Abstract
The classical idea that G-protein-coupled receptors (GPCRs) function as monomeric entities has been unsettled by the emerging concept of GPCR dimerization. Recent findings have indicated not only that many GPCRs exist as homodimers and heterodimers, but also that their oligomeric assembly could have important functional roles. Several studies have shown that dimerization occurs early after biosynthesis, suggesting that it has a primary role in receptor maturation. G-protein coupling, downstream signalling and regulatory processes such as internalization have also been shown to be influenced by the dimeric nature of the receptors. In addition to raising fundamental questions about GPCR function, the concept of dimerization could be important in the development and screening of drugs that act through this receptor class. In particular, the changes in ligand-binding and signalling properties that accompany heterodimerization could give rise to an unexpected pharmacological diversity that would need to be considered.
Keywords: G-protein-coupled receptor function, heterodimerization, homodimerization, oligomerization, regulation
Introduction
G-protein-coupled receptors (GPCRs) have classically been assumed to exist and function as monomeric entities, and the paradigms of ligand binding and signal transduction were based on this hypothesis. However, a growing body of biochemical and biophysical evidence indicates that some GPCRs can form both homodimers and heterodimers. Although their existence is now largely accepted (Angers et al, 2002; George et al, 2002), their functional importance remains more enigmatic and in some cases even controversial. The five stages of the GPCR life cycle that could be affected by dimerization are depicted in Fig 1. In this review, we focus our attention on the potential functional implications that have been proposed for GPCR dimerization at each of these stages, indicating, when possible, the aspects that will require additional studies before definitive conclusions can be drawn. Given that most current methods used do not strictly distinguish between dimers and larger oligomers, we refer systematically to the term 'dimer' because it represents the minimal oligomeric arrangement.
Figure 1. Potential roles of G-protein-coupled receptor (GPCR) dimerization during the GPCR life cycle.
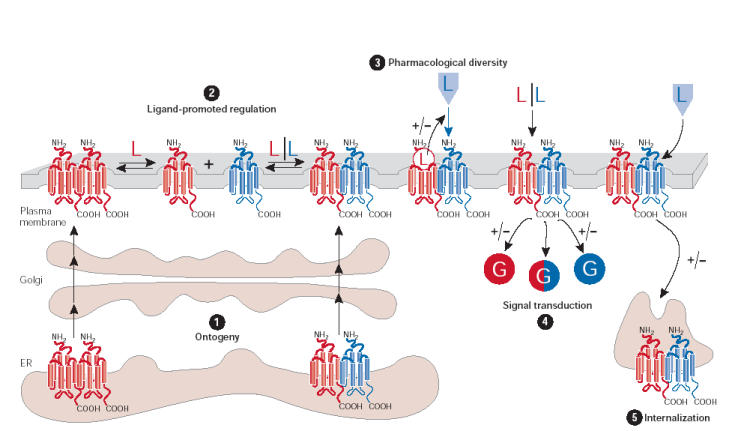
(1) In some cases, dimerization has been shown to have a primary role in receptor maturation and allows the correct transport of GPCRs from the endoplasmic reticulum (ER) to the cell surface. (2) Once at the plasma membrane, dimers might become the target for dynamic regulation by ligand binding. (3) It has been proposed that GPCR heterodimerization leads to both positive (+) and negative (−) ligand binding cooperativity, as well as (4) potentiating (+)/attenuating (−) signalling or changing G-protein selectivity. (5) Heterodimerization can promote the co-internalization of two receptors after the stimulation of only one protomer. Alternatively, the presence of a protomer that is resistant to agonist-promoted endocytosis, within a heterodimer, can inhibit the internalization of the complex. G, G protein; L, ligand.
