Abstract
The formation of the vertebrate neuromuscular junction (NMJ) requires the receptor tyrosine kinase MuSK and the adaptor molecule rapsyn. Here, we report that the phenotypes of mice deficient in these two molecules can be reproduced by RNA interference (RNAi) in rat muscle in vivo. Specifically, double-stranded RNA (dsRNA) targeting MuSK and rapsyn inhibited the formation of the NMJ in rat muscle fibres in vivo, while dsRNA targeting nonessential proteins did not have any effect. Moreover, plasmids that trigger RNAi to MuSK induced the disassembly of existing NMJs. These results thus demonstrate for the first time the functionality of dsRNA in silencing endogenous genes in adult mammalian muscle in vivo. Moreover, they show that MuSK is also required for the maintenance of the NMJ, offering a mechanistic explanation for the myasthenia gravis caused by auto-antibodies to MuSK.
Introduction
The molecular mechanisms responsible for the formation of synapses are best understood at the neuromuscular junction (NMJ) (reviewed in Sanes & Lichtman, 2001). Several lines of evidence demonstrate that nerve-derived agrin is required and sufficient for the assembly of the entire postsynaptic apparatus (Bezakova & Ruegg, 2003). Moreover, NMJ formation requires the musclespecific receptor tyrosine kinase MuSK (DeChiara et al, 1996) and the cytoplasmic adaptor molecule rapsyn (Gautam et al, 1995). All these data were generated by genetically engineering mice that are deficient in the particular protein.
A promising technique that may allow a more straightforward and faster assessment of gene function in vivo than current knockout techniques might be RNA interference (RNAi). This technique employs long double-stranded RNA (dsRNA) or 21–23-bp-long short interfering RNA (siRNA) that trigger specific silencing of gene expression (Tijsterman et al, 2002). However, long dsRNA has not successfully been applied to mammals because it seems to induce a general shutdown of translation and apoptosis of the cell (Paddison et al, 2002). This unspecific reaction is not observed with siRNA (Elbashir et al, 2001; Lewis et al, 2002; McCaffrey et al, 2002). The disadvantage of siRNA is that only some siRNAs are efficient in silencing gene transcription (McManus & Sharp, 2002) and that the effect of siRNA lasts only for a few days. In a further advancement of the technique, siRNA has been replaced by plasmids encoding short hairpin RNA (shRNA), which has enabled prolonged and stable suppression of gene expression in vivo (Brummelkamp et al, 2002; Yu et al, 2002; McCaffrey et al, 2003; Rubinson et al, 2003). Here we demonstrate that long dsRNA can be used in adult muscle to perturb the function of endogenous genes, and that prolonged exposure of muscle fibres to plasmids encoding hairpin RNA for MuSK induces the disassembly of existing NMJs.
Results
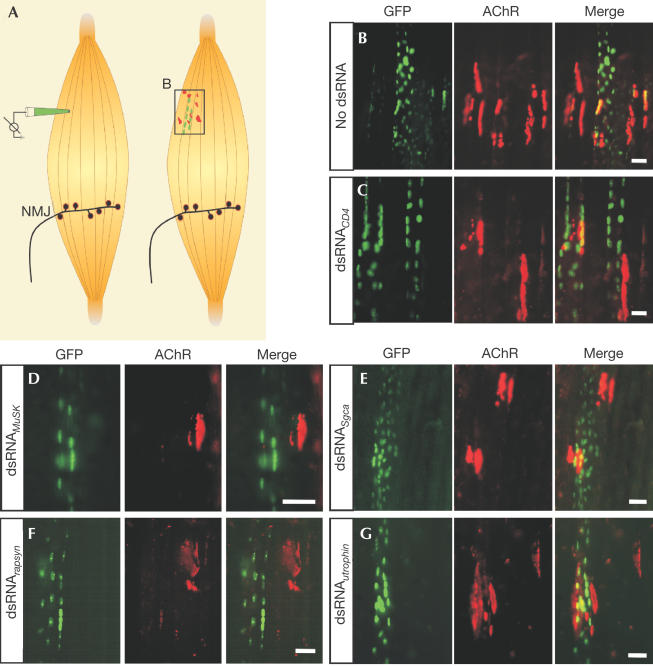
