Abstract
Deletion and point (L166P) mutations of DJ-1 have recently been shown to be responsible for the onset of familial Parkinson's disease (PD, PARK7). The aim of this study was to determine the role of DJ-1 in PD. We first found that DJ-1 eliminated hydrogen peroxide in vitro by oxidizing itself. We then found that DJ-1 knockdown by short interfering RNA rendered SH-SY5Y neuroblastoma cells susceptible to hydrogen peroxide-, MPP+- or 6-hydroxydopamine-induced cell death and that cells harbouring mutant forms of DJ-1, including L166P, became susceptible to death in parallel with the loss of oxidized forms of DJ-1. These results clearly showed that DJ-1 has a role in the antioxidative stress reaction and that mutations of DJ-1 lead to cell death, which is observed in PD.
Introduction
Parkinson's disease (PD) involves an irreversible degeneration of the dopaminergic nigrostriatal pathway. Genes responsible for rare familial early-onset PD, including α-synuclein (Polymeropoulos et al, 1997), Parkin (Kitada et al, 1998) and UCH-L1 (Leroy et al, 1998), have been identified, and they are thought to have a role in ubiquitin–proteasome dysfunction in PD. Various lines of evidence also suggest that oxidative stresses contribute to the cascade leading to dopaminergic cell degeneration in PD, but the mechanisms responsible for nigral dopaminergic cell death are not clear (Nicklas et al, 1987; Heikkila & Cohen, 1971; Lotharius & O'Malley, 2000; for a recent review, see Jenner, 2003). DJ-1 was identified by us as an oncogene product (Nagakubo et al, 1997). It was later found to be a positive regulator of the androgen receptor (Takahashi et al, 2001; Niki et al, 2003) and to be related to infertility (Wagenfeld et al, 1998, 2000; Welch et al, 1998; Klinefelter et al, 2002; Okada et al, 2002). Deletion and point (L166P) mutations of DJ-1 have recently been shown to be responsible for the onset of familial PD, PARK7 (Bonifati et al, 2003), and expression of DJ-1 was induced by oxidative stresses (Mitsumoto & Nakagawa, 2001; Mitsumoto et al, 2001; Srisomsap et al, 2002). The mechanisms underlying the onset of PD due to DJ-1 mutations, however, have not been clarified. In this study, we analysed the effects of DJ-1 on hydrogen-peroxide-induced cell death. We found that DJ-1 has a role in the antioxidative stress reaction and that mutations of DJ-1 lead to cell death.
Results And Discussion
Elimination of hydrogen peroxide by DJ-1
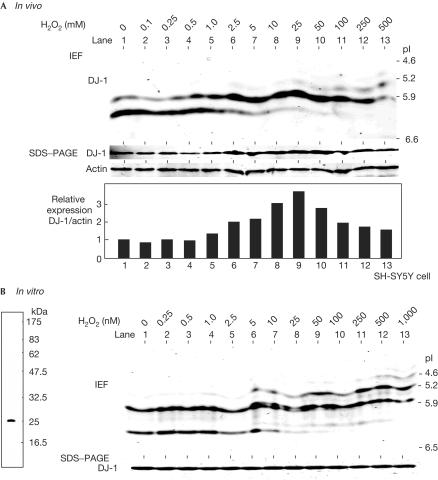
Expression of DJ-1 was induced in human ECV304 endothelial cells, mouse primary macrophages and human RD4 fibroblast cells that had been subjected to treatment with paraquat, endotoxin and iron, respectively, which yielded reactive oxygen species (Mitsumoto & Nakagawa, 2001; Mitsumoto et al, 2001; Srisomsap et al, 2002). To examine statistically the expression of DJ-1 in neuroblastoma cells, human neuroblastoma SH-SY5Y cells were treated with various concentrations of hydrogen peroxide for 12 h, and proteins prepared from cells were separated on SDS-containing polyacrylamide gel electrophoresis (PAGE) and isoelectric focusing gel over a pH range of 5–8 (Fig 1A). After blotting with an anti-DJ-1 antibody, the amounts of actin were not changed but DJ-1 was found to be induced by hydrogen peroxide at doses up to 25 μM in a dose-dependent manner, and the pI of DJ-1 was shifted to a more acidic point, from 6.7 to 5.7 (Fig 1A, SDS–PAGE and IEF, respectively). These phenomena were also observed in other cell lines, including human HeLa, monkey Cos1 and mouse NIH3T3 cells (data not shown, see later). Recombinant DJ-1 was expressed in and purified from Escherichia coli to near-homogeneity (Fig 1B, left panel). Recombinant DJ-1 was then reacted with various concentrations of hydrogen peroxide for 30 min, and its pI changes were examined (Fig 1B). Although the total amount of DJ-1 was not affected by hydrogen peroxide, a pI shift in DJ-1 to a more acidic point was observed. However, lower concentrations of hydrogen peroxide were found to be needed in in vitro reactions than in in vivo culture cells, suggesting that DJ-1 is directly affected by hydrogen peroxide.
