Abstract
Chemotaxis is the result of a refined interplay among various intracellular molecules that process spatial and temporal information. Here we present a modular scheme of the complex interactions between the front and the back of cells that allows them to navigate. First, at the front of the cell, activated Rho-type GTPases induce actin polymerization and pseudopod formation. Second, phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) is produced in a patch at the leading edge, where it binds pleckstrin-homology-domain-containing proteins, which enhance actin polymerization and translocation of the pseudopod. Third, in Dictyostelium amoebae, a cyclic-GMP-signalling cascade has been identified that regulates myosin filament formation in the posterior of the cell, thereby inhibiting the formation of lateral pseudopodia that could misdirect the cell.
Keywords: actin; myosin; cGMP; PtdIns(3,4,5)P3; modules
Introduction
Many cells sense the presence of extracellular chemical signals and guide their movement in the direction of the concentration gradient. This process, called chemotaxis, has a role in various functions such as finding food in prokaryotes, forming multicellular structures in protozoans, tracing bacterial infections by leukocytes and embryogenesis in metazoans (Baggiolini, 1998; Campbell & Butcher, 2000; Crone & Lee, 2002; Iijima et al, 2002). Cells that are exposed to chemoattractants can use temporal and spatial aspects of the chemoattractant gradient to orientate their movement. Prokaryotes use only the temporal component of the chemoattractant gradient, probably because, at 1–2 μm, they are too small to process spatial information. These cells exhibit a random walk, with movement steps in all directions. Cells frequently tumble and move in a new random direction. Tumbling frequency is decreased and movement in one direction is prolonged when cells experience an increase in chemoattractant concentration, that is, when moving up the gradient (Berg, 1988; Bourret & Stock, 2002). Eukaryotic chemotactic cells are about 10–20 μm in diameter, which allows them to process both spatial and temporal information: they measure the difference in chemoattractant concentration between the ends of the cell, and then move up the gradient of chemoattractant (Zigmond, 1977). Under starvation conditions, Dictyostelium cells emit the chemoattractant cyclic AMP, which causes them to aggregate and eventually form fruiting bodies. Dictyostelium cells are able to respond to a 2% difference in cAMP concentration between the front and the back of the cell, at a mean cAMP concentration that occupies only about 1% of its 40,000 cAMP receptors (Mato et al, 1975). The ability to sense and respond to shallow gradients of extracellular signals is remarkably similar in simple amoebae such as Dictyostelium and in mammalian leukocytes.
Here, we present data suggesting that chemotaxis is regulated by the interplay of at least three signalling pathways. These signalling pathways are presumably intricately connected to the basal mechanism of pseudopod formation (Pollard & Borisy, 2003). In the absence of chemoattractants, cells extend pseudopodia in a more or less random direction, probably as self-organizing structures (Bourne & Weiner, 2002). In the presence of a chemotactic gradient, this basic mechanism of pseudopod formation is biased to produce more pseudopodia at the front of the cell, as defined by the site of the highest chemoattractant concentration. This means that, during chemotaxis, cells become functionally polarized. In Dictyostelium cells that have been starved for 4 h, polarity is a rather transient state, because cells quickly extend a pseudopod at the back of the cell on gradient reversal (Swanson & Taylor, 1982; Chen et al, 2003; Kriebel et al, 2003). The polarity of neutrophils is more persistent, because gradient reversal usually leads to the formation of additional pseudopodia at the front by which cells make U-turns (Zigmond, 1977). Such a rigid polarization might improve chemotaxis by enhancing the persistence of movement in the gradient, but it also makes the cells less able to respond to changes in gradient direction. Interestingly, Dictyostelium cells starved for 7 h are more polar than those starved for a shorter period. At the onset of Dictyostelium cell aggregation, many potential aggregation centres emit cAMP waves, and cells experience successive cAMP gradients from different directions. After 7 h, the aggregation centres are fixed and the chemoattractant gradients become stable.