We investigated whether dsRNA-mediated RNAi could be used to study the function of genes in the formation of postsynaptic structures in muscle fibres in vivo. We injected 591–686-bp-long dsRNA, derived from different genes in conjunction with plasmids encoding neural agrin and green fluorescent protein fused to a nuclear localization signal (NLS_GFP), into nonsynaptic regions of rat soleus muscle (Fig 1A). As described previously (Cohen et al, 1997; Meier et al, 1997), injection of expression plasmids coding for neural agrin was sufficient to induce postsynaptic specializations in nonsynaptic regions (Fig 1A, right). These specializations are characterized by the accumulation of AChRs (red in Fig 1A) on the surface of injected (GFP-positive; green in Fig 1A) and neighbouring noninjected muscle fibres (Fig 1B). To test whether coinjection of dsRNA exerts any unspecific effect on protein synthesis, we injected dsRNA derived from cDNA encoding CD4 (Benoist & Mathis, 1999). As shown in Fig 1C, postsynaptic structures formed in the presence of dsRNACD4 were indistinguishable from controls and AChR clusters were found on injected and neighbouring muscle fibres. To test whether we could observe specific RNAi, we next coinjected dsRNA corresponding to MuSK (dsRNAMuSK). MuSK is an essential signalling component for NMJ formation that is activated by neural agrin (Glass et al, 1996). Thus, effective dsRNAMuSK should prevent the formation of postsynaptic specializations in response to neural agrin in the injected muscle fibre. Indeed, AChR clusters were only rarely detected on injected muscle fibres while they were readily detected on neighbouring, noninjected muscle fibres (Fig 1D). Thus, the effect of dsRNA remains restricted to the injected muscle fibres, indicating that dsRNA does not cross cell boundaries in mammalian muscle, a phenomenon that has been reported in Caenorhabditis elegans (Winston et al, 2002). As a further test for the specificity of the inhibitory activity of dsRNAMuSK, we also coinjected dsRNASgca derived from αsarcoglycan, a protein that is highly expressed in muscle fibres but is not necessary for the formation of NMJs (Duclos et al, 1998). In this case, AChR clusters were formed both on injected and neighbouring muscle fibres (Fig 1E). To test the universality of the method, we also examined the effect of dsRNArapsyn, which was derived from rapsyn, an adaptor molecule essential for the clustering of AChRs (Gautam et al, 1995). No AChR clusters were detected in dsRNArapsyn-containing muscle fibres, whereas such clusters were found on neighbouring fibres (Fig 1F). Finally, we injected dsRNAutrophin because utrophin is highly concentrated at the postsynaptic site of NMJs and at ectopic postsynaptic structures (Meier et al, 1997), but its inactivation in mice does not impinge on the initial formation of the NMJ (Deconinck et al, 1997; Grady et al, 1997). As expected, AChR clusters were still formed on injected muscle fibres (Fig 1G).
Figure 1.
Inhibition of the formation of postsynaptic structures by dsRNA. (A) Schematic representation of the injection of cDNA constructs at nonsynaptic regions of single muscle fibres of rat soleus muscle (left). The injection pipette contains expression plasmids NLS_GFP and neural agrin. dsRNA was added in RNAi experiments. Injected muscle fibres contain GFP-positive myonuclei (green) and aggregates of postsynaptic proteins including AChRs (red). The frame symbolizes the view shown in (B). Postsynaptic structures formed on the surface of the injected and on nearby muscle fibres when no dsRNA (B) or dsRNACD4 (C) was included. Injection of dsRNAMuSK (D) and dsRNArapsyn (F) prevents the formation of postsynaptic structures on injected muscle fibres but not on nearby fibres. Postsynaptic structures formed on muscle fibres injected with dsRNASgca (E) and dsRNAutrophin (G). Scale bars=50 μm.