Figure 1.
pI shift of DJ-1 after its treatment with hydrogen peroxide. (A) SH-SY5Y cells were treated with various concentrations of hydrogen peroxide for 12 h, and proteins in the extracts were then analysed by isoelectric focusing phoresis gel (IEF, upper panel) or PAGE containing SDS (SDS–PAGE in the lower panel) as described in Methods. Intensities of the bands of DJ-1 and actin were measured and are shown as ‘relative expression'. (B) In all, 1 μg of recombinant DJ-1 was reacted with various concentrations of hydrogen peroxide for 30 min at room temperature and subjected to isoelectric focusing Diet phoresis and SDS–PAGE electrophoresis as described in A (right panel). The recombinant DJ-1 used in this experiment was separated on PAGE containing SDS and stained with Coomassie brilliant blue R-250 (left panel).
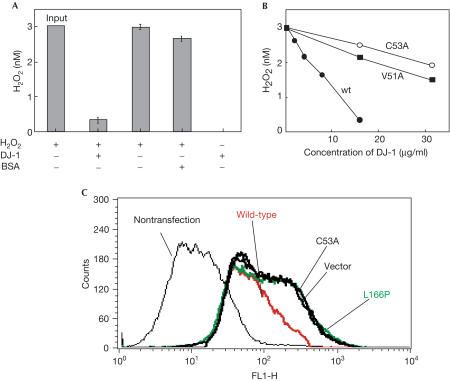
To examine the effect of DJ-1 on hydrogen peroxide, recombinant DJ-1 was reacted with hydrogen peroxide at concentrations five times greater than those of DJ-1 for 1 h at 30°C, and the concentrations of hydrogen peroxide were measured by the scopoletin method (Fig 2A). Although the control bovine serum albumin (BSA) was hardly affected, DJ-1 eliminated approximately 80% of hydrogen peroxide. Elimination of hydrogen peroxide by DJ-1 occurred in a time-dependent manner for up to 30 min (data not shown). We and others have analysed the structure of DJ-1 by X-ray crystallography and found that DJ-1 is present as a dimer (Honbou et al, 2003; Huai et al, 2003; Lee et al, 2003; Tao & Tong, 2003; Wilson et al, 2003). Based on this finding, two point-mutants of DJ-1, V51A and C53A, that disrupt the dimer formation of DJ-1 were constructed and tested for their ability to eliminate hydrogen peroxide in vitro (Fig 2B). Whereas wild-type DJ-1 eliminated hydrogen peroxide in a dose-dependent manner, the activity levels of the two mutants were only about 20% that of wild-type DJ-1, suggesting that the active form of DJ-1 is a dimer. Members of the family of peroxiredoxin proteins, such as thioredoxin peroxidase, eliminate hydrogen peroxide by the use of electrons provided by thioredoxin, and the resultant oxidized thioredoxin peroxidase is then reduced back to the active form (Jacquot et al, 2002; Wood et al, 2003). To determine whether DJ-1 is a peroxiredoxin protein, oxidized DJ-1 was reacted with thioredoxin or glutathione and its pI shift was measured. The results showed, however, that the pI of DJ-1 was not changed by these chemicals (data not shown), suggesting that DJ-1 is a new type of scavenger protein acting on hydrogen peroxide. To examine the elimination of hydrogen peroxide by DJ-1 in vivo, SH-SY5Y cells were transfected with wild-type and mutant forms of DJ-1. At 36 h after transfection, 10 μM hydrogen peroxide was added to the cells over a period of 60 min, and the cells were then reacted with 2′,7′-dichlorodihydrofluoroscein diacetate (DCFH-DA) for 30 min and analysed by flow cytometry (Fig 2C). Cells containing hydrogen peroxide have high fluorescence intensity and the peak is shifted to the left (compare cells that were not treated with cells transfected with a vector in Fig 2C). Whereas fluorescence-intensities of cells transfected with the two point-mutants V51A and C53A and another mutant of DJ-1, L166P, a mutant with a leucine-to-proline substitution found in a PARK7 patient (Bonifati et al, 2003), were shifted to the position of cells transfected with a control vector, that of cells transfected with wild-type DJ-1 was shifted back (Fig 2C), indicating that elimination of hydrogen peroxide by DJ-1 occurs in cultured cells.