Diffusion of intracellular messengers
During chemotaxis, pseudopodia are extended at the leading edge and retracted at the back of the cell. These processes are regulated by intracellular second messengers that are produced on the activation of chemoattractant receptors, and whose diffusion should meet two contradictory requirements. To establish and preserve a front-to-tail polarity, intracellular signalling molecules must not be allowed to diffuse very far into the cell. Conversely, the ability of the cells to detect shallow gradients requires the local integration of information from several occupied receptors, which implies that signalling molecules must diffuse over some distance. The complex mathematics of molecular diffusion can be reduced to a simple parameter, dispersion length, Ld = √(Dτ), which describes the average distance that a molecule with diffusion coefficient D travels from its source before it is degraded after a life-time (the life-time is the reciprocal of the degradation rate constant k; that is, 1/k; Postma & Van Haastert, 2001). A molecule will not move far from its source if it diffuses very slowly and/or is degraded very quickly. Soluble second messengers such as cGMP diffuse relatively quickly, so they cannot preserve spatial information unless their diffusion is restricted or their degradation is extremely fast (a life-time of less than 10 ms). With an observed life-time of about 10 s (see Valkema & Van Haastert, 1994), the dispersion length of cGMP in Dictyostelium can be estimated to be about 55 μm, which is several times the length of the cell (Table 1). Although such molecules cannot preserve spatial information in a 10-μm cell, they are well suited to provide temporal and global intracellular communication. Conversely, transmembrane proteins such as receptors diffuse extremely slowly, so that ligand-occupied receptors fully retain spatial information. However, with molecules whose diffusion is so sluggish, communication between the front and the back of cells would require hours. For example, the Dictyostelium cAMP–receptor complex has a life-time of about 1.4 s. In this short period, the cAMP–receptor complex diffuses about 0.2 μm, which is only as far as the closest receptor. Thus, receptors cannot even integrate spatial information locally. By contrast, membrane-localized lipids such as phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3), with a dispersion length of about 2 μm diffuse sufficiently slowly to preserve spatial information, but fast enough to allow the integration of information at the front of the cell.
Table 1.
Dispersion length of signalling components for Dictyostelium chemotaxis
| Compound | Diffusion coefficient, D (μm2 s−1) | Life-time, τ (s) | Dispersion length, Ld = √(Dτ) (μm) |
|---|---|---|---|
| cGMP | 300 | 10 | 55 |
| G protein in cytosol | 10 | 20 | 14 |
| PtdIns(3,4,5)P3 | 1 | 5 | 2.2 |
| G protein in membrane | 0.1 | 20 | 1.4 |
| Receptor–cAMP complex | 0.027 | 1.4 | 0.20 |
Some measurements of cells might help in interpreting these data. The diameter of a Dictyostelium cell is about 10 μm and a pseudopod has a size of 2–3 μm. A Dictyostelium cell contains about 40,000 cAMP receptors distributed over a surface area of about 400 μm2, yielding an average distance between receptors of 0.1 μm. At threshold cAMP concentrations (1 nM) only about 400 receptors are occupied with cAMP, and the average distance between occupied receptors is about 1 μm. References: diffusion of cGMP, Chen et al. (1999); cGMP degradation in vivo, Valkema & Van Haastert (1994); diffusion of phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3), Almeida & Vaz (1995); degradation of PtdIns(3,4,5)P3 in vivo, Huang et al. (2003); diffusion of G protein in cytosol as diffusion of cytosolic GFP, Potma et al. (2001); degradation of GαGTP from interconversions of cAMP-binding sites, Van Haastert et al. (1986); diffusion of G proteins in membrane with lipid anchor slower than diffusion of lipid, degradation of G proteins in membrane as degradation of soluble G-protein and diffusion of receptor–cAMP complex, Ueda et al. (2001); life-time of receptor–cAMP complex, Van Haastert et al. (1986).
Heterotrimeric and small GTP-binding proteins form another interesting group of signal transducers, because they can either diffuse in the cytosol (such as many activated G protein α-subunits) or localize to the plasma membrane through lipid anchors (such as some G protein α-subunits, most G protein β-subunits and many small G proteins). In the cytosol, the diffusion of these proteins is rather fast, whereas when they are anchored to the plasma membrane, their diffusion is about 100-fold slower. In view of the average life-time of the active GTP-bound state of G proteins, it is expected that a soluble G protein has a dispersion length of about 10–20 μm, whereas a membrane-anchored G protein (or its subunits) will disperse about 1.5 μm.
Thus, soluble second messengers and small proteins are well suited to the transmission of temporal information through global signalling but their diffusion is too fast to retain spatial information. Transmembrane proteins, in contrast, are too sluggish. However, phospholipids and membrane lipid-anchored proteins have the required diffusion properties to preserve and integrate spatial information locally within a few micrometres, which corresponds to the size of a pseudopod.
