Abstract
Cleavage of the cohesin subunit Scc1p/Mcd1p/Rad21 permits sister chromatid separation and is considered to trigger anaphase onset. It has also been suggested that the cohesin complex is essential for chromosome condensation and for assembling fully functional kinetochores. Here, we used vertebrate cells conditionally deficient in Scc1 to probe cohesin function in mitosis. Cells lacking cohesin arrest in prometaphase, with many chromosomes failing to align at a metaphase plate and high levels of the spindle assembly checkpoint protein, BubR1, at all kinetochores. We show that the structural integrity of chromosomes is normal in the absence of Scc1. Furthermore, specific inhibition of topoisomerase II, which is required for decatenation of replicated chromosomes, can bypass the cohesin requirement for metaphase chromosome alignment and spindle checkpoint silencing. Since the kinetochore effects of Scc1 deficiency can be compensated for by topoisomerase II inhibition, we conclude that Scc1 is not absolutely required for kinetochore assembly or function, and that its principal role in allowing the onset of anaphase is the establishment of sufficient inter-sister tension to allow biorientation.
Introduction
To ensure the faithful transmission of chromosomes in mitosis, replicated chromatids remain paired until both sister kinetochores attach to opposite poles of the mitotic spindle, a state known as biorientation. This pairing requires cohesin, a multiprotein complex that ensures proper sister chromatid pairing until anaphase onset (Nasmyth, 2002).
Genetic and biochemical studies in yeast, Xenopus and human systems have shown that the cohesin complex contains two SMC (structural maintenance of chromosomes) proteins, Smc1 and Smc3, and two non-SMC proteins, Scc1 and Scc3 (Hirano, 2002; Nasmyth, 2002). Cleavage of the cohesin subunit Scc1p/Mcd1p/Rad21 (hereafter Scc1) by separase triggers sister chromatid separation (Uhlmann et al, 2000; Hauf et al, 2001). Cohesin complexes localize to the centromeres in yeasts (Blat & Kleckner, 1999; Tanaka et al, 1999) and vertebrates (Gregson et al, 2002). However, the association of the Scc1 subunit with chromosome arms persists until anaphase onset in Saccharomyces cerevisiae (Guacci et al, 1997; Michaelis et al, 1997; Uhlmann et al, 1999), but is lost during prophase in metazoan cells (Waizenegger et al, 2000; Warren et al, 2000).
Several observations have suggested that cohesin might be required for kinetochore assembly or function during mitosis. Fission yeast cohesin mutants exhibit prolonged Bub1 binding to centromeres and appear to be defective in kinetochore–microtubule interactions (Toyoda et al, 2002). Chicken DT40 cells, conditionally null for Scc1, arrest in mitotic prometaphase following Scc1 repression, with most chromosomes properly aligned on the metaphase plate but with a significant number scattered throughout the spindle (Sonoda et al, 2001). Depletion of the Drosophila Scc1 orthologue Drad21 by RNA interference also results in mitotic defects (Vass et al, 2003). In the absence of Scc1, the chromosomal passenger INCENP was mislocalized in both chicken and Drosophila cells (Sonoda et al, 2001; Vass et al, 2003). Finally, the expression of an N-terminally truncated form of human Scc1 leads to mitotic abnormalities (Hoque & Ishikawa, 2002).
An additional role for cohesin might be in establishing chromosome higher-order structure. Chromosome condensation defects have been described in Scc1-deficient yeast (Guacci et al, 1997; Lavoie et al, 2002). We have recently shown that mitotic chromosome formation involves two separable steps: condensation of the chromatin and formation of a stable higher-order structure (Hudson et al, 2003). Condensin is required only for the latter. Although Scc1-deficient DT40 cells underwent chromatin condensation, and appeared to bind condensin normally (Sonoda et al, 2001), we could not exclude the possibility that, as in yeast, cohesin might be required for some aspect of the chromosome higher-order structure.
Here we show that the structural integrity of mitotic chromosomes remains intact in the absence of Scc1. We also demonstrate that specific inhibition of topoisomerase II (topo II) can bypass the cohesin requirement in metaphase chromosome alignment and silencing of the spindle assembly checkpoint. These findings show that the cohesin complex is not absolutely required for kinetochore function in mitotic chromosomes.
Results
In this study, we have used a conditionally null chicken DT40 cell line, genotype Scc1−/−/−tet-Scc1 (Sonoda et al, 2001). In standard culture media, the cells are healthy and will be referred to as Scc1ON. The addition of doxycycline shuts off expression of the Scc1 transgene, and under these conditions cells will be referred to as Scc1OFF.
