Summary
Evolution has programmed us for early survival and reproduction but has left us vulnerable to disease in old age. In our present affluent environment, we are better adapting to these improved conditions.
Time and again we see not only does our mean life expectancy keep increasing (Fig 1), but also that this linear increase means that all predictions of our maximum life expectancy so far have turned out to be gross underestimates (Oeppen & Vaupel, 2002). Today, citizens in developed countries can easily expect to live beyond the age of 75 years—81 for women—but if we take Fig 1 as an indication, they will probably reach an even higher age. This ongoing demographic trend is caused by an incremental decrease in the probability of disease, disability and death at old age. Because the number of births is decreasing, old people constitute the fastest growing segment of developed countries' populations. This trend not only has repercussions for individuals or families that today can easily span four generations, but also has serious implications for our affluent societies. Ever-ageing populations are already creating concerns—and ongoing debates—about the future of retirement funds, health care and regulations of the labour market, such as the age of retirement. To deal with these social problems and make predictions about the future of populations, it is therefore necessary to understand the biological reasons behind this demographic trend towards ever higher life expectancy.
Figure 1.
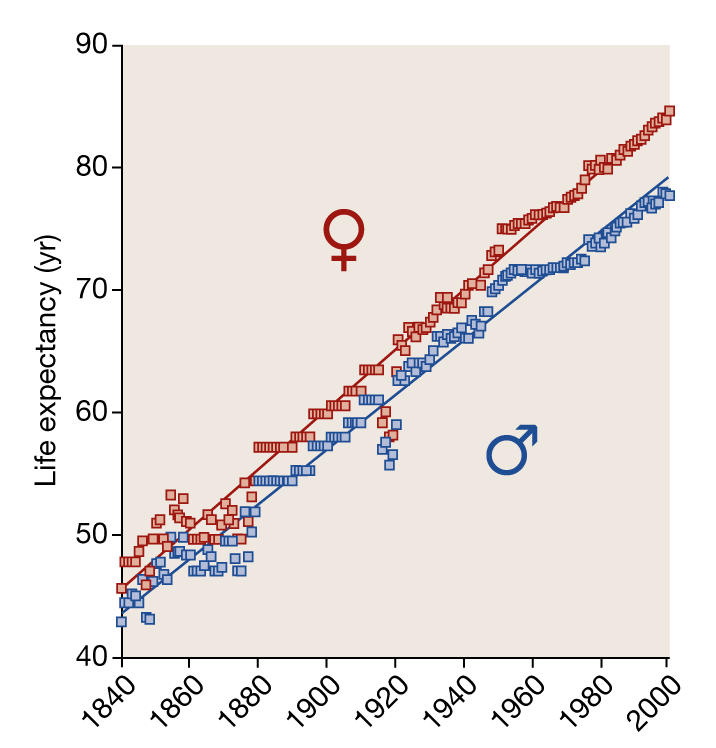
Increase in life expectancy in record-holding countries from 1840 to 2000 (based on data from Oeppen & Vaupel, 2002). The data fit a straight line, indicating that there is no reason to expect the increase in life expectancy to level off in the foreseeable future. Life expectancy for women increases by 2.4 years with every decade of calendar time, in comparison with 2.2 years for men.
In most countries, the increase in the gross national product and the increase in mean life expectancy are closely linked, illustrating that higher life expectancy is caused by an improvement of the environmental conditions in which we live. Starting at about the second half of the nineteenth century—although at different time points for various nations—child mortality decreased markedly as mortality from famine and disease decreased to a minimum (Omran, 2001). However, if improved environmental conditions affected only the young, we would expect the increase in life expectancy to level off at some point. This expectation clearly does not match the steady, linear increase in life expectancy that has been observed since 1840 (Fig 1) and there is no sign that it will slow down soon. The abundant resources that have helped to overcome child mortality have also led to better survival at middle and old age. This can be best illustrated with the impressive decrease in death from coronary heart disease—although, again, this has occurred at a different pace in different countries.
The abundant resources that have helped to overcome child mortality have also led to better survival at middle and old age
But not only can we expect to live increasingly to the maximum of human lifespan, the maximum itself keeps increasing. In fact, this trend has accelerated over the past few decades, with the record set by a French woman who lived to the age of 122. The demographic data suggest that the limit of our biological design has not yet been reached. It is a tantalizing question, then, how our bodies manage to keep up as we continuously challenge the end of life—why is it that we still live longer?