Intracellular signalling at the front of the cell
Several excitation–inhibition models for chemotaxis have been proposed to explain the conversion of a shallow gradient of chemo-attractant to activation at the front of the cell. These models all use a local activator and a global inhibitor in some way (Haugh & Lauffenburger, 1997; Parent & Devreotes, 1999; Meinhardt & Gierer, 2000; Postma & Van Haastert, 2001; Iijima et al, 2002; Levchenko & Iglesias, 2002). However, the primary intracellular activator has not been fully identified. One of the proposed candidates is PtdIns(3,4,5)P3. During chemotaxis, several proteins that contain pleckstrin homology (PH) domains translocate from the cytosol to the plasma membrane at the leading edge of both Dictyostelium cells and leukocytes (Parent et al, 1998; Meili et al, 1999; Servant et al, 2000). These proteins include the cytosolic regulator of adenylyl cyclase (Crac) and protein kinase B (Akt/PKB). Several indications suggest that, in vivo, the PH-domain-containing proteins bind to PtdIns(3,4,5)P3 but not to other products of the phosphatidylinositol-3-OH kinase (PI(3)K) pathway such as PtdIns(3,4)P2. First, chemoattractants induce the accumulation of PtdIns(3,4,5)P3 with the appropriate kinetics in both Dictyostelium and neutrophils (Traynor-Kaplan et al, 1989; Huang et al, 2003). Second, the inactivation of the gene that encodes the phosphatase and tensin homologue (PTEN) leads to higher PtdIns(3,4,5)P3 concentrations and enhanced chemoattractantstimulated translocation of PH-domain-containing proteins (Iijima & Devreotes, 2002; Huang et al, 2003). Third, in Dictyostelium the concentration of PtdIns(3,4)P2 is very low (H.M.L., D. Blero, C. Erneux and P.J.M.V.H., unpublished observations).
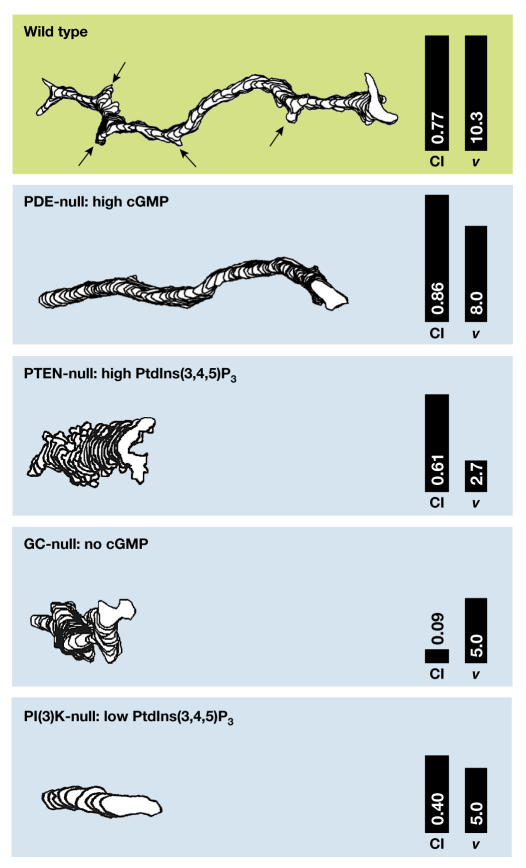
PtdIns(3,4,5)P3 is synthesized by the PI(3)Ks PI3K1 and PI3K2 in Dictyostelium and by PI(3)Kγ in neutrophils (Zhou et al, 1995; Hirsch et al, 2000; Huang et al, 2003), and is degraded by the 3-phosphatase PTEN (Iijima & Devreotes, 2002) and by multiple 5-phosphatases (Loovers et al, 2002). Recent studies have shown that PI3K1 and PI3K2 translocate from the cytosol to the front of Dictyostelium cells, whereas PTEN becomes localized to the back (Funamoto et al, 2002; Iijima & Devreotes, 2002). In pten-null cells, the absence of 3-phosphatase activity leads to an increase in the membrane area with bound PHCrac–GFP (the PH domain of Crac fused to green fluorescent protein) and also results in an expansion of the region from which F-actin-filled pseudopodia are extended (Iijima & Devreotes, 2002). Thus, where PtdIns(3,4,5)P3 is formed in the cell, its diffusion kinetics and its apparent ability to induce F-actin formation make PtdIns(3,4,5)P3 an ideal second messenger for chemotaxis. However, the chemotactic defect of Dictyostelium pi3k1−/pi3k2− or mammalian PI(3)Kγ-null cells is only partial and results in a decrease in speed rather than in poor directionality of cell movement (Fig 1). These modest defects are also observed in wild-type cells that have been treated with moderate concentrations of the PI(3)K inhibitors LY294002 and wortmannin (Funamoto et al, 2002; Iijima et al, 2002; Wang et al, 2002; Huang et al, 2003). Furthermore, it has been shown in Dictyostelium that the amino-terminal segment of PI(3)K expressed in pi3k-null cells translocates to the leading edge, suggesting that signalling molecules upstream of PI(3)K are already highly localized (Funamoto et al, 2002).