Scc1 depletion does not affect condensin function
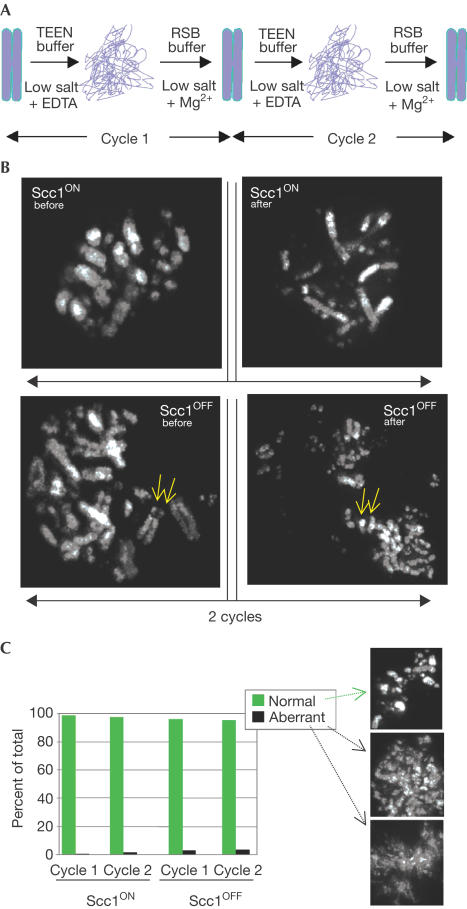
We recently developed an assay that probes the stable higher-order structure of mitotic chromosomes. This assay relies on the ability of chromosomes to recover their morphology following complete unfolding of the chromatin to the 10 nm fibre (‘beads-on-a-string') (Hudson et al, 2003). In this assay, chromosomes are first unfolded by exposure to a low ionic strength solution lacking divalent cations and containing EDTA (Fig 1A). They are then refolded by the addition of a low ionic strength buffer containing 5 mM Mg2+ (Cole, 1967; Earnshaw & Laemmli, 1983). We define the term ‘structural integrity' as the ability of chromosomes to remain morphologically indistinguishable at the level of light microscopy during repeated cycles of swelling and shrinking. We previously showed that condensin is essential for this structural integrity (Hudson et al, 2003).
Figure 1.
Scc1-deficient chromosomes have a stable higher-order structure. (A) Assay for mitotic chromosome structural integrity (Hudson et al, 2003). Note that this is a morphological assay and might not detect the possible loss or alteration of associated proteins during the refolding cycles. (B) Even though the sister chromatids (arrows) show an abnormal degree of premature separation, cohesin (Scc1)-depleted chromosomes have normal structural integrity. (C) Quantitation of the results presented in panel (B). Examples of normally refolded and aberrantly refolded chromosomes are shown at the right. Scc1-depleted chromosomes recover normally.
In order to investigate whether the loss of cohesin might have an impact on this structural integrity of mitotic chromosomes, we performed the assay on chromosomes of Scc1ON and Scc1OFF cells. As shown in Fig 1B, even though the loss of Scc1 caused sister chromatids to separate, it did not perturb the ability of chromosomes to recover their condensed morphology following unfolding of the chromatin. These data are quantified in Fig 1C. In contrast, 95 and 100% of condensin-deficient chromosomes are abnormal at cycles 1 and 2, respectively, in this assay (Hudson et al, 2003). Thus, the loss of cohesin does not disrupt significantly the structural integrity of mitotic chromosomes.
Topo II inhibition rescues chromosome biorientation
Scc1OFF cells in mitosis accumulate in prometaphase, with most macrochromosomes aligned at a metaphase plate but with a number of the microchromosomes scattered throughout the spindle (Fig 2A) (Sonoda et al, 2001). This phenotype is consistent with defective kinetochore function, but could equally well be explained by defects in the ability of sister kinetochores to orient properly to opposite spindle poles. To test whether cohesin is indeed required for kinetochore function, we have artificially imposed cohesion in Scc1OFF DT40 cells by inhibiting topo II. Topo II activity is thought to be required to separate catenated sister chromatids during mitosis in yeast (DiNardo et al, 1984; Uemura & Yanagida, 1986; Holm et al, 1989) and mammalian cells (Gorbsky, 1994; Ishida et al, 1994; Sumner, 1995).