In fact, human bodies have never been intended to be perfect and thus immortal, because there is no evolutionary need for it. Irrespective of the species studied, animals that live in their natural habitat do not grow old, because the risk of mortality from environmental factors—disease or predators—is high and the probability of long-term survival is limited. For instance, features of ageing in animals can be observed only in artificial, protected environments—lions with grey manes are present in zoos but not in the African savannahs. For a true understanding of the ageing process in man, it is therefore crucial to realize that the natural habitat of Homo sapiens is far different from our present, affluent environment. The human species stems from the African continent where tens of thousands of years ago it was highly exceptional to reach an age of more than 40 years (Fig 2). The fact that long-term survival in our natural habitat is limited led Peter Medawar to conclude that old people can contribute little to the next generation and to the evolution of the human population genome (Medawar, 1952). Ageing remains in the shadow of evolution and is for that reason not programmed into our genome.
It is a tantalizing question, then, how our bodies manage to keep up as we continuously challenge the end of life—why is it that we still live longer?
Figure 2.
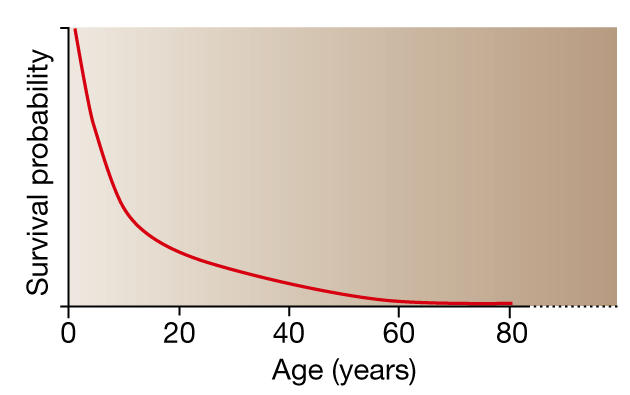
Survival curve for populations in their natural habitat. Simply because older individuals have been exposed to environmental hazards for longer, the likelihood of getting old is limited. Young individuals outnumber old, and old individuals outnumber those even older. The force of natural selection thus progressively weakens with increasing age, and ageing remains in the evolutionary shadow.
It provides a useful insight into the ageing process to realize that the DNA in our germ cells is the result of an uninterrupted chain of events and modifications that, over millions of years, links us to the earliest forms of life on Earth. In contrast to ever-renewing DNA, our bodies are only temporary and disposable carriers of genetic material, and continuously accumulate damage from wear and tear. As time goes by, the risk of mortality grows, and it is in fact the increase in the probability of death that defines the ageing process. Absence of ageing is only attainable if we have an unlimited ability to maintain and repair our bodies, an ability that prevents permanent damage from occurring and keeps our bodies in perfect condition. It easily follows why there has never been a need for such a perfect body. Once we have children who have reached reproductive age we have fulfilled our duties and there is, from an evolutionary point of view, no need to live any longer.
Some will find the thought of being a disposable vessel, to be thrown away after use, unbearable and will refer to the great knowledge, experience and abilities of grandparents to contribute to the survival of the young, while referring to their undisputable position in our temporary societies. It should be remembered, however, that this is only a social construct. In our natural habitat in the middle of Africa, under adverse conditions, the number of old people was small and could therefore not have materially contributed to the survival of the lineage.
In 1977, Thomas Kirkwood took this idea a major step further and proposed that investment in maintenance and repair comes at the cost of investment in reproduction (Kirkwood, 1977). His theory is of an illuminating simplicity. Too little investment in the maintenance and repair of our bodies will lead to premature death and a low probability of having progeny; our biological fitness will thus be low. Too much investment in maintenance and repair, however, will also lead to a decrease in reproductive success, because resources are not unlimited. Every species trades investments in maintenance and repair against investments in reproduction to optimize evolutionary fitness under the specific environmental conditions in which they live. The theory helps us to understand why species that suffer high mortality from their environment invest a great deal in reproduction to prevent extinction, whereas species under less environmental pressure invest more in maintenance and repair and live longer—although at the cost of reproductive success.