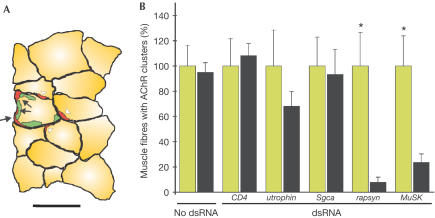
For quantification, we counted the number of AChR clusters on injected and on neighbouring, noninjected muscle fibres as schematically shown in Fig 2A. From Fig 2B, this quantification demonstrates that injection of dsRNAMuSK or dsRNArapsyn resulted in a highly significant reduction of AChR clusters in the injected muscle fibres, whereas ‘no dsRNA', dsRNACD4, dsRNAutrophin and dsRNASgca did not inhibit AChR cluster formation. In summary, these results demonstrate that dsRNA, when injected into single, adult muscle fibres in vivo, knocks the expression of the targeted protein down to the extent that it mimics the phenotype of the corresponding knockout mouse.
Figure 2.
Quantification of the number of AChR clusters formed on injected and neighbouring muscle fibres. (A) Camera lucida drawing of a crosssection through an injection site, 2 weeks after injection. In this particular case, the injection cocktail contained expression constructs for neural agrin and NLS_GFP, and dsRNAutrophin. In the cross-section shown, AChR clusters (red) were found along the circumference of the injected (GFP-positive nuclei; green) and the neighbouring, noninjected muscle fibres. AChR clusters on injected fibres are marked with solid arrows, and AChR clusters on neighbouring fibres are marked with open arrowheads. Bar=50 μm. (B) Quantification of three independent experiments for each experimental condition. For each muscle, the number of AChR clusters in neighbouring, noninjected muscle fibres (green) was normalized to 100%. Significant differences (P<0.01; Wilcoxon test) between the number of AChR clusters on noninjected, neighbouring (green) and injected (black) muscle fibres are indicated by asterisks. For experimental details, see supplementary information online.
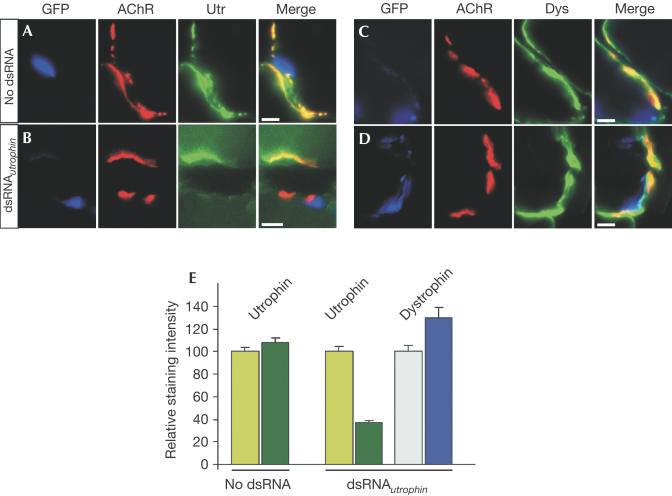
To measure the effect of dsRNA-induced RNAi on a particular gene directly, we next quantified the amount of utrophin and dystrophin found at ectopic postsynapses. As shown earlier, agrin-induced formation of postsynaptic specializations at ectopic sites requires local transcription of synaptic proteins in the myonuclei underlying these sites (Briguet & Ruegg, 2000; Moore et al, 2001). Moreover, agrin-induced ectopic postsynaptic structures form de novo, which makes the protein levels at the ectopic sites grossly independent of protein turnover. In controls, all postsynaptic structures induced by neural agrin contained a high concentration of utrophin (Fig 3A), whereas the staining was less intense at AChR clusters of muscle fibres injected with dsRNAutrophin (Fig 3B). When we stained for dystrophin, which may compensate for utrophin (Deconinck et al, 1997; Grady et al, 1997), we found that staining for dystrophin was very similar in injected and neighbouring muscle fibres (Fig 3C,D). Quantification of the fluorescence intensity for utrophin and dystrophin in the different experimental paradigms is shown in Fig 3E. No difference between the staining intensity for utrophin at AChR clusters formed on injected and neighbouring muscle fibres was observed in controls (no dsRNA). The levels of utrophin at AChR clusters of dsRNAutrophin-injected muscle were significantly lower (<40%) than at AChR clusters in neighbouring fibres. The low level of utrophin that is still detected at the ectopic postsynaptic sites of dsRNAutrophin-containing muscle fibres might be due to either some utrophin expressed in nonsynaptic regions and/or the aggregation of utrophin diffused from the NMJ. We also could not detect any unspecific suppression of gene products that are not concentrated at AChR clusters (supplementary Fig 1 online). Note that the level of dystrophin is slightly increased in the muscle fibres injected with dsRNAutrophin compared to neighbouring fibres. This lends support to the idea that dystrophin compensates for the lack of utrophin in the knockout mice.