Ontogeny
GPCR biosynthesis and transport along the secretory pathway are poorly characterized, but their exit from the endoplasmic reticulum (ER) is a crucial step that controls their cell surface expression (Petaja-Repo et al, 2000). Only correctly folded receptors escape the ER quality-control system and are allowed to exit, whereas incompletely folded or misfolded proteins are retained and eventually degraded (Petaja-Repo et al, 2001). For many proteins, oligomeric assembly has an important function in ER quality control because it masks specific retention signals or hydrophobic patches that would otherwise retain the proteins in the ER (Reddy & Corley, 1998). For GPCRs, the necessity of dimerization for correct transport to the plasma membrane has been clearly shown for the metabotropic γ-aminobutyric acid b receptor (GbR), which is composed of two subunits GbR1 and GbR2 (Marshall et al, 1999). When expressed alone, GbR1 is retained intracellularly as an immature protein because it has a carboxy-terminal ER retention motif (Margeta-Mitrovic et al, 2000), whereas GbR2 reaches the cell surface but is not functional. Following their co-expression, heterodimerization masks the GbR1 ER retention signal, allowing the proper targeting of a functional heterodimeric GbR to the plasma membrane. Although a general role for heterodimerization and/or homodimerization in GPCR quality control and ER export has not yet been established, studies using cellular fractionation and fluorescence or bioluminescence resonance energy transfer (FRET and BRET, respectively) have revealed that several GPCRs dimerize in the ER (Issafras et al, 2002; Overton & Blumer, 2002; Terrillon et al, 2003; Floyd et al, 2003). Consistent with the idea that GPCR dimerization occurs early in the secretory pathway is the observation that truncated mutants of vasopressin V2R (Zhu & Wess, 1998), D3 dopamine (Karpa et al, 2000), GnRH gonadotropin-releasing hormone (Grosse et al, 1997) and CCR5 chemokine (Benkirane et al, 1997; Shioda et al, 2001) receptors, as well as rhodopsin mutants (Colley et al, 1995) behave as dominant-negatives of their respective wild-type receptors by preventing their expression on the cell surface. As the physical interaction between wild-type and mutant receptors was confirmed by co-immunoprecipitation in some of these studies (Benkirane et al, 1997; Zhu & Wess, 1998; Karpa et al, 2000), the dominant-negative action was taken as evidence that early heterodimerization between wild-type and mutant receptors leads to their ER retention. For naturally occurring mutations this could have pathophysiological consequences. For example, it has been suggested that the loss of cellsurface expression of CCR5 observed following its co-expression with the ER-retained CCR5Δ32 mutant contributes to the delayed onset of AIDS in HIV-infected patients that harbour a CCR5/CCR5Δ32 genotype (Benkirane et al, 1997). A series of rhodopsin mutants was also proposed to cause retinal degeneration in Drosophila by interfering with the maturation of the wild-type photoreceptor (Colley et al, 1995).
Ligand-promoted regulation
A role for dimerization in GPCR ontogeny does not exclude the possibility that, once the receptor has reached the cell surface, its oligomeric state could be dynamically regulated. Whether receptor activation can promote or inhibit dimerization and/or favour exchanges between protomers is a central question with wide implications for the mechanisms of receptor activation and regulation. Unfortunately, no general consensus has yet been established. Whereas several studies suggest that ligand binding can regulate the dimer by either promoting (Rodriguez-Frade et al, 1999; Angers et al, 2000; Rocheville et al, 2000a,b; Cornea et al, 2001; Horvat et al, 2001; Kroeger et al, 2001; Wurch et al, 2001; Patel et al, 2002; Zhu et al, 2002; Hunzicker-Dunn et al, 2003; Roess & Smith, 2003) or inhibiting (Gines et al, 2000; Cheng & Miller, 2001; Latif et al, 2002) its formation, many others conclude that homodimerization and heterodimerization are constitutive processes that are not modulated by ligand binding (Overton & Blumer, 2000; Ayoub et al, 2002; Issafras et al, 2002; Jensen et al, 2002; Babcock et al, 2003; Canals et al, 2003; Dinger et al, 2003; Floyd et al, 2003; Guo et al, 2003; Stanasila et al, 2003; Terrillon et al, 2003; Trettel et al, 2003). Although such discrepancies might reflect intrinsic differences in the behaviour of the individual receptors considered in each study, they might also result from interpretational difficulties linked to the techniques used. In many cases, the extent of receptor co-immunoprecipitation, determined by western blot analysis, was used to assess the effects of ligands on the dimerization state. Unfortunately, such approaches are not highly quantitative, and changes in the immunoreactivity detected could result from agonist-induced conformational changes that alter epitope recognition rather than from varying amounts of receptor dimers. In other cases, ligand-induced increases or decreases in BRET or FRET were interpreted as ligand-regulated dimerization. However, given that the efficacy of BRET and FRET is highly dependent on the relative distance and orientation between the donor and acceptor fluorophores, ligand-promoted changes in energy transfer could reflect either a modulation of the dimerization process or changes in the conformation of pre-existing dimers. Additional experimental approaches will be required to determine unambiguously whether or not the dimerization process is subjected to ligand-promoted regulation.