Figure 2.
Elimination of hydrogen peroxide by DJ-1. (A) In all, 3 nM of hydrogen peroxide was reacted with 0.5 nM of DJ-1 for 1 h at 30°C, and the concentration of hydrogen peroxide was measured as described in Methods. (B) In all, 3 nM of hydrogen peroxide was reacted with various amounts of wild-type, V51A and C53A DJ-1 for 1 h at 30°C, and the concentrations of hydrogen peroxide were measured as described in Methods. (C) SHSY-5Y cells in 10-cm dishes were transfected with 5 μg each of plasmids used for the establishment of cell lines by the lipofectamine method. At 36 h after transfection, cells were treated with 10 μM hydrogen peroxide for 60 min and then with 5 μM DCFH-DA for 30 min, and analysed by flow cytometry. BSA, bovine serum albumin.
Abrogation of hydrogen-peroxide-induced cell death by DJ-1
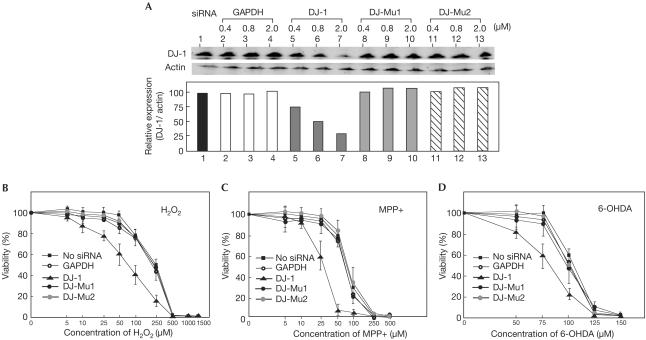
Reactive oxygen species such as hydrogen peroxide induce cell death, and these effects are related to the onset of PD (Heikkila & Cohen, 1971; Nicklas et al, 1987; Lotharius & O'Malley, 2000; for a recent review, see Jenner, 2003). To determine the roles of DJ-1 in cells treated with hydrogen peroxide, siRNA against DJ-1 was transfected into SH-SY5Y cells to knock down the expression of endogenous DJ-1, and viabilities of cells in the presence of various doses of hydrogen peroxide were measured 36 h after transfection (Fig 3). The endogenous levels of DJ-1 and actin in SHsY5Y cells transfected with wild-type or mutated siRNAs against DJ-1 or against GAPDH, a negative control, were first measured by western blotting with anti-DJ-1 and anti-actin antibodies, and the level of DJ-1 was normalized to that of actin (Fig 3A). Although the mutated siRNAs against DJ-1 or siRNA against GAPDH did not affect the amount of DJ-1, the amount of DJ-1 was reduced to 30% of that without siRNA. As the efficiency of transfection of siRNA into SHSY-5Y cells was about 80%, the expression of DJ-1 in cells containing siRNA against DJ-1 was almost knocked down. Under these conditions, the death of SH-SY5Y cells transfected with siRNA against DJ-1 was accelerated compared to that of nontransfected SH-SY5Y cells and that of cells transfected with mutated siRNAs against DJ-1 or siRNA against GAPDH; concentrations of hydrogen peroxide that gave ID50 in cells transfected with siRNA against DJ-1 and with mutated siRNA against DJ-1 were 70±10 and 230±8 μM, respectively (Fig 3B). The same activity of DJ-1 against cell death was also observed in mouse NIH3T3 cells (data not shown). The effect of DJ-1 on the viability of cells in the presence of dopaminergic neurotoxins, 1-methyl-4-phenylpyridium (MPP+) and 6-hydroxydopamine (6-OHDA), both of which induce oxidative stress in cells (Heikkila & Cohen, 1971; Nicklas et al, 1987; Lotharius & O'Malley, 2000), was also examined, and results similar to those for cells treated with hydrogen peroxide were obtained (Fig 3C,D, respectively). These results clearly indicate that DJ-1 has a role in oxidative-stress-induced cell death.