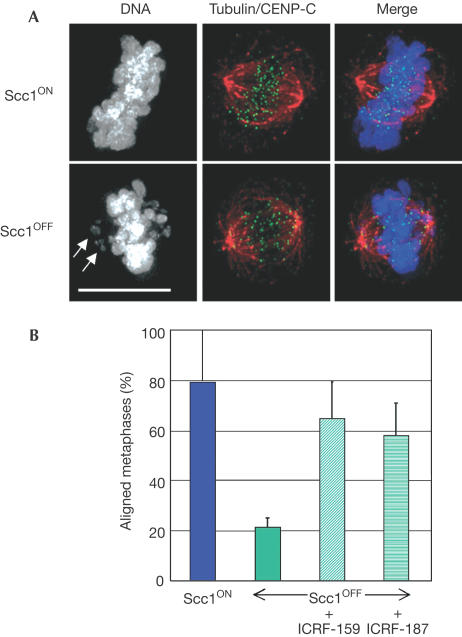
Figure 2.
Successful alignment of Scc1-deficient chromosomes at a metaphase plate following treatment with topo II inhibitors. (A) A metaphase cell was defined as a cell with its chromosomes all aligned on the plate, as shown at the top, whereas cells with chromosomes scattered about the plate (arrows, lower panels) were defined as prometaphase. Cells on polylysine-treated slides were fixed and incubated with antibodies to α-tubulin (red) and CENP-C (green), along with DAPI (grey in left panels). Scale bar, 10 μm. (B) Histogram showing the relative percentage of metaphase Scc1OFF cells following the treatment of synchronized cells with the topo II inhibitors ICRF-159 or ICRF-187 for 1 h before analysis. Cells in which the spindle axis was not parallel to the slide were excluded from this analysis. Data shown are the mean±standard deviation of at least three experiments in which at least 25 cells with appropriately aligned spindles were scored blind. Levels of alignment under all conditions differed significantly from those observed in Scc1OFF conditions (χ2-test; P<0.01). A control experiment on 25 Scc1ON metaphases showed 85 and 84% aligned at metaphase after treatment with ICRF-159 and ICRF-187, respectively.
To test whether topo II inhibition might improve metaphase chromosome alignment, we treated Scc1OFF DT40 cells with ICRF-159 or ICRF-187, which are highly specific bisdioxopiperazine drugs that catalytically inhibit topo II without causing a significant G2–M arrest (Andoh & Ishida, 1998). It was necessary to perform these analyses on synchronized DT40 cells treated only for short periods, as long-term exposure to either drug interfered with mitotic chromosome condensation (Gorbsky, 1994) and caused dramatic levels of apoptosis (data not shown). Both drugs significantly improved metaphase chromosome alignment in Scc1OFF cells (Fig 2B), and in high-resolution images sister kinetochores could be seen to be bioriented (Fig 3E arrows in insets). We conclude that cohesin is not essential for microtubule binding by kinetochores or for chromosome biorientation on the mitotic spindle. Our data are consistent with results recently obtained in budding yeast, where inhibition of topo II was able to suppress partially defects in sister kinetochore biorientation following depletion of Scc1 (T. Tanaka, personal communication).
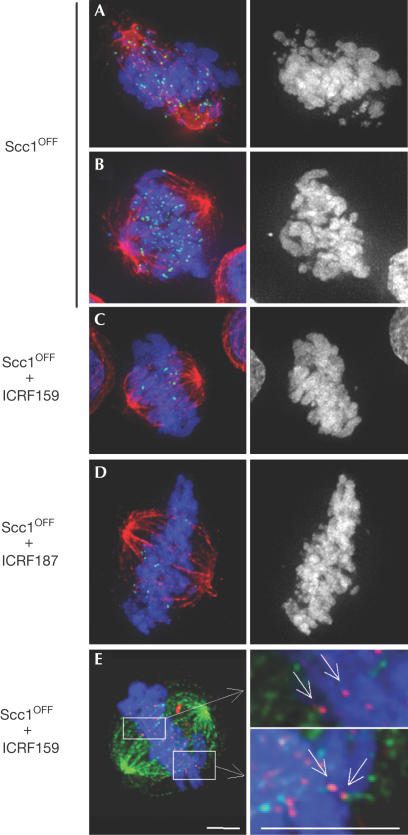
Figure 3.