Figure 3.
Staining of ectopic postsynaptic structures for utrophin and dystrophin. (A) No difference in the staining intensity for utrophin was seen when dsRNA was omitted. (B) Although AChR clusters were formed on the muscle that contained dsRNAutrophin (GFP-positive), these clusters were often devoid of any utrophin. (C) Dystrophin was more uniformly distributed along the entire plasmalemmal membrane, with only some enrichment at AChR clusters. (D) In dsRNAutrophin-expressing muscle fibres, dystrophin was found at sites of AChR accumulation. (E) Quantification of the amount of utrophin (green bars) and dystrophin (blue bars) in neighbouring (light colours) and injected muscle fibres (dark colours). Bars in (A–D)=5 μm.
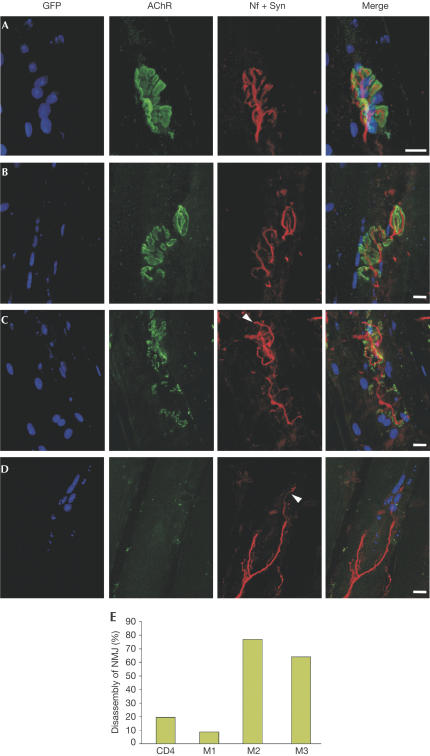
In a further step, we asked whether RNAi could also be used to study the requirement of genes for the stability of the nerve–muscle synapse. To address this question, we chose to target MuSK because (i) it is required for the formation of postsynapses and (ii) auto-antibodies to MuSK cause myasthenia gravis (Hoch et al, 2001). This disease is characterized by muscle weakness and loss of AChRs, suggesting that NMJs might disassemble. As NMJs represent only 0.1% of the total surface of a muscle fibre, dsRNA injection into single muscle fibres did not allow a global and sustained perturbation of gene expression at the NMJs. We therefore used plasmids that express functional long dsRNA or siRNA (Brummelkamp et al, 2002; Yu et al, 2002; Yi et al, 2003). Three different MuSK–shRNA plasmids were designed while only one long hairpin RNA was used. As a control, plasmids derived from CD4 were transfected. On examining the electroporated muscles after 2 weeks, we could not detect a clear effect on the structure of their NMJs (data not shown), and we therefore concentrated our examination to 6 weeks. In CD4-targeted muscle fibres, NMJs overlying GFP-positive myonuclei were indistinguishable from NMJs on muscle fibres that were not transfected (Fig 4A). The alterations of the postsynaptic structures after applying MuSK shRNA plasmids ranged from fragmentation (Fig 4B) to severe disassembly of postsynaptic AChR clusters (Fig 4C). In response to the abrogation of postsynapse integrity, presynaptic nerve terminals began to sprout (arrowheads in Fig 4C,D). In some severe cases (Fig 4D), the entire postsynaptic structure was lost and only the remaining motor nerve terminal indicated that an NMJ had been present before. In all GFP-negative muscle fibres, NMJs were not different from nontreated animals (data not shown). Quantification revealed that NMJs were not altered by electroporation of shRNA plasmids directed to CD4 and to one sequence of MuSK (M1). Two other shRNA plasmids to MuSK (M2 and M3) showed a clear effect on NMJ structure (Fig 4E). Similarly, plasmids encoding functional long dsRNA to MuSK led to the disassembly of postsynaptic AChR clusters (supplementary Fig 2 online).