Figure 1.
Chemotaxis of Dictyostelium mutants. The tracks of Dictyostelium mutants moving for 10 min in a cAMP gradient were obtained by computer-assisted analysis. The arrows in the upper panel indicate the formation of lateral pseudopodia, which are absent from phosphodiesterase (PDE)-null cells. The numbers in each panel refer to the chemotaxis index (CI), which is the efficiency of orientating in the gradient, and the speed of movement (v, in μm s−1). Data are from Bosgraaf et al (2002) and Iijima & Devreotes (2002), and are corrected for the differences between the wild types in these three reports. GC, guanylyl cyclase; PI(3)K, phosphatidylinositol-3-OH kinase; PTEN, phosphatase and tensin homologue.
Another candidate for the initial intracellular activator are small G proteins. In neutrophils, it has been shown that chemoattractant-induced stimulation of Rho GTPase-family members has an essential role in cellular polarization (by means of Cdc42) and F-actin formation (by means of Rac1) (Wang et al, 2002; Srinivasan et al, 2003; Xu et al, 2003). Small GTP-binding proteins are positively and negatively regulated by guanine exchange factors (GEFs) and GTPase-activating proteins (GAPs), respectively (Etienne-Manneville & Hall, 2002). It is conceivable that the interplay between GEFs and GAPs causes a local accumulation of active small GTP-binding proteins at the future front of the cell, which induces actin polymerization and pseudopod formation. A similar signal transduction cascade with a putative small GTP-binding protein has been proposed for Dictyostelium (Chung et al, 2001a).
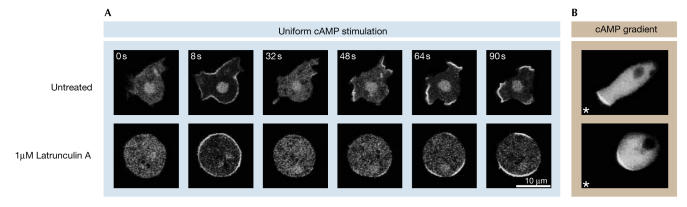
If the PI(3)K/PTEN system is not responsible for the primary processing of the chemoattractant gradient, what is the function of PtdIns(3,4,5)P3 synthesis and the translocation of PH-domain-containing proteins to the leading edge? Strikingly, Dictyostelium cells stimulated with uniform cAMP concentrations exhibit a biphasic PHCrac–GFP response: there is first a uniform translocation of PHCrac–GFP to the cell boundary, which is followed by a second persistent translocation of PHCrac–GFP to smaller patches (Postma et al, 2003). These patches of PHCrac–GFP are also observed in latrunculin-treated cells (Fig 2). Latrunculin A inhibits actin filaments and leads to symmetrical spherical cells without pseudopodia (Parent et al, 1998), which indicates that patches of PHCrac–GFP are formed in the absence of actin filaments, pseudopodia or other visible spatial cues. The patches that form under conditions of uniform cAMP distribution have essentially the same properties with respect to size, life-time and intensity as the single patch of PHCrac–GFP that is formed at the leading edge of cells in a cAMP gradient (Fig 2). PHCrac–GFP patches are self-organizing; that is, PHCrac–GFP patches are triggered by cAMP but the cAMP concentration has no effect on the size, intensity or life-time of the patches. About 10 s after a patch is formed, a pseudopod is extended from the area of the cell with a PHCrac–GFP patch. Furthermore, pseudopodia with PHCrac–GFP patches show movement steps that are about double in size those of pseudopodia without PHCrac–GFP patches. In uniform cAMP, patches of PHCrac–GFP are induced by very low cAMP concentrations, the response does not show perfect adaptation, and PHCrac–GFP patch formation is readily reversible on the removal and readdition of cAMP (Chen et al, 2003; Postma et al, 2003; M.P., J. Roelofs, J. Goedhart, H.M.L., A.J.W.G. Visser and P.J.M.V.H., unpublished observations). We propose two functions for the PtdIns(3,4,5)P3 synthesis that occurs at these PHCrac–GFP patches at the leading edge. First, the absence of adaptation implies that cells constantly monitor changes in the direction of the chemoattractant gradient that triggers new patches of PtdIns(3,4,5)P3. Second, the induction of PtdIns(3,4,5)P3 patches by very low cAMP concentrations and the increased movement steps of pseudopodia with patches suggest that the PI(3)K pathway increases the efficiency of the cells to detect and process shallow gradients.