Decrease in kinetochore-associated BubR1 in Scc1-deficient cells following topo II inhibitor treatment. (A, B) All Scc1OFF cells in early mitosis have levels of BubR1 accumulation at kinetochores characteristic of prometaphase for both aligned and nonaligned chromosomes. (C, D) Treatment of Scc1OFF cells with the topoisomerase inhibitors ICRF-159 (C) and ICRF-187 (D) promotes the alignment of chromosomes at a metaphase plate and results in a downregulation of BubR1 levels at the kinetochores. Synchronized, Scc1-deficient cells were treated as for Fig 2. Staining is shown with antibodies to α-tubulin (red) and BubR1 (green), along with DAPI (grey in the right panels). (E) Treatment of Scc1OFF cells with ICRF-159 promotes the bipolar attachment of sister kinetochores to opposite spindle poles. Synchronized, Scc1-deficient cells were treated as for Fig 2. Staining is shown with antibodies to α-tubulin (green) and CENP-C (red), along with DAPI (blue). Scale bars, 5 μm.
Topo II inhibition restores tension at kinetochores
The accumulation of Scc1OFF cells in prometaphase is probably due to activation of the spindle assembly checkpoint, as kinetochores of all chromosomes in these cells exhibit high levels of BubR1 (Fig 3A,B). This accumulation of BubR1 at kinetochores could reflect either defects in kinetochore structure or an inability to shut off the spindle assembly checkpoint when sister kinetochores are linked by chromatin lacking cohesin. To examine whether the improved metaphase alignment induced by inhibition of topo II was sufficient to silence this checkpoint signal, we stained ICRF-159- or ICRF-187-treated Scc1OFF cells for BubR1. Both treatments greatly reduced the levels of kinetochore-associated BubR1 (Fig 3C,D).
Discussion
Scc1 is dispensable for mitotic chromosome structure
Our findings confirm and extend previous observations (Losada et al, 1998; Sonoda et al, 2001) by showing that Scc1 is not required for mitotic chromatin condensation or for the structural integrity (as defined by the assay shown in Fig 1) of vertebrate mitotic chromosomes. These results are in contrast to the close interdependence between cohesin and chromosome condensation seen in budding yeast (Guacci et al, 1997; Lavoie et al, 2002). The key condensin component ScII/SMC2 localizes to the chromosome scaffold in Scc1OFF cells (Sonoda et al, 2001). Since condensin loss in vertebrate mitotic chromosomes significantly impairs the structural integrity of mitotic chromosomes (Hudson et al, 2003), these observations suggest that condensin in Scc1-depleted cells is functional. Therefore, our findings provide support for a model in which the condensation of vertebrate mitotic chromosomes is largely independent of cohesin.
Cohesin not absolutely required for kinetochore function
The present results also resolve an ongoing controversy about the role of cohesin in kinetochore assembly and function. Previous studies had suggested that cohesin might be required for the assembly of fully functional kinetochores (Tanaka et al, 2000; Sonoda et al, 2001; Hoque & Ishikawa, 2002; Toyoda et al, 2002). Here, we have shown that if topo II is inhibited using the highly specific inhibitors ICRF-159 or ICRF-187, chromosomes deficient in cohesin can (i) bind microtubules from opposite spindle poles, (ii) bind elevated levels of BubR1 when not properly aligned at the spindle midzone, (iii) congress to a metaphase plate and (iv) downregulate BubR1 levels at the kinetochore (and presumably therefore silence the metaphase spindle assembly checkpoint) once properly bioriented. Thus, by these four criteria, fully functional kinetochores can be assembled in the effective absence of Scc1.
Speculation: role of topo II in sister chromatid cohesion?
Our results raise the possibility that topo II could have a role in regulating levels of tension between sister kinetochores during normal prometaphase and metaphase. The enzyme is concentrated in the centromeric heterochromatin at the appropriate time (Rattner et al, 1996; Christensen et al, 2002; Tavormina et al, 2002) and can be phosphorylated by Aurora B kinase (Morrison et al, 2002). The role of topo II at centromeres remains open; however, the present results showing that topo II activity can apparently influence the activation of the spindle assembly checkpoint raise the possibility that regulation of topo II activity might modulate the development of spindle tension across sister kinetochores. Indeed, sumoylation of topo II in budding yeast appears to have a key role in regulating centromeric cohesion (Bachant et al, 2002). Furthermore, abnormal regulation of topo II activity may perturb anaphase onset. Regardless of the role of topo II, our data show that the role of cohesin in achieving chromosome biorientation is indeed to establish cohesion (Nasmyth, 2002) and not to perform some secondary function at the kinetochore.