The past two decades have brought ample experimental evidence for this trade-off, also known as the 'disposable soma theory'. Experiments with the fruitfly Drosophila melanogaster proved the general existence of trade-offs between longevity and reproduction, for both females and males. A selection regime that favours flies that retained fertility at later ages resulted in populations with increased lifespans, reduced fertility early in life and enhanced resistance to a variety of stresses, suggesting that the mechanisms underlying the increase in lifespan involve greater investments in somatic durability. Direct selection for longevity, by exploiting the dependence on temperature of the fruitfly lifespan, also produced long-lived populations with significantly reduced fertility, underpinning a genetic cause for the trade-off (Zwaan et al, 1995).
In contrast to ever-renewing DNA, our bodies are only temporary and disposable carriers of genetic material, and continuously accumulate damage from wear and tear
Similar trade-offs have been described for the worm Caenorhabditis elegans. A series of point mutations in the insulin signalling pathway—regulating metabolism, stress resistance and cell growth—are associated with increases in lifespan of up to 200%, but at the cost of reproductive success. This explains why these mutants do not last in their natural habitat, where only the evolutionarily fittest survive. The climax of evidence for the existence of a trade-off between reproduction and longevity, however, is provided by experiments in which germline precursor cells are removed. As a result, the worm's lifespan increases markedly (Arantes-Oliveira et al, 2002, 2003).
Few would accept that the disposable soma theory is also applicable to humans on the basis of experimental work in fruitflies and worms. Some years ago, our group at Leiden University therefore set out to find arguments for a trade-off between longevity and reproduction in Homo sapiens. A major methodological problem in such an epidemiological analysis is to exclude the fact that specific environmental conditions determine the number of offspring and better survival, causing spurious correlations. Instead of adjusting for differences in socio-economic class, we relied on the genealogies of the British aristocracy that for centuries embodied the upper crust of society, and thus provided us with a unique, uniform population sample for which environmental conditions were equal within a certain time frame (Westendorp & Kirkwood, 1998).
When we plotted the age of death of aristocratic women against the number of children that they had, the number of children was small when women died at an early age, increased with age at death reaching a plateau through the sixth, seventh and eighth decades of life, and decreased again for women who died at ages of 80 years and over (Fig 3). In line with the disposable soma theory, postmenopausal women who reached very old age therefore had significantly fewer children than those who died in middle age. Clearly, the relationship between the number of children and lifespan cannot be the consequence of deliberate family planning, because considerations of family size took place when these women were in their twenties to forties and would be unaware of their age at death. Apparently, women whose bodies had better durability because of greater investment in maintenance and repair lived longer, but at the cost of reproductive success. This study of the British aristocracy was carried further by Oeppen, who argued that the initial analysis did not take into account the fact that premenopausal women might have died at an early age because they had invested more in fertility than in maintenance. In an updated version of the historical data set, he found even stronger statistical evidence for this trade-off when he modelled maintenance at the cost of reproduction over the whole trajectory of life (Doblhammer & Oeppen, 2003).
Figure 3.

Progeny number for married aristocratic women from different birth cohorts as a function of age at death (Westendorp & Kirkwood, 1998). When only postmenopausal women (that is, aged 60 years and over) were included in the analysis, the decrease in number of progeny with age at death was statistically significant for both the period before 1700 and the period after 1700.
In parallel, we set out to find molecular mechanisms that would support the disposable soma theory. One obvious candidate is immunity, because the adverse conditions in our natural habitat necessitate large investments in an adequate immune system to fight abundant infections and therefore reach reproductive age. We studied the levels of two major cytokines—interleukin-10 (IL-10) and tumour necrosis factor (TNF)—in first-degree relatives of patients who suffered meningococcal disease, an infection that is widely present in Africa and occasionally surfaces in developed countries (Westendorp et al, 1997). Cytokines are signalling molecules for cell–cell interactions, and include compounds such as TNF that initiate an inflammatory response to fight infection, and regulatory signals such as IL-10 to switch off the inflammatory response and prevent collateral damage after the infection has been overcome. Their activity has been shown to be under tight genetic control and we therefore assumed that families of those patients who had died would have a distinct pattern of cytokine activity (Fig 4). Almost without exception, the level of pro-inflammatory TNF in all of these cases was low, and the level of the anti-inflammatory IL-10 was high. Our interpretation of these data is that subjects with an innate propensity towards anti-inflammatory responses are at an increased risk of death from infection.