Figure 4.
Whole mounts of NMJs, 6 weeks after in vivo electroporation of mouse soleus muscle with siRNA plasmids. Electroporated muscle fibres are marked by NLS_GFP (GFP). AChRs were stained to visualize postsynapses, and a mixture of antibodies to neurofilament (Nf) and synaptophysin (Syn) was used to label the presynaptic motor neurons. (A) NMJs are not altered by an siRNA plasmid to CD4. (B–D) Disassembly of NMJs by siRNA plasmids to MuSK. See text for details. Scale bars=15 μm. (E) Quantification of the effect of plasmid-mediated siRNA (see supplementary information online). CD4, siRNA plasmid targeting CD4; M1, M2 and M3, three different siRNA plasmids targeting MuSK. Note that M1 does not show any effect, which is consistent with observations by others that most but not all siRNA constructs are functional (McManus & Sharp, 2002).
Discussion
Although dsRNA can mediate RNAi in cultured mammalian cells (Elbashir et al, 2001), its specific effect is overwritten by the activation of the dsRNA-dependent interferon response, which triggers general inhibition of protein translation and induces apoptosis of cells (Paddison et al, 2002). Here, we used dsRNA and show for the first time that dsRNA-induced RNAi is highly reproducible and sequence specific. For example, dsRNA directed to utrophin clearly reduced the amount of utrophin at the ectopic postsynapses but did not affect the expression levels of its homologue dystrophin. Second, the use of dsRNA did not cause inhibition of protein translation in general, indicated by the fact that the number of AChR clusters formed on muscle fibres injected with dsRNA directed to CD4, αsarcoglycan or utrophin was not different from muscle fibres that were not injected with any dsRNA. Moreover, we could not detect any off-target effect (Jackson et al, 2003, but see also Chi et al, 2003; Semizarov et al, 2003) because the amount of several proteins was not affected by the expression of dsRNA (supplementary Fig 1 online). Third, the effect of dsRNA was confined to the injected muscle fibre and did not spread across cell boundaries. This allowed comparison of perturbed and nonaffected muscle fibres in the same muscle and easy control of the method.
We did not investigate why we found no evidence for a general silencing of translation in dsRNA-injected muscle fibres. It could well be that muscle fibres do not respond to dsRNA in this unspecific way. Indeed, vector-mediated delivery of dsRNA has also been shown to induce sequence-specific RNAi in cultured C2C12 cells, a cell line that forms myotubes. As in our case, the silencing of endogenous genes was not accompanied by a global effect on translation (Yi et al, 2003). To restrict expression of long dsRNA to skeletal muscle, we used the muscle creatine kinase promoter (Moll et al, 2001) instead of the U6 promoter used for shRNA plasmids, to drive expression of long hairpin RNA. Our work also introduces two alternative methods for applying RNA interference. While electroporation is the method of choice to reach many muscle fibres and allows assessment of gene function in the entire muscle fibre including the NMJ, microinjection of single muscle fibres allows one to fine-tune the experimental settings. In particular, the amount of dsRNA and expression plasmids can be well controlled. Moreover, the distance of the injection site from the endogenous NMJ can be chosen. All these parameters influence the outcome of the experiment, and thus microinjection is well suited to study the molecular details of how postsynaptic structures are induced in vivo. In summary, both methods will facilitate the functional characterization of unknown genes in muscle in vivo, which is a significant advantage over the current methods using conventional gene-targeting techniques in mice. Moreover, this method may also be beneficial for studies aimed at the treatment of particular diseases.