Figure 2.
Localization of phosphatidylinositol-3,4,5-trisphosphate by using PHCrac–GFP. (A) Dictyostelium cells were stimulated in a perfusion chamber with a uniform cAMP concentration of 1 μM. In untreated cells, PHCrac–GFP transiently translocates to the entire membrane in about 8 s, which is followed by persistent translocation of PHCrac–GFP to small patches. About 10 s after a PHCrac–GFP patch appears, a pseudopod is formed at the position of a PHCrac–GFP patch (Postma et al, 2003). In the lower panels, cells were pretreated for 5 min with 1 μM latrunculin A, which induces the depolymerization of actin, the inhibition of pseudopod formation and the rounding up of the cell. A uniform cAMP concentration still induces a localized response with patches of PHCrac–GFP in these spherical cells. (B) Cells were stimulated locally with cAMP by using a micropipette. The position of the pipette is indicated by an asterisk (data from Parent et al, 1998; Parent & Devreotes, 1999). The multiple patches induced by uniform cAMP have the same size and life-time as the single patch induced by the cAMP gradient, and they closely correlate with pseudopod formation. Crac, cytosolic regulator of adenylyl cyclase; GFP, green fluorescent protein; PH, pleckstrin homology.
Intracellular signalling at the back of the cell
The suppression of pseudopodia at the back of the cell is important for efficient chemotaxis, and this seems to be mediated by myosin filaments in the cortex at the back of the cell, both in Dictyostelium and in leukocytes (Stites et al, 1998; Eddy et al, 2000; Xu et al, 2003). Previous work in Dictyostelium suggests that intracellular cGMP is a good candidate for the messenger that induces myosin filament formation and the suppression of lateral pseudopodia (Liu & Newell, 1993). Recently, the genes that encode guanylyl cyclases, cGMP-phosphodiesterases and cGMP-target proteins were identified, and these have been used to resolve the function of cGMP signalling in Dictyostelium chemotaxis (Bosgraaf et al, 2002; Goldberg et al, 2002; Roelofs et al, 2002). Cells that lack two phosphodiesterases contain very high concentrations of cGMP, which are accompanied by an increase in the number of myosin filaments in the cortex and the suppression of lateral pseudopodia (Fig 1). Cells without guanylyl cyclases or without the cGMP targets show very poor chemotaxis (Bosgraaf et al, 2002). Although cGMP clearly mediates myosin filament formation, cGMP might have other functions that are unknown at present, because the deletion of guanylyl cyclases has a more drastic effect on chemotaxis than the deletion of myosin (Wessels et al, 1988; Bosgraaf et al, 2002). In addition, cGMP might not be the only mediator of myosin filament formation. A small residual myosin response remains detectable in guanylyl-cyclase-null cells (Bosgraaf et al, 2002), which might be mediated by the activation of PAKa, a member of the p21-activated kinase family, by PI(3)K (Chung & Firtel, 1999; Chung et al, 2001b). In neutrophils, myosin filament formation seems to be mediated by a Rhostimulated kinase (Xu et al, 2003) rather than by a cGMPstimulated kinase. Although these pathways are different in neutrophils and in Dictyostelium, the end result is essentially identical: myosin filaments are formed in the back and at the sides of the cell that mediate the contraction of the back and inhibit the formation of lateral pseudopodia.