...improvements in our environmental conditions have, through genetic adaptation, an indirect effect on the patterns of disease
Figure 4.
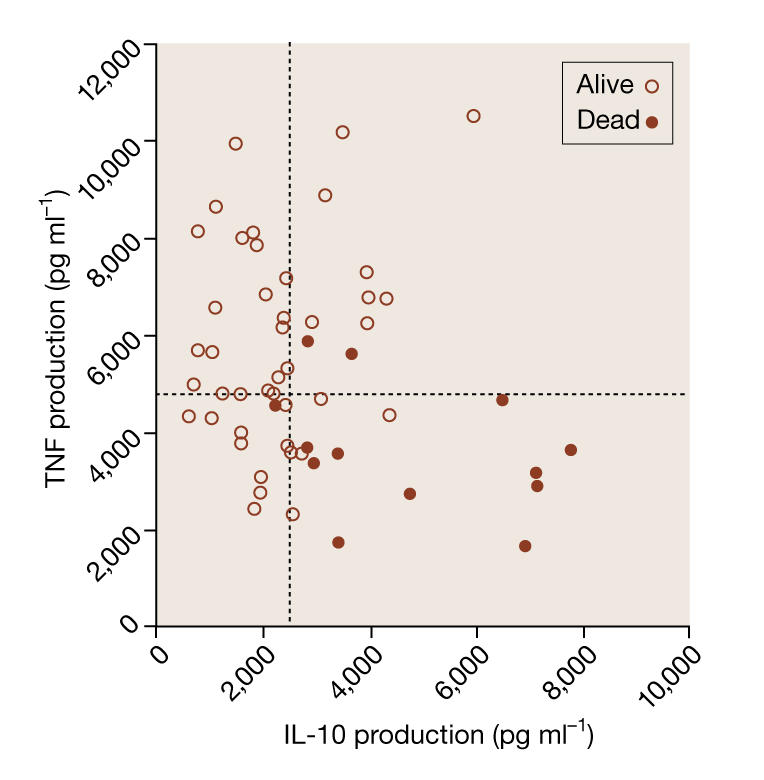
Symbols represent the family mean levels of the pro-inflammatory cytokine TNF and the anti-inflammatory cytokine IL-10 (Westendorp et al, 1997). Open circles represent cytokine production in families of patients who survived meningococcal infection, and filled circles represent the production of cytokines of dead patients. Dotted lines indicate medians of the family estimates for both cytokines.
In contrast with fighting infection, which requires a strong inflammatory host response, reproductive success depends on a tolerant immune response. About half of a baby's tissue antigens have paternal origin, so at the fetal–maternal interface, immune reactions must be suppressed to allow pregnancy to proceed. We therefore compared the cytokine profiles of women with impaired fertility, as defined by having at least three consecutive spontaneous abortions, with the profiles of women of normal fecundity (Westendorp et al, 2001). Reproductive success was associated with a tolerant profile—low TNF and high IL-10—whereas an inflammatory profile was associated with habitual abortion. The probability of normal fecundity increased up to 16-fold when the women's cytokine levels were characterized by high anti-inflammatory and low pro-inflammatory profiles.
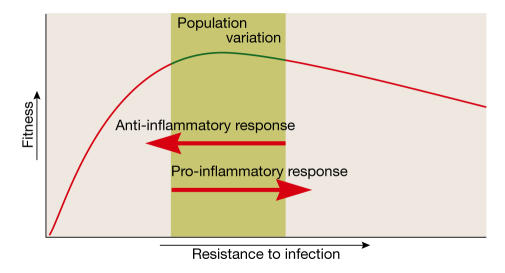
These data on cytokine profiles help to elucidate two phenomena. First, they can explain why British aristocrats, who lived longer, were less likely to have successful pregnancies. Their innate immune system favoured resistance to infection but at the same time prevented pregnancy from proceeding, a trade-off that was even stronger in times when the environmental conditions were relatively poor. Second, it explains why a genotype associated with impaired fertility might have persisted in spite of its obvious disadvantage with regard to evolutionary fitness. Selection for resistance to infection is traded against selection for fertility, resulting in a compromise that is optimal for the fitness of the species in a specific environment (Fig 5).