Another aspect of our work is that we provide direct evidence that MuSK expression is necessary to warrant the integrity of the NMJ. The recent discovery that auto-antibodies to MuSK cause myasthenia gravis (Hoch et al, 2001) is suggestive of a role of MuSK in warranting the integrity of postsynaptic structures. However, no direct evidence has yet been provided. We describe here that MuSK perturbation causes pronounced disassembly of the entire NMJ. MuSK perturbation also resulted in the sprouting of the presynaptic nerve terminal, indicating that a compact postsynaptic structure is also required to maintain presynaptic integrity. Denervation of NMJs may be a secondary consequence in patients who suffer from myasthenia gravis caused by MuSK auto-antibodies.
Methods
shRNA plasmids and injections Full-length chick agrin cDNA and the NLS_GFP construct have been described previously (Denzer et al, 1995; Jones et al, 1999). Vectors encoding shRNAs were constructed according to Yu et al (2002) using the loop sequence TTCAAGAGA (Brummelkamp et al, 2002). The murine 21 nt target sequences correspond to nucleotides 125–145 (M1), 352–372 (M2) and 525–545 (M3) of MuSK (NCBI accession: NM_010944) and 494–514 of CD4 (M36850). Injection into rat muscle fibres was performed as described (Meier et al, 1997).
dsRNA preparation PCR-generated transcription templates contained T7 or T3 promoter sequences on the 5′ end of the sense or antisense template. RNAs were synthesized using the Megascripts kit (Ambion) and annealed as described (Wianny & Zernicka-Goetz, 2000). The target sequences in rats correspond to nucleotides 5–608 of MuSK (U34985), 44–687 of rapsyn according to murine homologue gene (NM_009023), 114–704 of CD4 (M15768), 55–740 of αsarcoglycan according to murine homologue gene (NM_009161) and 2132–2747 of utrophin (AJ002967). PCR products of rapsyn, α-sarcoglycan and utrophin were sequenced.
Electroporation of cDNA into muscle fibres A 5–10 μl mix of cDNAs (2 μg/μl of each construct) was injected into the soleus muscle of C57BL/6 mice (>6 months). Electroporation was performed as described previously (Gehl & Mir, 1999) using an ECM 830 electroporation system (BTX). Eight pulses were applied for 20 ms and at a frequency of 1 Hz. The voltage was set to 200 V/cm. After 2–6 weeks, the electroporated muscle was analysed.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Drs G. Bezakova, T. Meier and W. Filipowicz for their comments, Dr S. Lin for his help in the quantification and the members of the laboratory for fruitful discussions. X.C.K. is supported by a fellowship from Hoffmann-LaRoche Ltd. Additional support was provided by the Swiss National Science Foundation, the Kanton of Basel-Stadt and the Swiss Foundation for Research on Muscle Diseases to M.A.R.
References
- Benoist C, Mathis D (1999) in Fundamental Immunology, Paul WE (ed) Vol. 1, pp 367–409. Philadelphia: Lippincott–Raven [Google Scholar]
- Bezakova G, Ruegg MA (2003) New insights into the roles of agrin. Nat Rev Mol Cell Biol 4: 295–308 [DOI] [PubMed] [Google Scholar]
- Briguet A, Ruegg MA (2000) The ets transcription factor GABP is required for postsynaptic differentiation in vivo. J Neurosci 20: 5989–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Chi JT, Chang HY, Wang NN, Chang DS, Dunphy N, Brown PO (2003) Genomewide view of gene silencing by small interfering RNAs. Proc Natl Acad Sci USA 100: 6343–6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Rimer M, Lomo T, McMahan UJ (1997) Agrin-induced postsynaptic apparatus in skeletal muscle fibers in vivo. Mol Cell Neurosci 9: 237–253 [DOI] [PubMed] [Google Scholar]
- DeChiara TM et al. (1996) The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85: 501–512 [DOI] [PubMed] [Google Scholar]