Figure 3.
Acceleration of hydrogen-peroxide-induced cell death in DJ-1-knockdown cells. (A) SH-SY5Y cells were transfected with siRNAs targeting GAPDH and DJ-1. At 36 h after transfection, the proteins in the extracts were blotted with an anti-DJ-1 antibody or anti-actin antibody as described in Methods. The intensities of the bands in western blotting shown in A (upper panel) were measured by an Odyssey system, and the intensity of DJ-1 was normalized to that of actin (lower panel). SH-SY5Y cells were transfected with 2 μM of siRNAs targeting GAPDH and DJ-1. At 36 h after transfection, cells were reacted with various concentrations of hydrogen peroxide (B), MPP+ (C) or 6-OHDA (D) for 12 h, and their viabilities were measured by an MTT assay.
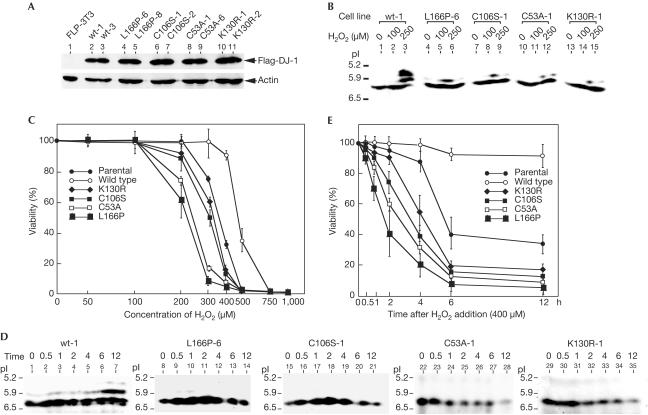
The role of DJ-1 in hydrogen-peroxide-induced cell death was further confirmed by experiments using established NIH3T3 cell lines harbouring exogenously added Flag-tagged wild type or various mutants of DJ-1. The Flp-In system (InVitrogen) was used to establish these cell lines: CMV promoter-based expression vectors surrounded by Flp sequences at both the 5′ and 3′ ends were inserted into a site containing the FRT sequence of a chromosome in mouse NIH3T3 cells by Flp recombinase that had been supplied by co-transfection of its expression vector. All the wild-type DJ-1 and mutant DJ-1 proteins were thereby expressed on the same backgrounds in cells, and these were confirmed by western blotting with anti-Flag and anti-actin antibodies (Fig 4A). Two other mutants of DJ-1 were used in addition to the C53A and L166P mutants: C106S, a mutant with a cysteine-to-serine substitution corresponding to a catalytic site for protease activity, as the X-ray crystal structure suggests that DJ-1 has protease activity (Honbou et al, 2003); and K130R, a mutant with a lysine-to-arginine substitution corresponding to a major sumoylation site. When pI shifts of wild-type DJ-1 and mutants of DJ-1 were measured after the addition of two doses of hydrogen peroxide to these cell lines, only small changes to acidic points were observed in all the mutants of DJ-1 compared with that of wild-type DJ-1 (Fig 4B). Viabilities of these cell lines in the presence of hydrogen peroxide were then determined (Fig 4C). Contrary to the case of DJ-1-knockdown cells, cells harbouring exogenously added wild-type DJ-1 were much more resistant to hydrogen peroxide than parental nontransfected cells. Conversely, of the cell lines harbouring mutants, the cell line harbouring L166P was found to be most sensitive to hydrogen peroxide, and the C53A, C106S and K130R lines followed in terms of sensitivity to hydrogen peroxide. To examine the effect of DJ-1 statistically on the viability of cells in the presence of 400 μM hydrogen peroxide, a time-course experiment was carried out using these cell lines (Fig 4D,E). Although pI shifts of wild-type DJ-1 began at 0.5 h after the addition of hydrogen peroxide and continued up to 12 h, only small changes to acidic points were observed in all the mutants of DJ-1 at this concentration of hydrogen peroxide (Fig 4D). Furthermore, all the cell lines, except for the cell line harbouring wild-type DJ-1, began to die 30 min after hydrogen peroxide treatment and continued to die up to 6 h after (Fig 4E). The cell line harbouring L166P was again most sensitive to hydrogen peroxide, and the cell line harbouring wild-type DJ-1 was resistant to hydrogen-peroxide-induced cell death at a concentration of 400 μM. About 50% of parental cells had died at 6 h after hydrogen peroxide