In any case, the structural data available strongly suggest that at least some GPCRs can form dimers in the absence of ligand stimulation. For instance, crystallization of the extracellular amino-terminal ligand-binding domains of the metabotropic glutamate receptor, mGluR1 (Kunishima et al, 2000), and the Wnt receptor, frizzled (Dann et al, 2001), revealed that they exist as dimers in the absence of their ligands. More recently, Palczewski and co-workers used atomic force microscopy to show that rhodopsin and opsin form constitutive dimers in dark-adapted native retinal membranes (Fotiadis et al, 2003; Liang et al, 2003).
Pharmacological diversity
The concept that GPCR heterodimerization could have a role in pharmacological diversity was first indicated by studies on the δ- and κ-opioid receptors (Jordan & Devi, 1999). Co-expression of both receptors led to the formation of a stable heterodimer with a very low affinity for either the δ- or the κ-selective ligand alone. However, high affinity was restored following the combination of the two ligands, suggesting the occurrence of positive cooperativity. Although the direct link between heterodimerization itself and the changes in pharmacological properties has not been formally established, positive or negative ligand-binding cooperativity that occurs after receptor co-expression has been interpreted as resulting from receptor heterodimerization for many other GPCRs. These include the metabotropic GbR GbR1/GbR2 (Galvez et al, 2001), opioid δ/μ (Gomes et al, 2000), muscarinic m2/m3 (Maggio et al, 1999), somatostatin SSTR5/dopamine D2 (Rocheville et al, 2000a) and adenosine A2A/dopamine D1 (Franco et al, 2000) receptors. If this is a general phenomenon, such heterodimerization between pharmacologically distinct receptors could underlie a level of pharmacological diversity that would have far-reaching implications for drug development. In particular, it could provide new opportunities for the development of more selective compounds that would target specific heterodimers without affecting the individual protomers (George et al, 2002). Obviously, testing all possible combinations from existing GPCRs represents a daunting task that cannot be achieved with the current methods. So, establishing the rules that dictate the selectivity of interactions between receptors and determining their occurrence in native tissues will be essential before heterodimerization can be systematically incorporated into drug screening campaigns.