Methods
Cell culture and microscopy
DT40 cells were cultured and synchronized as described (Sonoda et al, 2001). ICRF drugs were from Adria SP (Albuquerque, NM) and were dissolved in 0.2 M HCl. Working concentrations were 10 μg/ml. Nocodazole was used at 0.25 μg/ml and colcemid at 0.1 μg/ml. For immunostaining, whole cells were fixed with 4% formaldehyde and permeabilized with 0.15% Triton X-100 (both in cytoskeleton buffer: 137 mM NaCl, 5 mM KCl, 1.1 mM Na2HPO4, 0.4 mM KH2PO4, 2 mM MgCl2, 2 mM EGTA, 5 mM PIPES, 5.5 mM glucose). DAPI was used at 1 μg/ml. Analysis of chromosome structure by treatment of cells with chromosome compacting/unfolding buffers was performed as described (Hudson et al, 2003). The micrographs shown consist of single-plane projections of deconvolved, three-dimensional data sets taken with an Olympus IX-70 microscope driven by the SoftWorx system (Applied Precision, Issaquah, WA).
Antibodies
Polyclonal anti-CENP-C antiserum WCE30B was raised in rabbit against an N-terminal peptide CMAERLDHLKKYYRAR, synthesized at the Centre Hospitalier de l'Université Laval (Québec, Canada). After purification over protein A-agarose, serum was used at 1:500 dilution. Anti-BubR1 antibody (Nishihashi et al, 2002) was raised against amino acids 650–765 of the chicken protein N-terminally fused to glutathione S-transferase and was used at 1:500 dilution. Monoclonal anti-α-tubulin B512 from Sigma was used for immunofluorescence at 1:2,000 dilution. Fluorescently labelled secondary antibodies from Jackson Immunoresearch Laboratories (West Grove, PA) were used at 1:200 dilution.
Acknowledgments
Financial support to E.S. and S.T. was provided in part by CREST, JST and Center of Excellence (COE) grants from the Ministry of Education, Science and Culture of Japan. C.M. received an EMBO long-term postdoctoral fellowship and is now supported by a Science Foundation Ireland Investigator award. H.D. received a Wellcome Trust Prize Studentship. Work in W.C.E's laboratory is supported by the Wellcome Trust, of which he is a Principal Research Fellow.
References
- Andoh T, Ishida R (1998) Catalytic inhibitors of DNA topoisomerase II. Biochim Biophys Acta 1400: 155–171 [DOI] [PubMed] [Google Scholar]
- Bachant J, Alcasabas A, Blat Y, Kleckner N, Elledge SJ (2002) The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol Cell 9: 1169–1182 [DOI] [PubMed] [Google Scholar]
- Blat Y, Kleckner N (1999) Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell 98: 249–259 [DOI] [PubMed] [Google Scholar]
- Christensen MO, Larsen MK, Barthelmes HU, Hock R, Andersen CL, Kjeldsen E, Knudsen BR, Westergaard O, Boege F, Mielke C (2002) Dynamics of human DNA topoisomerases IIα and IIβ in living cells. J Cell Biol 157: 31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A (1967) Chromosome structure. Theor Biophys 1: 305–375 [Google Scholar]
- DiNardo S, Voelkl K, Sternglanz R (1984) DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci USA 81: 2616–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC, Laemmli UK (1983) Architecture of metaphase chromosomes and chromosome scaffolds. J Cell Biol 96: 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky GJ (1994) Cell cycle progression and chromosome segregation in mammalian cells cultured in the presence of the topoisomerase II inhibitors ICRF-187 [(+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane; ADR-529] and ICRF-159 (Razoxane). Cancer Res 54: 1042–1048 [PubMed] [Google Scholar]
- Gregson HC, Van Hooser AA, Ball AR Jr, Brinkley BR, Yokomori K (2002) Localization of human SMC1 protein at kinetochores. Chromosome Res 10: 267–277 [DOI] [PubMed] [Google Scholar]
- Guacci V, Koshland D, Strunnikov A (1997) A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell 91: 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Waizenegger IC, Peters JM (2001) Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293: 1320–1323 [DOI] [PubMed] [Google Scholar]
- Hirano T (2002) The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion, and repair. Genes Dev 16: 399–414 [DOI] [PubMed] [Google Scholar]
- Holm C, Stearns T, Botstein D (1989) DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol Cell Biol 9: 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque MT, Ishikawa F (2002) Cohesin defects lead to premature sister chromatid separation, kinetochore dysfunction, and spindle-assembly checkpoint activation. J Biol Chem 277: 42306–42314 [DOI] [PubMed] [Google Scholar]