- Deconinck AE, Potter AC, Tinsley JM, Wood SJ, Vater R, Young C, Metzinger L, Vincent A, Slater CR, Davies KE (1997) Postsynaptic abnormalities at the neuromuscular junctions of utrophin-deficient mice. J Cell Biol 136: 883–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer AJ, Gesemann M, Schumacher B, Ruegg MA (1995) An amino-terminal extension is required for the secretion of chick agrin and its binding to extracellular matrix. J Cell Biol 131: 1547–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos F et al. (1998) Progressive muscular dystrophy in alphasarcoglycan-deficient mice. J Cell Biol 142: 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498 [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP (1995) Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature 377: 232–236 [DOI] [PubMed] [Google Scholar]
- Gehl J, Mir LM (1999) Determination of optimal parameters for in vivo gene transfer by electroporation, using a rapid in vivo test for cell permeabilization. Biochem Biophys Res Commun 261: 377–380 [DOI] [PubMed] [Google Scholar]
- Glass DJ et al. (1996) Agrin acts via a MuSK receptor complex. Cell 85: 513–523 [DOI] [PubMed] [Google Scholar]
- Grady RM, Merlie JP, Sanes JR (1997) Subtle neuromuscular defects in utrophin-deficient mice. J Cell Biol 136: 871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A (2001) Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 7: 365–368 [DOI] [PubMed] [Google Scholar]
- Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS (2003) Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol 21: 635–637 [DOI] [PubMed] [Google Scholar]
- Jones G, Moore C, Hashemolhosseini S, Brenner HR (1999) Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J Neurosci 19: 3376–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DL, Hagstrom JE, Loomis AG, Wolff JA, Herweijer H (2002) Efficient delivery of siRNA for inhibition of gene expression in postnatal mice. Nat Genet 32: 107–108 [DOI] [PubMed] [Google Scholar]
- McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA (2002) RNA interference in adult mice. Nature 418: 38–39 [DOI] [PubMed] [Google Scholar]
- McCaffrey AP, Nakai H, Pandey K, Huang Z, Salazar FH, Xu H, Wieland SF, Marion PL, Kay MA (2003) Inhibition of hepatitis B virus in mice by RNA interference. Nat Biotechnol 21: 639–644 [DOI] [PubMed] [Google Scholar]
- McManus MT, Sharp PA (2002) Gene silencing in mammals by small interfering RNAs. Nat Rev Genet 3: 737–747 [DOI] [PubMed] [Google Scholar]
- Meier T, Hauser DM, Chiquet M, Landmann L, Ruegg MA, Brenner HR (1997) Neural agrin induces ectopic postsynaptic specializations in innervated muscle fibers. J Neurosci 17: 6534–6544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Barzaghi P, Lin S, Bezakova G, Lochmuller H, Engvall E, Muller U, Ruegg MA (2001) An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature 413: 302–307 [DOI] [PubMed] [Google Scholar]
- Moore C, Leu M, Muller U, Brenner HR (2001) Induction of multiple signaling loops by MuSK during neuromuscular synapse formation. Proc Natl Acad Sci USA 98: 14655–14660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddison PJ, Caudy AA, Hannon GJ (2002) Stable suppression of gene expression by RNAi in mammalian cells. Proc Natl Acad Sci USA 99: 1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinson DA et al. (2003) A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 33: 401–406 [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci 2: 791–805 [DOI] [PubMed] [Google Scholar]
- Semizarov D, Frost L, Sarthy A, Kroeger P, Halbert DN, Fesik SW (2003) Specificity of short interfering RNA determined through gene expression signatures. Proc Natl Acad Sci USA 100: 6347–6352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijsterman M, Ketting RF, Plasterk RH (2002) The genetics of RNA silencing. Annu Rev Genet 36: 489–519 [DOI] [PubMed] [Google Scholar]
- Wianny F, Zernicka-Goetz M (2000) Specific interference with gene function by doublestranded RNA in early mouse development. Nat Cell Biol 2: 70–75 [DOI] [PubMed] [Google Scholar]
- Winston WM, Molodowitch C, Hunter CP (2002) Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295: 2456–2459 [DOI] [PubMed] [Google Scholar]
- Yi CE, Bekker JM, Miller G, Hill KL, Crosbie RH (2003) Specific and potent RNA interference in terminally differentiated myotubes. J Biol Chem 278: 934–939 [DOI] [PubMed] [Google Scholar]
- Yu JY, DeRuiter SL, Turner DL (2002) RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA 99: 6047–6052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information