Signal transduction
The first convincing evidence that GPCR dimerization has a crucial function in signal transduction came from studies on the GbR. Even though GbR1 harbours the binding site for γ-aminobutyric acid (GABA), its co-expression with GbR2 was found to be essential for the formation of a receptor that can couple functionally to the G-protein signalling cascade (Margeta-Mitrovic et al, 2000; Galvez et al, 2001). This is not just because GbR2 is required for the expression of GbR1 on the cell surface (see above), as a mutant form of GbR1 that lacks its ER retention signal and can reach the cell surface on its own still requires GbR2 for functional activity (Margeta-Mitrovic et al, 2000). Using a combination of chimeric receptor constructs of GbR1 and GbR2, Pin and colleagues proposed a transactivation model in which GbR1 binds GABA while GbR2 activates the G protein (Galvez et al, 2001). More recently, it has also been proposed that heterodimerization is obligatory for the formation of functional taste receptors. Indeed, sweet (Nelson et al, 2001) and l-amino-acid (Nelson et al, 2002) taste responses were strictly dependent on the co-expression of T1R3 and either T1R2 or T1R1, respectively. Even if these three cases are the only ones for which obligatory heterodimerization has been firmly established, such oligomerization has often been invoked to explain the changes in signalling properties that result from the co-expression of distinct receptors. Signalling potentiation resulting from heterodimerization has been suggested for the opioid δ/κ (Jordan & Devi, 1999), opioid δ/μ (Gomes et al, 2000), chemokine CCR5/CCR2 (Mellado et al, 2001), somatostatin SSTR5/dopamine D2 (Rocheville et al, 2000a), angiotensin AT1/bradykinin B2 (AbdAlla et al, 2000) and metabotropic glutamate 1α/adenosine A1 (Ciruela et al, 2001) receptors, whereas signal attenuation has been described for the somatostatin SST2a/SST3 (Pfeiffer et al, 2001), adenosine A1/dopamine D1 (Gines et al, 2000), angiotensin AT1/bradykinin B2 (AbdAlla et al, 2000) and yeast α-mating factor wild-type/mutant (Overton & Blumer, 2000) receptors. Heterodimerization has also been proposed to promote changes in the selectivity of some GPCRs towards the different G-protein subfamilies (Gs, Gi, Gq and G12). In particular, a loss of Gi coupling has been reported following co-expression of μ- and δ-opioid receptors (George et al, 2000; Charles et al, 2003) and in cells that co-express CCR5 and CCR2 chemokine receptors (Mellado et al, 2001). Although the formation of heterodimers was confirmed in all the above studies by either co-immunoprecipitation or resonance-energy-transfer approaches, cross-talk regulation involving downstream components of the individual signalling pathways activated by each receptor cannot be formally excluded. So the contribution of heterodimerization to the observed phenotypes cannot be proved unambiguously.
The classical model of G-protein activation is based on the premise that one receptor interacts with one heterotrimeric G protein at a time. However, this concept should be re-examined in the context of GPCR dimerization. Does one dimer activate one or two G proteins? Structural studies of the receptor–G-protein interface have led to the identification of several points of contact between the G protein and the receptor on both α- and βγ-subunits (Hamm, 2001). However, the crystallographic structure of rhodopsin revealed that its cytoplasmic surface is too small to accommodate these points of contact simultaneously. This led to the proposition that two receptor molecules might be necessary to satisfy the binding requirements of a single G protein (Hamm, 2001; Liang et al, 2003). An elegant reconstitution study recently supported this idea. Chemical cross-linking followed by size-exclusion chromatography, mass spectroscopy and neutron scattering measurements demonstrated clearly that the complex formed between the purified, activated leukotriene B4 receptor BLT1 and Gαi2β1γ2 corresponds to a pentameric assembly of one heterotrimeric G protein and one dimeric receptor (Baneres & Parello, 2003). It remains to be determined whether this arrangement of one GPCR dimer interacting with a single heterotrimeric G protein is the functional complex in cells.
Internalization
Several recent studies have suggested that heterodimerization could affect agonist-promoted GPCR endocytosis, a well-characterized process classically involved in signal attenuation. For many documented heterodimers, stimulation of only one of the protomers was sufficient to promote co-internalization of the two receptors. These include: SSTR1/SSTR5 somatostatin (Rocheville et al, 2000b), δ-opioid/β2 adrenergic (β2AR; Jordan et al, 2001), α2A/β1 adrenergic (Xu et al, 2003), α1A/α1b adrenergic (Stanasila et al, 2003), SSTR2A somatostatin/μ opioid (Pfeiffer et al, 2002) and A2A adenosine/D2 dopamine (Hillion et al, 2002) receptors. In the last two cases, the co-internalization was also associated with a cross-desensitization of the signalling activities. By contrast, receptors that do not undergo efficient agonist-promoted endocytosis were found to act as dominant-negatives for endocytosis-prone receptors after heterodimerizaton. For example, the κ-opioid receptor inhibited the endocytosis of both δ-opioid receptors (Jordan & Devi, 1999) and the β2AR (Jordan et al, 2001), whereas the β1AR prevented the agonist-promoted internalization of the β2AR (Lavoie et al, 2002). In some of these studies, a nonspecific effect on the endocytosis of all GPCRs was ruled out by showing that the internalization of non-related GPCRs was not affected (Stanasila et al, 2003). Although of significant potential interest, the physiological consequences of these observations on the regulation of GPCR desensitization/resensitization cycles remain to be determined.
