lockman, R. R. Colwell, and S. W. Joseph. 1979. Ecology, serology, and enterotoxin production of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 37:91-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirchman, D., and R. Mitchell. 1982. Contribution of particle-bound bacteria to total microheterotrophic activity in five ponds and two marshes. Appl. Environ. Microbiol. 43:200-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirchman, D. L. 1993. Particulate detritus and bacteria in marine environments, p. 321-341. In T. E. Ford (ed.), Aquatic microbiology: an ecological approach. Blackwell Scientific Publishing, Oxford, United Kingdom.
- 31.Levine, M. M., R. Black, and M. L. Clements. 1984. Pathogenesis of enteric infections caused by Vibrio, p. 109-122. In R. R. Colwell (ed.), Vibrios in the environment. John Wiley & Sons, Inc., New York, N.Y.
- 32.Levinton, J. S. 1982. Marine ecology. Prentice-Hall, Inc., Englewood Cliffs, N.J.
- 33.Lightner, D. V., T. A. Bell, R. M. Redman, L. L. Mohney, J. M. Natividad, A. Rukyani, and A. Poernomo. 1992. A review of some major diseases of economic significance in penaeid prawns/shrimp in the Americas and Indopacific, p. 57-80. In I. M. Shariff, R. P. Subasinghe, and J. R. Arthur (ed.), Diseases in asian aquaculture. Fish Health Section, Asian Fisheries Society, Manila, Philippines.
- 34.McLaughlin, J. C. 1995. Vibrio, p. 465-476. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, D.C.
- 35.Nalin, D. R., V. Daya, A. Ried, M. M. Levine, and L. Cisneros. 1979. Adsorption and growth of Vibrio cholerae to chitin. Infect. Immun. 25:768-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rath, J., K. Y. Wu, G. J. Herndl, and E. F. DeLong. 1998. High phylogenetic diversity in a marine-snow-associated bacterial assemblage. Aquat. Microb. Ecol. 14:261-269. [Google Scholar]
- 37.Roszak, D. B., and R. R. Colwell. 1987. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 51:365-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakar, B. L., G. B. Nair, B. K. Sircar, and S. C. Pal. 1983. Incidence and level of Vibrio parahaemolyticus associated with freshwater plankton. Appl. Environ. Microbiol. 46:288-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimodori, S., T. Moriya, O. Kobashi, D. Faming, and K. Amako. 1989. Extraction from prawn shells of substances cryoprotective for Vibrio cholerae. Appl. Environ. Microbiol. 55:2726-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simidu, U., K. Ashino, and E. Kaneko. 1971. Bacterial flora of phyto- and zoo-plankton in the inshore waters of Japan. Can. J. Microbiol. 17:1157-1160. [DOI] [PubMed] [Google Scholar]
- 41.Simon, M., A. L. Alldredge, and F. Azam. 1990. Bacterial carbon dynamics on marine snow. Mar. Ecol. Prog. Ser. 65:205-211. [Google Scholar]
- 42.Sochard, M. R., D. F. Wilson, B. Austin and R. R. Colwell. 1979. Bacteria associated with the surface and gut of marine copepods. Appl. Environ. Microbiol. 37:750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamplin, M. L., A. L. Gauzens, A. Huq, D. A. Sack, and R. R. Colwell. 1990. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl. Environ. Microbiol. 56:1977-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unanue, M., B. Ayo, L. Azua, I. Barcina, and J. Irriberri. 1992. Temporal variability of attached and free-living bacteria in coastal waters. Microb. Ecol. 23:27-39. [DOI] [PubMed] [Google Scholar]
- 45.Venkateswaran, K., S. W. Kim, H. Nakano, T. Onbè, and H. Hashimoto. 1989. The association of Vibrio parahaemolyticus serotypes with zooplankton and its relationship with bacterial indicators of pollution. Syst. Appl. Microbiol. 11:194-201. [Google Scholar]
- 46.Venkateswaran, K., T. Takai, I. M. Navarro, H. Nakano, H. Hashimoto, and R. Siebeling. 1989. Ecology of Vibrio cholerae non-01 and Salmonella spp. and role of zooplankton in their seasonal distribution in Fukuyama coastal waters, Japan. Appl. Environ. Microbiol. 55:1591-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu, H.-S., N. Roberts, F. L. Singleton, R. W. Attwell, D. J. Grimes, and R. R. Colwell. 1982. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 8:313-323. [DOI] [PubMed] [Google Scholar]
- 48.Yu, C., A. M. Lee, B. L. Bassler, and S. Roseman. 1991. Chitin utilization by marine bacteria. A physiological function for bacterial adhesion to immobilized carbohydrates. J. Biol. Chem. 266:24260-24267. [PubMed] [Google Scholar]