In Dictyostelium, chemoattractant-induced cGMP formation is tightly regulated by adaptation, such that only an increment in the chemoattractant concentration leads to a higher concentration of cGMP (Van Haastert & Van der Heijden, 1983). These temporal properties of cGMP formation could be important for chemotaxis. As long as cells move up the gradient, the cGMP concentration is higher, leading to the inhibition of pseudopodia at the back of the cell. When the chemoattractant concentration no longer changes with time, or when the gradient reverses, the cGMP concentration declines, and soon the back of the cell regains the ability to form pseudopodia. This temporal component of chemotactic signalling in Dictyostelium is similar to the temporal mechanism of chemotaxis in prokaryotes (Berg, 1988).
As mentioned above, it is unlikely that cGMP can form spatial gradients inside cells because a cGMP molecule can diffuse on average five cell lengths before it is degraded. What prevents the formation of myosin filaments at the front, if cGMP cannot form a gradient in the cell? Recent studies show that myosin heavy-chain kinase A (MHCK-A) translocates to the leading edge (Liang et al, 2002; Steimle et al, 2002). It is known that phosphorylated myosin is bent and does not form filaments (de la Roche & Cote, 2001), suggesting that myosin filaments at the leading edge fall apart. It seems that temporal and spatial mechanisms collaborate: cGMP induces myosin filaments globally as long as the chemoattractant concentration increases with time, whereas filaments dissociate at the front of the cell as a result of the local activity of MHCK-A. In this way, myosin filaments become restricted to the back of the cell, where they retract the uropod and suppress lateral pseudopodia.
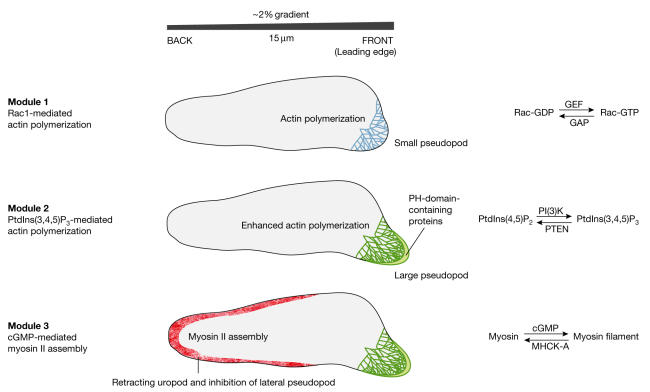
In conclusion, our present knowledge, which has been gleaned from different systems such as mammalian neutrophils and protozoan amoebae, suggests that gradient sensing during chemotaxis does not depend on a single molecular mechanism that informs cells where to go, but instead consists of several modules of interconnected signalling networks (Fig 3). Efficient chemotaxis is the result of a refined interplay between these modules to transmit and integrate spatial information (such as PtdIns(3,4,5)P3) and temporal information (for example cGMP). The spatial signals are formed in a highly nonlinear way and spread relatively slowly so that high local concentrations appear in patches. Temporal signals spread swiftly and serve as global inhibitors; their formation might be subject to adaptation so that they are only generated when the stimulus concentration increases with time. These properties of chemotaxis are surprisingly similar in Dictyostelium and leukocytes, cells that diverged during evolution about a billion years ago. Through future research it will be exciting to uncover additional modules of regulatory interactions that add to the wonderful complexity of cellular navigation systems.
Figure 3.
Modules in chemotaxis. The first module provides the formation of active small G proteins of the Rho/Rac group, leading to actin polymerization and a small pseudopod at the front of the cell. It is proposed that the differential spatial activation of stimulatory guanine exchange factors (GEFs) and inhibitory GTPase-activating proteins (GAPs) leads to the accumulation of GTP-bound G protein at the higher chemoattractant concentrations. The polarity induced by the first module is used by the second module to trigger a patch of phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) at the leading edge, which enhances actin polymerization and extension of the pseudopod. The third module inhibits the formation of pseudopodia in the back and at the sides of the cell, and induces retraction of the uropod. An increase in cGMP concentration leads to myosin filament formation throughout the cortex, while myosin becomes phosphorylated and is depolymerized in the front of the cell by a specific kinase (myosin heavy-chain kinase A (MHCK-A)) that translocates to the leading edge. Other pathways (PAKa in Dictyostelium and Rho in neutrophils) might also contribute to myosin filament formation.
Acknowledgments
We thank J. Knol for valuable discussions and critical reading of the manuscript. This research was supported by the Netherlands Organization for Scientific Research.