Physiological relevance
Until now, heterologous expression systems have been the preferred models to study the functional consequences of GPCR dimerization, which raises the question of physiological relevance. For instance, the artificial co-expression of receptors that are never expressed together in the same cells in vivo could lead to erroneous conclusions. In addition, although great care has been taken in many studies to maintain expression levels within physiological ranges, the high expression levels that are sometimes achieved with these systems could lead to spurious interactions. This possibility has been elegantly illustrated in a recent study, which reported that δ-opioid receptors and β2AR could form heterodimers but only when expressed at high levels (Ramsay et al, 2002). Nevertheless, the potential physiological importance of heterodimerization is supported by studies in cells that endogenously co-express the GPCRs under consideration. For example, the synergistic binding or signalling documented in heterologous expression systems between the δ- and μ-opioid (Gomes et al, 2000), the metabotropic glutamate1α and adenosine A1 (Ciruela et al, 2001) as well as the angiotensin AT1 and the bradykinin B2 (AbdAlla et al, 2000) receptors were also observed in neuroblastoma cells, cortical neurons and smooth muscle cells, respectively. More recently, the blockade of either the AT1 receptor or the β2AR with selective antagonists was found to inhibit the signalling of both receptors simultaneously in freshly isolated mouse cardiomyocytes (Barki-Harrington et al, 2003), a phenomenon linked to the ability of the two receptors to heterodimerize.
Conclusion
Although first met with healthy scepticism, the concept that GPCRs can exist as dimeric entities is now largely accepted. As summarized here, mounting evidence now suggests that the homodimerization and heterodimerization of GPCRs could be important in aspects of their biology that range from ontogeny to the regulation of their pharmacological and signalling properties. However, many more studies will be required for the following: (1) to establish the general physiological importance of dimerization in native systems, (2) to identify the dimerization interface and elucidate the three-dimensional organization of the dimers, (3) to find out the rules that dictate the selectivity of interactions between receptors, (4) to determine the stoichiometry of interaction between receptors and their partner proteins, and (5) to assess whether higher-order oligomeric complexes that include more than two receptors exist. Gathering this information will be a challenging task that will require the further development of innovative approaches permitting both the study of protein–protein interactions in living cells and the determination of the three-dimensional structures of transmembrane proteins.

Acknowledgments
We are grateful to M. Lagaeé for critical reading of the manuscript. M.B. holds a Canada Research Chair in signal transduction and molecular pharmacology. Work in the Bouvier laboratory is supported by the Canadian Institute for Health Research.