Figure 5.
Schematic diagram showing how pro-inflammatory responses that are beneficial because they increase the probability of surviving fatal infections are traded off against anti-inflammatory immune responses that allow pregnancies to proceed. This results in a compromise that is optimal for the fitness of the species in a specific environment.
The evolutionary theory could thus explain two concepts: the forces of natural selection progressively weaken with increasing adult age, and the acquisition of longevity involves significant investments in somatic durability. The first suggests that genes with deleterious effects late in life can accumulate within the gene pool. The second suggests that resources invested in longevity might be at the expense of reproduction. These mechanisms are not mutually exclusive, and generalized trade-offs between early-life and late-life fitness are also implied by the pleiotropic-genes hypothesis. According to Williams, genes that have beneficial effects early in life can have deleterious effects later in life (Williams, 1957).
Despite its protective role in infection, inflammation is potentially harmful; for instance, strong pro-inflammatory responses can cause tissue damage at the site of infection. Although we are programmed to resist infection, in affluent countries the burden of disease has now shifted away from infectious diseases towards chronic diseases that are typically expressed in old age. Accordingly, fatal infections still account for the majority of deaths in less-developed parts of the world, especially at younger ages, but cardiovascular disease has become the leading cause of mortality in ageing populations, accounting for 30% of all deaths worldwide each year. Contrary to general opinion, cardiovascular disease is not a 'new' disease due to our modern, affluent lifestyle, but the epidemic has developed as a result of improved survival, because we all become increasingly susceptible to this disease when we reach middle age. Further arguments for this reasoning can be found in Egyptian mummies of people who had reached old age. The bodies of these exceptional men and women who died in their fifties and sixties all show symptoms of widespread atherosclerosis (Magee, 1998). Similar observations can be made in the relative few who reach old age in developing countries in contemporary time. Indeed, the causes of death at old age in developed and developing countries are similar, indicating that in the second half of human life, death from cardiovascular disease is taking the lead, under both affluent and adverse environmental conditions (Bonow et al, 2002). Under adverse conditions, relatively few will reach old age to suffer from cardiovascular disease, but it becomes epidemic in developing countries as soon as death from infection and malnutrition disappears and life expectancy rapidly increases.
There is clear evidence that inflammation contributes to the development of cardiovascular disease (Libby et al, 2002). For example, levels of C-reactive protein, a marker of inflammation, have been associated with coronary artery disease, angina and infarction. Moreover, results from population-based studies have demonstrated that increased levels of markers of inflammation, such as cytokines, adhesion molecules and acute-phase reactants, are associated with cardiovascular events. Because our immune system has evolved under the constant attack of pathogens, we are evolutionarily programmed for an inflammatory response to resist infection. In old age, however, the protective effect of this inflammatory response trades off with the increased risk of death from cardiovascular events, thereby reducing life expectancy. This idea is confirmed by our own data that at very old age those with high inflammatory responses are at a higher risk of stroke and vascular dementia.
In our modern affluent societies, death from infection has become rare, which is causing various transitions at the epidemiological and demographic levels. The change in the causes of death—from infection to cardiovascular disease and cancer—is known as the 'epidemiological transition', following the improvements we have made to our environment that have altered the biology of disease. Nowadays, virtually everybody can expect to survive up to middle age, when he or she will typically suffer from chronic diseases such as atherosclerosis and cancer. Consequently, whereas in former times society was dominated numerically by youth—as it still is in developing countries—in our present societies the old outnumber the young. This change in the population structure is known as the 'demographic transition'.