- Hudson DF, Vagnarelli P, Gassmann R, Earnshaw WC (2003) Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Dev Cell 5: 323–336 [DOI] [PubMed] [Google Scholar]
- Ishida R, Sato M, Narita T, Utsumi KR, Nishimoto T, Morita T, Nagata H, Andoh T (1994) Inhibition of DNA topoisomerase II by ICRF-193 induces polyploidization by uncoupling chromosome dynamics from other cell cycle events. J Cell Biol 126: 1341–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie BD, Hogan E, Koshland D (2002) In vivo dissection of the chromosome condensation machinery: reversibility of condensation distinguishes contributions of condensin and cohesin. J Cell Biol 156: 805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Hirano M, Hirano T (1998) Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev 12: 1986–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis C, Ciosk R, Nasmyth K (1997) Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91: 35–45 [DOI] [PubMed] [Google Scholar]
- Morrison C, Henzing AJ, Jensen ON, Osheroff N, Dodson H, Kandels-Lewis SE, Adams RR, Earnshaw WC (2002) Proteomic analysis of human metaphase chromosomes reveals topoisomerase II α as an Aurora B substrate. Nucleic Acids Res 30: 5318–5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K (2002) Segregating sister genomes: the molecular biology of chromosome separation. Science 297: 559–565 [DOI] [PubMed] [Google Scholar]
- Nishihashi A, Haraguchi T, Hiraoka Y, Ikemura T, Regnier V, Dodson H, Earnshaw WC, Fukagawa T (2002) CENP-I is essential for centromere function in vertebrate cells. Dev Cell 2: 463–476 [DOI] [PubMed] [Google Scholar]
- Rattner JB, Hendzel MJ, Furbee CS, Muller MT, Bazett-Jones DP (1996) Topoisomerase-II-α is associated with the mammalian centromere in a cell-cycle and species-specific manner and is required for proper centromere/kinetochore structure. J Cell Biol 134: 1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E et al. (2001) Scc1/Rad21/Mcd1 is required for sister chromatid cohesion and kinetochore function in vertebrate cells. Dev Cell 1: 759–770 [DOI] [PubMed] [Google Scholar]
- Sumner AT (1995) Inhibitors of topoisomerase II delay progress through mitosis and induce a doubling of the DNA content in CHO cells. Exp Cell Res 217: 440–447 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Cosma MP, Wirth K, Nasmyth K (1999) Identification of cohesin association sites at centromeres and along chromosome arms. Cell 98: 847–858 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Fuchs J, Loidl J, Nasmyth K (2000) Cohesin ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation. Nat Cell Biol 2: 492–499 [DOI] [PubMed] [Google Scholar]
- Tavormina PA, Come MG, Hudson JR, Mo YY, Beck WT, Gorbsky GJ (2002) Rapid exchange of mammalian topoisomerase II α at kinetochores and chromosome arms in mitosis. J Cell Biol 158: 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda Y, Furuya K, Goshima G, Nagao K, Takahashi K, Yanagida M (2002) Requirement of chromatid cohesion proteins rad21/scc1 and mis4/scc2 for normal spindle–kinetochore interaction in fission yeast. Curr Biol 12: 347–358 [DOI] [PubMed] [Google Scholar]
- Uemura T, Yanagida M (1986) Mitotic spindle pulls but fails to separate chromosomes in type II DNA topoisomerase mutants: uncoordinated mitosis. EMBO J 5: 1003–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F, Lottspeich F, Nasmyth K (1999) Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400: 37–42 [DOI] [PubMed] [Google Scholar]
- Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K (2000) Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103: 375–386 [DOI] [PubMed] [Google Scholar]
- Vass S, Cotterill S, Valdeolmillos AM, Barbero JL, Lin E, Warren WD, Heck MM (2003) Depletion of drad21/scc1 in Drosophila cells leads to instability of the cohesin complex and disruption of mitotic progression. Curr Biol 13: 208–218 [DOI] [PubMed] [Google Scholar]
- Waizenegger IC, Hauf S, Meinke A, Peters JM (2000) Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103: 399–410 [DOI] [PubMed] [Google Scholar]
- Warren WD et al. (2000) The Drosophila RAD21 cohesin persists at the centromere region in mitosis. Curr Biol 10: 1463–1466 [DOI] [PubMed] [Google Scholar]