References
- AbdAlla S, Lother H, Quitterer U (2000) AT1–receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 407: 94–98 [DOI] [PubMed] [Google Scholar]
- Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, Bouvier M (2000) Detection of β2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc Natl Acad Sci USA 97: 3684–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Salahpour A, Bouvier M (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435 [DOI] [PubMed] [Google Scholar]
- Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R (2002) Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem 277: 21522–21528 [DOI] [PubMed] [Google Scholar]
- Babcock GJ, Farzan M, Sodroski J (2003) Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem 278: 3378–3385 [DOI] [PubMed] [Google Scholar]
- Baneres JL, Parello J (2003) Structure-based analysis of GPCR function: evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. J Mol Biol 329: 815–829 [DOI] [PubMed] [Google Scholar]
- Barki-Harrington L, Luttrell LM, Rockman HA (2003) Dual inhibition of β-adrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor–receptor interaction in vivo. Circulation 108: 1611–1618 [DOI] [PubMed] [Google Scholar]
- Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT (1997) Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by Ccr5δ32. J Biol Chem 272: 30603–30606 [DOI] [PubMed] [Google Scholar]
- Canals M et al. (2003) Adenosine A2A-dopamine D2 receptor–receptor heteromerization. Qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem 278: 46741–46749 [DOI] [PubMed] [Google Scholar]
- Charles AC, Mostovskaya N, Asas K, Evans CJ, Dankovich ML, Hales TG (2003) Coexpression of δ-opioid receptors with μ receptors in GH3 cells changes the functional response to μ agonists from inhibitory to excitatory. Mol Pharmacol 63: 89–95 [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Miller LJ (2001) Agonist-dependent dissociation of oligomeric complexes of G protein-coupled cholecystokinin receptors demonstrated in living cells using bioluminescence resonance energy transfer. J Biol Chem 276: 48040–48047 [DOI] [PubMed] [Google Scholar]
- Ciruela F et al. (2001) Metabotropic glutamate 1α and adenosine A1 receptors assemble into functionally interacting complexes. J Biol Chem 276: 18345–18351 [DOI] [PubMed] [Google Scholar]
- Colley NJ, Cassill JA, Baker EK, Zuker CS (1995) Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proc Natl Acad Sci USA 92: 3070–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea A, Janovick JA, Maya-Nunez G, Conn PM (2001) Gonadotropin-releasing hormone receptor microaggregation. rate monitored by fluorescence resonance energy transfer. J Biol Chem 276: 2153–2158 [DOI] [PubMed] [Google Scholar]
- Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ (2001) Insights into Wnt binding and signalling from the structures of two frizzled cysteine-rich domains. Nature 412: 86–90 [DOI] [PubMed] [Google Scholar]
- Dinger MC, Bader JE, Kobor AD, Kretzschmar AK, Becksickinger AG (2003) Homodimerization of neuropeptide Y receptors investigated by fluorescence resonance energy transfer in living cells. J Biol Chem 278: 10562–10571 [DOI] [PubMed] [Google Scholar]
- Floyd DH, Geva A, Bruinsma SP, Overton MC, Blumer KJ, Baranski TJ (2003) C5a receptor oligomerization. II. Fluorescence resonance energy transfer studies of a human G protein-coupled receptor expressed in yeast. J Biol Chem 278: 35354–35361 [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K (2003) Atomic-force microscopy: rhodopsin dimers in native disc membranes. Nature 421: 127–128 [DOI] [PubMed] [Google Scholar]
- Franco R et al. (2000) Evidence for adenosine/dopamine receptor interactions: indications for heteromerization. Neuropsychopharmacology 23: S50–S59 [DOI] [PubMed] [Google Scholar]
- Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, Prezeau L, Pin JP (2001) Allosteric interactions between GB1 and GB2 subunits are required for optimal GABAB receptor function. EMBO J 20: 2152–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF (2000) Oligomerization of μ- and δ-opioid receptors. Generation of novel functional properties. J Biol Chem 275: 26128–26135 [DOI] [PubMed] [Google Scholar]
- George SR, O'Dowd BF, Lee SP (2002) G-protein-coupled receptor oligomerization and its potential for drug discovery. Nature Rev Drug Discov 1: 808–820 [DOI] [PubMed] [Google Scholar]