treatment. As the half-life of DJ-1 was estimated to be about 6 h (Macedo et al, 2003; Miller et al, 2003), a reduced amount of DJ-1 in cells may affect cell susceptibility to hydrogen-peroxide-induced cell death. The possibility that different susceptibilities of cell lines to hydrogen-peroxide-induced cell death were due to different activities of antioxidant enzymes, and not to DJ-1, was ruled out by measuring the enzyme activities of superoxide dismutase, glutathione peroxidase and catalase in the cell lines that had been treated with hydrogen peroxide (see supplementary information online). The results showed that the enzyme activities of all the cell lines were similarly induced by treatment with hydrogen peroxide. These results clearly indicate that DJ-1 has a role in oxidative stress and that mutations of DJ-1 lead to oxidative-stress-induced cell death.
Figure 4.
Acceleration of hydrogen-peroxide-induced cell death in cells harbouring various mutants of DJ-1, including L166P, which is observed in PARK7 patients. (A) Expression levels of Flag-DJ-1 and actin in NIH3T3 cell lines harbouring Flag-tagged wild-type DJ-1 and various mutants of DJ-1 were examined by western blotting. Two different cell lines were used for each construct. (B) NIH3T3 cell lines were reacted with 100 and 250 μM of hydrogen peroxide for 12 h. Proteins in cells were then subjected to isoelectric focusing phoresis and blotted with an anti-DJ-1 antibody. (C) NIH3T3 cell lines were reacted with various concentrations of hydrogen peroxide for 24 h and their viabilities were measured by an MTT assay. (D) NIH3T3 cell lines were reacted with 400 μM of hydrogen peroxide. Proteins in cells at various times after treatment with hydrogen peroxide were then subjected to isoelectric focusing phoresis and blotted with an anti-DJ-1 antibody. (E) NIH3T3 cell lines were reacted with 400 μM hydrogen peroxide, and cell viabilities were measured by an MTT assay at various times after treatment with hydrogen peroxide.
DJ-1 is involved in transcriptional regulation, tumorigenesis, fertilization and early onset of PD (PARK7), and its expression is induced by oxidative stresses. The mechanisms underlying these phenomena, however, have not been clarified. In this study, we found that DJ-1 eliminated hydrogen-peroxide by oxidizing itself and that this activity was a prerequisite for protection of cells against hydrogen-peroxide-induced cell death, as was demonstrated by an experiment using DJ-1-knockdown cells. We also found that mutations of DJ-1 abrogated this activity, leading to hypersusceptibility to cell death. The X-ray crystal structure revealed that DJ-1 functions as a dimer and possesses protease activity. The finding that cell lines containing mutants of DJ-1 became hypersusceptible to cell death is explained as follows. There are heterodimers formed by endogenously present wild-type DJ-1 in NIH3T3 cells and mutant DJ-1 and there are homodimers formed by mutants such as L166P and K130R, which still possess the β-helix that is necessary for dimerization. The antioxidative stress activities of these hetero- and homodimers are lost or weakened. Proper localization in cells is also thought to be necessary for DJ-1 to exert its functions, because L166P is localized in the mitochondria (Bonifati et al, 2003), whereas wild-type DJ-1 is localized in the cytoplasm and nucleus. Localization of proteins is determined by their interaction with other proteins, modification of proteins such as sumoylation or proper conformation of proteins, and these situations may also be true for DJ-1.