References
- Almeida PFF, Vaz WLC (1995) Lateral Diffusion in Membranes. Elsevier, Amsterdam, the Netherlands [Google Scholar]
- Baggiolini M (1998) Chemokines and leukocyte traffic. Nature 392: 565–568 [DOI] [PubMed] [Google Scholar]
- Berg HC (1988) A physicist looks at bacterial chemotaxis. Cold Spring Harb Symp Quant Biol 53: 1–9 [DOI] [PubMed] [Google Scholar]
- Bosgraaf L, Russcher H, Smith JL, Wessels D, Soll DR, Van Haastert PJM (2002) A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J 21: 4560–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Weiner O (2002) A chemical compass. Nature 419: 21. [DOI] [PubMed] [Google Scholar]
- Bourret RB, Stock AM (2002) Molecular information processing: lessons from bacterial chemotaxis. J Biol Chem 277: 9625–9628 [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Butcher EC (2000) Chemokines in tissuespecific and microenvironment-specific lymphocyte homing. Curr Opin Immunol 12: 336–341 [DOI] [PubMed] [Google Scholar]
- Chen C, Nakamura T, Koutalos Y (1999) Cyclic AMP diffusion coefficient in frog olfactory cilia. Biophys J 76: 2861–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Janetopoulos C, Huang YE, Iijima M, Borleis J, Devreotes P (2003) Two phases of actin polymerization display different dependencies on PtdIns(3,4,5)P3 accumulation and have unique roles during chemotaxis. Mol Biol Cell 14: 5028–5037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Firtel RA (1999) PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis. J Cell Biol 147: 559–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Funamoto S, Firtel RA (2001a) Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem Sci 26: 557–566 [DOI] [PubMed] [Google Scholar]
- Chung CY, Potikyan G, Firtel RA (2001b) Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol Cell 7: 937–947 [DOI] [PubMed] [Google Scholar]
- Crone SA, Lee KF (2002) The bound leading the bound: target-derived receptors act as guidance cues. Neuron 36: 333–335 [DOI] [PubMed] [Google Scholar]
- de la Roche MA, Cote GP (2001) Regulation of Dictyostelium myosin I and II. Biochim Biophys Acta 1525: 245–261 [DOI] [PubMed] [Google Scholar]
- Eddy RJ, Pierini LM, Matsumura F, Maxfield FR (2000) Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J Cell Sci 113: 1287–1298 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420: 629–635 [DOI] [PubMed] [Google Scholar]
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA (2002) Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109: 611–623 [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Bosgraaf L, Van Haastert PJM, Smith L (2002) Identification of four candidate cGMP targets in Dictyostelium. Proc Natl Acad Sci USA 99: 6749–6754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh JM, Lauffenburger DA (1997) Physical modulation of intracellular signaling processes by locational regulation. Biophys J 72: 2014–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP (2000) Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 287: 1049–1053 [DOI] [PubMed] [Google Scholar]
- Huang YE, Iijima M, Parent CA, Funamoto S, Firtel RA, Devreotes PN (2003) Receptor mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol Biol Cell 14: 1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Devreotes P (2002) Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109: 599–610 [DOI] [PubMed] [Google Scholar]
- Iijima M, Huang YE, Devreotes P (2002) Temporal and spatial regulation of chemotaxis. Dev Cell 3: 469–478 [DOI] [PubMed] [Google Scholar]
- Kriebel PW, Barr VA, Parent CA (2003) Adenylyl cyclase localization regulates streaming during chemotaxis. Cell 112: 549–560 [DOI] [PubMed] [Google Scholar]
- Levchenko A, Iglesias PA (2002) Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys J 82: 50–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Licate L, Warrick H, Spudich J, Egelhoff T (2002) Differential localization in cells of myosin II heavy chain kinases during cytokinesis and polarized migration. BMC Cell Biol 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Newell PC (1993) Role of cyclic GMP in signal transduction to cytoskeletal myosin. Symp Soc Exp Biol 47: 283–295 [PubMed] [Google Scholar]
- Loovers H, Veenstra K, Snippe H, Pesesse X, Erneux C, Van Haastert PJM (2002) A diverse family of inositol 5-phosphatases playing a role in growth and development in Dictyostelium discoideum. J Biol Chem 278: 5652–5658 [DOI] [PubMed] [Google Scholar]