- Gines S et al. (2000) Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci USA 97: 8606–8611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA (2000) Heterodimerization of μ and δ opioid receptors: a role in opiate synergy. J Neurosci 20: RC110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse R, Schoneberg T, Schultz G, Gudermann T (1997) Inhibition of gonadotropin-releasing hormone receptor signaling by expression of a splice variant of the human receptor. Mol Endocrinol 11: 1305–1318 [DOI] [PubMed] [Google Scholar]
- Guo W, Shi L, Javitch JA (2003) The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem 278: 4385–4388 [DOI] [PubMed] [Google Scholar]
- Hamm HE (2001) How activated receptors couple to G proteins. Proc Natl Acad Sci USA 98: 4819–4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion J et al. (2002) Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem 277: 18091–18097 [DOI] [PubMed] [Google Scholar]
- Horvat RD, Roess DA, Nelson SE, Barisas BG, Clay CM (2001) Binding of agonist but not antagonist leads to fluorescence resonance energy transfer between intrinsically fluorescent gonadotropin-releasing hormone receptors. Mol Endocrinol 15: 695–703 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Barisas G, Song J, Roess DA (2003) Membrane organization of luteinizing hormone receptors differs between actively signaling and desensitized receptors. J Biol Chem 278: 42744–42749 [DOI] [PubMed] [Google Scholar]
- Issafras H, Angers S, Bulenger S, Blanpain C, Parmentier M, Labbe-Jullie C, Bouvier M, Marullo S (2002) Constitutive agonist-independent CCR5 oligomerization and antibody-mediated clustering occurring at physiological levels of receptors. J Biol Chem 277: 34666–34673 [DOI] [PubMed] [Google Scholar]
- Jensen AA, Hansen JL, Sheikh SP, Brauner-Osborne H (2002) Probing intermolecular protein–protein interactions in the calciumsensing receptor homodimer using bioluminescence resonance energy (BRET). Eur J Biochem 269: 5076–5087 [DOI] [PubMed] [Google Scholar]
- Karpa KD, Lin R, Kabbani N, Levenson R (2000) The dopamine D3 receptor interacts with itself and the truncated D3 splice variant D3nf: D3-D3nf interaction causes mislocalization of D3 receptors. Mol Pharmacol 58: 677–683 [DOI] [PubMed] [Google Scholar]
- Kroeger KM, Hanyaloglu AC, Seeber RM, Miles LE, Eidne KA (2001) Constitutive and agonist-dependent homo-oligomerization of the thyrotropin-releasing hormone receptor. Detection in living cells using bioluminescence resonance energy transfer. J Biol Chem 276: 12736–12743 [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K (2000) Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407: 971–977 [DOI] [PubMed] [Google Scholar]
- Latif R, Graves P, Davies TF (2002) Ligand-dependent inhibition of oligomerization at the human thyrotropin receptor. J Biol Chem 277: 45059–45067 [DOI] [PubMed] [Google Scholar]
- Lavoie C et al. (2002) β1/β2-adrenergic receptor heterodimerization regulates β2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem 277: 35402–35410 [DOI] [PubMed] [Google Scholar]
- Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A (2003) Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J Biol Chem 278: 21655–21662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio R, Barbier P, Colelli A, Salvadori F, Demontis G, Corsini GU (1999) G protein-linked receptors: pharmacological evidence for the formation of heterodimers. J Pharmacol Exp Ther 291: 251–257 [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY (2000) A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron 27: 97–106 [DOI] [PubMed] [Google Scholar]
- Marshall FH, Jones KA, Kaupmann K, Bettler B (1999) GABAB receptors-the first 7TM heterodimers. Trends Pharmacol Sci 20: 396–399 [DOI] [PubMed] [Google Scholar]
- Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, Fernandez S, Martin de Ana A, Jones DR, Toran JL, Martinez A (2001) Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J 20: 2497–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS (2001) Mammalian sweet taste receptors. Cell 106: 381–390 [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS (2002) An amino-acid taste receptor. Nature 416: 199–202 [DOI] [PubMed] [Google Scholar]