...we can foresee that the next generations of Homo sapiens will have even longer lifespans but at the cost of impaired fertility
But these changes do not stop there; in fact, we are witnessing a second transition in both epidemiology and demography in developed countries. Whereas in the ancestral, adverse environment, only children who were genetically outfitted to resist infection were able to survive, nowadays this force of selection has faded away. At present, newborns will survive to reproductive age and have progeny, which might have a great impact on the distribution of the population genome—in an affluent environment, those with a tolerant immune response will also survive and propagate. This adaptation of the innate immune system to improved environmental conditions is not illogical and is in line with evolutionary theory. And as the population genome adapts, the pattern of disease will become different. Hence, improvements in our environmental conditions have, through genetic adaptation, an indirect effect on the patterns of disease. This could best be described as the 'second epidemiological transition', to emphasize the difference from the first epidemiological transition that was caused by the direct effects of improved health care, sanitation and sufficient nutrition.
Similarly, patterns of fertility follow this increase in mean life expectancy, and this is known as the 'second demographic transition'. Not long ago, only a minority of women gave birth to a majority of the offspring—estimates around 1900 show that about 10% of the women gave birth to more than half of the newborns. Nowadays, all women contribute equally to the next generation, and even sub-fertile women can have one or more children. Furthermore, mothers tend to become pregnant later in life. Apart from the impact on society, this could have profound biological effects as well. As the evolutionary pressures that favoured high fertility decrease, more resources can be invested in maintenance and repair, thus increasing both mean life expectancy and maximum lifespan. I mentioned above that a selection regime favouring flies that retained fertility at later ages resulted in populations with reduced fertility early in life but with increased lifespans. The enhanced resistance to a variety of stresses suggests that their bodies were less disposable. If we take the lead from these flies and from the demographic and epidemiological transitions taking place, we can foresee that the next generations of Homo sapiens will have even longer lifespans but at the cost of impaired fertility. This is not due to alleged phyto-oestrogens or endocrine disruptors in our food chain that impair fertility or even more improvements being made to our current environment, but because our human population genome is on the drift.

References
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C ( 2002) Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295: 502–505 [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Berman JR, Kenyon C ( 2003) Healthy animals with extreme longevity. Science 302: 611. [DOI] [PubMed] [Google Scholar]
- Bonow RO, Smaha LA, Smith SC Jr, Mensah GA, Lenfant C ( 2002) World Heart Day 2002: the international burden of cardiovascular disease: responding to the emerging global epidemic. Circulation 106: 1602–1605 [DOI] [PubMed] [Google Scholar]
- Doblhammer G, Oeppen J ( 2003) Reproduction and longevity among the British peerage: the effect of frailty and health selection. Proc R Soc Lond B 270: 1541–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TBL ( 1977) Evolution of ageing. Nature 270: 301–304 [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A ( 2002) Inflammation and atherosclerosis. Circulation 105: 1135–1143 [DOI] [PubMed] [Google Scholar]
- Magee R ( 1998) Arterial disease in antiquity. Med J Aust 169: 663–666 [PubMed] [Google Scholar]
- Medawar PB ( 1952) An Unsolved Problem of Biology. Lewis, London, UK [Google Scholar]
- Oeppen J, Vaupel JW ( 2002) Broken limits to life expectancy. Science 296: 1029–1031 [DOI] [PubMed] [Google Scholar]
- Omran AR ( 2001) The epidemiologic transition. A theory of the epidemiology of population change. Bull Wld Hlth Org 79: 161–170 [PMC free article] [PubMed] [Google Scholar]
- Westendorp RG, Langermans JA, Huizinga TW, Verweij CL, Sturk A ( 1997) Genetic influence on cytokine production in meningococcal disease. Lancet 349: 170–173 [DOI] [PubMed] [Google Scholar]
- Westendorp RG, Kirkwood TB ( 1998) Human longevity at the cost of reproductive success. Nature 396: 743–746 [DOI] [PubMed] [Google Scholar]
- Westendorp RG, van Dunne FM, Kirkwood TB, Helmerhorst FM, Huizinga TW ( 2001) Optimizing human fertility and survival. Nature Med 7: 873. [DOI] [PubMed] [Google Scholar]
- Williams GC ( 1957) Pleiotropy, natural selection and the evolution of senescence. Evolution 11: 398–411 [Google Scholar]
- Zwaan BJ, Bijlsma R, Hoekstra RF ( 1995) Direct selection of lifespan in Drosophila melanogaster. Evolution 49: 649–659 [DOI] [PubMed] [Google Scholar]