- Mato JM, Losada A, Nanjundiah V, Konijn TM (1975) Signal input for a chemotactic response in the cellular slime mold Dictyostelium discoideum. Proc Natl Acad Sci USA 72: 4991–4993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA (1999) Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J 18: 2092–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H, Gierer A (2000) Pattern formation by local self-activation and lateral inhibition. BioEssays 22: 753–760 [DOI] [PubMed] [Google Scholar]
- Parent CA, Devreotes PN (1999) A cell's sense of direction. Science 284: 765–770 [DOI] [PubMed] [Google Scholar]
- Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN (1998) G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95: 81–91 [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465 [DOI] [PubMed] [Google Scholar]
- Postma M, Van Haastert PJM (2001) A diffusion-translocation model for gradient sensing by chemotactic cells. Biophys J 81: 1314–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma M, Roelofs J, Goedhart J, Gadella TWJ, Visser AJWG, Van Haastert PJM (2003) Uniform cAMP stimulation of Dictyostelium cells induces localized patches of signal transduction and pseudopodia. Mol Biol Cell 14: 5019–5027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potma EO, de Boeij WP, Bosgraaf L, Roelofs J, Van Haastert PJM, Wiersma DA (2001) Reduced protein diffusion rate by cytoskeleton in vegetative and polarized Dictyostelium cells. Biophys J 81: 2010–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs J, Smith JL, Van Haastert PJM (2002) cGMP signalling: different ways to create a pathway. Trends Genet 19: 132–134 [DOI] [PubMed] [Google Scholar]
- Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR (2000) Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287: 1037–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR (2003) Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol 160: 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimle PA, Licate L, Cote GP, Egelhoff TT (2002) Lamellipodial localization of Dictyostelium myosin heavy chain kinase A is mediated via F-actin binding by the coiled-coil domain. FEBS Lett 516: 58–62 [DOI] [PubMed] [Google Scholar]
- Stites J, Wessels D, Uhl A, Egelhoff T, Shutt D, Soll DR (1998) Phosphorylation of the Dictyostelium myosin II heavy chain is necessary for maintaining cellular polarity and suppressing turning during chemotaxis. Cell Motil Cytoskel 39: 31–51 [DOI] [PubMed] [Google Scholar]
- Swanson JA, Taylor DL (1982) Local and spatially coordinated movements in Dictyostelium discoideum amoebae during chemotaxis. Cell 28: 225–232 [DOI] [PubMed] [Google Scholar]
- Traynor-Kaplan AE, Thompson BL, Harris AL, Taylor P, Omann GM, Sklar LA (1989) Transient increase in phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol trisphosphate during activation of human neutrophils. J Biol Chem 264: 15668–15673 [PubMed] [Google Scholar]
- Ueda M, Sako Y, Tanaka T, Devreotes P, Yanagida T (2001) Single-molecule analysis of chemotactic signaling in Dictyostelium cells. Science 294: 864–867 [DOI] [PubMed] [Google Scholar]
- Valkema R, Van Haastert PJM (1994) A model for cAMP-mediated cGMP response in Dictyostelium discoideum. Mol Biol Cell 5: 575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert PJM, Van der Heijden PR (1983) Excitation, adaptation, and deadaptation of the cAMP-mediated cGMP response in Dictyostelium discoideum. J Cell Biol 96: 347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert PJM, de Wit RJ, Janssens PM, Kesbeke F, DeGoede J (1986) G-protein-mediated interconversions of cellsurface cAMP receptors and their involvement in excitation and desensitization of guanylate cyclase in Dictyostelium discoideum. J Biol Chem 261: 6904–6911 [PubMed] [Google Scholar]
- Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR (2002) Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nature Cell Biol 4: 513–518 [DOI] [PubMed] [Google Scholar]
- Wessels D, Soll DR, Knecht D, Loomis WF, De Lozanne A, Spudich J (1988) Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Dev Biol 128: 164–177 [DOI] [PubMed] [Google Scholar]
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR (2003) Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114: 201–214 [DOI] [PubMed] [Google Scholar]
- Zhou K, Takegawa K, Emr SD, Firtel RA (1995) A phosphatidylinositol (PI) kinase gene family in Dictyostelium discoideum: biological roles of putative mammalian p110 and yeast Vps34p PI 3-kinase homologs during growth and development. Mol Cell Biol 15: 5645–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH (1977) Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol 75: 606–616 [DOI] [PMC free article] [PubMed] [Google Scholar]