Methods
Isoelectric focusing and western blotting. SH-SY5Y and NIH3T3 cells, as well as NIH3T3 cells harbouring wild-type or mutants of DJ-1, were cultured in Dulbecco's modified Eagle's medium supplemented with 10% calf serum. The cells were then treated with various concentrations of hydrogen peroxide for 12 or 24 h, and cell extracts were prepared in a mixture containing 2% NP-40 and phosphate-buffered saline (PBS). Proteins in the extracts were then separated in pH 5–8 ranges of isoelectric focusing phoresis gel or 12.5% PAGE containing SDS, transferred onto nitrocellulose membranes, and blotted with an anti-DJ-1 polyclonal antibody or anti-actin antibody (MAB1501R, Chemicon). The proteins that reacted with primary antibodies were visualized with IRDye800-conjugated or Alexa Fluor680-conjugated secondary antibodies using an infrared imaging system (Odyssey, LI-COR). When recombinant DJ-1 was used in the experiment, GST-free DJ-1 was prepared as described previously.
Establishment of cell lines harbouring wild-type DJ-1 or mutants of DJ-1. KpnI–XhoI fragments containing the CMV promoter and Flag-tagged wild-type or mutant DJ-1s were inserted into KpnI–XhoI sites of pcDNA5/FRT. These plasmids were cotransfected with pOG44, an expression vector for Flp recombinase, into Flp-In™3T3 cells (InVitrogen) by the calcium phosphate precipitation method, and the cells were cultured in the medium in the presence of 100 μg/ml zeocin and 100 μg/ml hygromycin for 14 days. The cells that were resistant to both drugs were then selected, and expression of Flag-DJ-1 was examined by western blotting with an anti-Flag antibody (M2, Sigma).
Cell viability assay. Cells were cultured in a 96-well plate and treated with various amounts of hydrogen peroxide for 12 or 24 h. Cell viability was then measured by an MTT assay using a cell counting kit 8 (DOJINDO).
Knockdown of DJ-1 in cells. The nucleotide sequences for wild-type and mutated siRNA (DJ-1, DJ-Mu1 and DJ-Mu2) targeting DJ-1 were as follows: DJ-1: 5′-UGGAGACGGUCAUCCCUGUdTdT-3′ (upper strand) and 3′-dTdTACCUCUGCCAGUAGGGACA-5′ (lower strand); DJ-Mu1: 5′-UCCAGACGGUCAUCCCUGUdTdT-3′ (upper strand) and 3′-dTdTAGGUCUGCCAGUAGGGACA-5′ (lower strand); DJ-M2: 5′-UGGAGACGGAGAUCCCUGUdTdT-3′ (upper strand) and 3′-dTdTACCUCUGCCUCUAGGGACA-5′ (lower strand). The siRNA targeting GAPDH was purchased from Greiner (Japan). Various amounts of siRNA were transfected into SH-SY5Y cells using Oligofectamine reagent (InVitrogen) according to the supplier's manual.
Measurement of hydrogen peroxide. Various amounts of hydrogen peroxide were reacted in 200 μl mixtures with recombinant DJ-1 for 1 h at 30°C, and 1 ml of PBS, 400 μl of 10 μM scopoletin and 400 μl of 32.5 U/ml horseradish peroxidase were then added to the mixture. After 5 min at 25°C, fluorescence at Ex 366 nm and Em 460 nm was measured.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400074s1.pdf).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Yoko Misawa and Kiyomi Takaya for their technical assistance. This work was supported by grants-in-aid from the Ministry of Education, Science, Culture, Sports and Technology of Japan.