- Overton MC, Blumer KJ (2000) G-protein-coupled receptors function as oligomers in vivo. Curr Biol 10: 341–344 [DOI] [PubMed] [Google Scholar]
- Overton MC, Blumer KJ (2002) The extracellular N-terminal domain and transmembrane domains 1 and 2 mediate oligomerization of a yeast G protein-coupled receptor. J Biol Chem 277: 41463–41472 [DOI] [PubMed] [Google Scholar]
- Patel RC et al. (2002) Ligand binding to somatostatin receptors induces receptorspecific oligomer formation in live cells. Proc Natl Acad Sci USA 99: 3294–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M (2000) Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human δ opioid receptor. J Biol Chem 275: 13727–13736 [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M (2001) Newly synthesized human δ opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem 276: 4416–4423 [DOI] [PubMed] [Google Scholar]
- Pfeiffer M, Koch T, Schroder H, Klutzny M, Kirscht S, Kreienkamp HJ, Hollt V, Schulz S (2001) Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of Sst3 receptor function by heterodimerization with Sst2A. J Biol Chem 276: 14027–14036 [DOI] [PubMed] [Google Scholar]
- Pfeiffer M, Koch T, Schroder H, Laugsch M, Hollt V, Schulz S (2002) Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J Biol Chem 277: 19762–19772 [DOI] [PubMed] [Google Scholar]
- Ramsay D, Kellett E, McVey M, Rees S, Milligan G (2002) Homo- and hetero-oligomeric interactions between G-protein-coupled receptors in living cells monitored by two variants of bioluminescence resonance energy transfer (BRET): hetero-oligomers between receptor subtypes form more efficiently than between less closely related sequences. Biochem J 365: 429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PS, Corley RB (1998) Assembly, sorting, and exit of oligomeric proteins from the endoplasmic reticulum. BioEssays 20: 546–554 [DOI] [PubMed] [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC (2000a) Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science 288: 154–157 [DOI] [PubMed] [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC (2000b) Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J Biol Chem 275: 7862–7869 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Frade JM, Vila-Coro AJ, de Ana AM, Albar JP, Martinez A, Mellado M (1999) The chemokine monocyte chemoattractant protein-1 induces functional responses through dimerization of its receptor CCR2. Proc Natl Acad Sci USA 96: 3628–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roess DA, Smith SM (2003) Self-association and raft localization of functional luteinizing hormone receptors. Biol Reprod 69: 1765–1770 [DOI] [PubMed] [Google Scholar]
- Shioda T, Nakayama EE, Tanaka Y, Xin X, Liu H, Kawana-Tachikawa A, Kato A, Sakai Y, Nagai Y, Iwamoto A (2001) Naturally occurring deletional mutation in the C-terminal cytoplasmic tail of CCR5 affects surface trafficking of CCR5. J Virol 75: 3462–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanasila L, Perez JB, Vogel H, Cotecchia S (2003) Oligomerization of the α1a- and α1b-adrenergic receptor subtypes: potential implications in receptor internalization. J Biol Chem 278: 40239–40251 [DOI] [PubMed] [Google Scholar]
- Terrillon S, Durroux T, Mouillac B, Breit A, Ayoub MA, Taulan M, Jockers R, Barberis C, Bouvier M (2003) Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol Endocrinol 17: 677–691 [DOI] [PubMed] [Google Scholar]
- Trettel F, Di Bartolomeo S, Lauro C, Catalano M, Ciotti TM, Limatola C (2003) Ligand-independent CXCR2 dimerization. J Biol Chem 278: 40980–40988 [DOI] [PubMed] [Google Scholar]
- Wurch T, Matsumoto A, Pauwels PJ (2001) Agonist-independent and -dependent oligomerization of dopamine D2 receptors by fusion to fluorescent proteins. FEBS Lett 507: 109–113 [DOI] [PubMed] [Google Scholar]
- Xu J, He J, Castleberry AM, Balasubramanian S, Lau AG, Hall RA (2003) Heterodimerization of α2A- and β1-adrenergic receptors. J Biol Chem 278: 10770–10777 [DOI] [PubMed] [Google Scholar]
- Zhu CC, Cook LB, Hinkle PM (2002) Dimerization and phosphorylation of thyrotropin-releasing hormone receptors are modulated by agonist stimulation. J Biol Chem 277: 28228–28237 [DOI] [PubMed] [Google Scholar]
- Zhu X, Wess J (1998) Truncated V2 vasopressin receptors as negative regulators of wild-type V2 receptor function. Biochemistry 37: 15773–15784 [DOI] [PubMed] [Google Scholar]