References
- Bonifati V et al. (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science 299: 256–259 [DOI] [PubMed] [Google Scholar]
- Heikkila R, Cohen G (1971) Inhibition of biogenic amine uptake by hydrogen peroxide: a mechanism for toxic effects of 6-hydroxydopamine. Science 172: 1257–1258 [DOI] [PubMed] [Google Scholar]
- Honbou K et al. (2003) The crystal structure of DJ-1, a protein related to male fertility and Parkinson's disease. J Biol Chem 278: 31380–31384 [DOI] [PubMed] [Google Scholar]
- Huai Q et al. (2003) Crystal structure of DJ-1/RS and implication on familial Parkinson's disease. FEBS Lett 549: 171–175 [DOI] [PubMed] [Google Scholar]
- Jacquot JP et al. (2002) Thioredoxins and related proteins in photosynthetic organisms: molecular basis for thiol dependent regulation. Biochem Pharmacol 64: 1065–1069 [DOI] [PubMed] [Google Scholar]
- Jenner P (2003) Oxidative stress in Parkinson's disease. Ann Neurol 53(Suppl 3): S26–S36 [DOI] [PubMed] [Google Scholar]
- Kitada T et al. (1998) Mutations in the Parkin gene cause autosomal recessive juvenile Parkinsonism. Nature 392: 605–608 [DOI] [PubMed] [Google Scholar]
- Klinefelter GR et al. (2002) Localization of the sperm protein SP22 and inhibition of fertility in vivo and in vitro. J Androl 23: 48–63 [DOI] [PubMed] [Google Scholar]
- Lee SJ et al. (2003) Crystal structures of human DJ-1 and Escherichia coli Hsp31 that share an evolutionarily conserved domain. J Biol Chem 278: 44552–44559 [DOI] [PubMed] [Google Scholar]
- Leroy E et al. (1998) The ubiquitin pathway in Parkinson's disease. Nature 395: 451–452 [DOI] [PubMed] [Google Scholar]
- Lotharius J, O'Malley KL (2000) The Parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J Biol Chem 275: 38581–38588 [DOI] [PubMed] [Google Scholar]
- Macedo MG et al. (2003) The DJ-1L166P mutant protein associated with early onset Parkinson's disease is unstable and forms higher order protein complexes. Hum Mol Genet 12: 2807–2816 [DOI] [PubMed] [Google Scholar]
- Miller DW et al. (2003) L166P mutant DJ-1, causative for recessive Parkinson's disease, is degraded through the ubiquitin–proteasome system. J Biol Chem 278: 36588–36595 [DOI] [PubMed] [Google Scholar]
- Mitsumoto A, Nakagawa Y (2001) DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic Res 35: 885–893 [DOI] [PubMed] [Google Scholar]
- Mitsumoto A et al. (2001) Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic Res 35: 301–310 [DOI] [PubMed] [Google Scholar]
- Nagakubo D et al. (1997) DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Comm 31: 509–513 [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Youngster SK, Kindt MV, Heikkila RE (1987) MPTP, MPP+ and mitochondrial function. Life Sci 40: 721–729 [DOI] [PubMed] [Google Scholar]
- Niki T, Takahashi-Niki K, Taira T, Iguchi-Ariga SMM, Ariga H (2003) DJBP: a novel DJ-1-binding protein, negatively regulates the androgen receptor by recruiting histone deacetylase complex, and DJ-1 antagonizes this inhibition by abrogation of this complex. Mol Cancer Res 1: 247–261 [PubMed] [Google Scholar]
- Okada M et al. (2002) DJ-1, a target protein for an endocrine disrupter, participates in the fertilization in mice. Biol Pharm Bull 25: 853–856 [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH et al. (1997) Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276: 2045–2047 [DOI] [PubMed] [Google Scholar]
- Srisomsap C et al. (2002) Detection of cathepsin B up-regulation in neoplastic thyroid tissues by proteomic analysis. Proteomics 2: 706–712 [DOI] [PubMed] [Google Scholar]
- Takahashi K et al. (2001) DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx α to the receptor. J Biol Chem 276: 37556–37563 [DOI] [PubMed] [Google Scholar]
- Tao X, Tong L (2003) Crystal structure of human DJ-1, a protein associated with early-onset Parkinson's disease. J Biol Chem 278: 31372–31379 [DOI] [PubMed] [Google Scholar]
- Wagenfeld A, Yeung CH, Strupat K, Cooper TG (1998) Shedding of a rat epididymal sperm protein associated with infertility induced by ornidazole and α-chlorohydrin. Biol Reprod 8: 1257–1265 [DOI] [PubMed] [Google Scholar]
- Wagenfeld A et al. (2000) Expression and cellular localization of contraception-associated protein. J Androl 21: 954–963 [PubMed] [Google Scholar]
- Welch JE, Barbee RR, Roberts NL, Suarez JD, Klinefelter GR (1998) SP22: a novel fertility protein from a highly conserved gene family. J Androl 19: 385–393 [PubMed] [Google Scholar]
- Wilson MA, Collins JL, Hod Y, Ringe D, Petsko GA (2003) The 1.1-Å resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson's disease. Proc Natl Acad Sci USA 100: 9256–9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ZA, Schroder E, Robin Harris J, Poole LB (2003) Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 28: 32–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information