Summary
Meeting on Signal Transduction Determining the Fate of Stem Cells
Introduction
Many scientific meetings have been organized to present research findings on stem-cell sources, their pluripotent nature and potential therapeutic applications for treating disease. The theme of this meeting was the signalling pathways that control stem-cell maintenance and differentiation. In his keynote address, A. Spiegel (Bethesda, MD, USA) emphasized the high therapeutic potential of stem cells, and also the need to understand more about their unique cell and molecular biology to harness their therapeutic application. Although embryonic, haematopoietic and hepatic stem cells from humans are the obvious therapeutics, stem-cell research in various organisms from flies to humans was presented at this meeting.

The meeting “Signal Transduction Determining the Fate of Stem Cells” was held at Montana State University in Bozeman, Montana, between August 9 and 12, 2003. The meeting was sponsored by the American Society for Cell Biology and was organized by G.L. Johnson and N. Terada.
Signalling of stem-cell proliferation and survival
A common feature of stem cells, regardless of origin and type, is their ability to undergo self-renewal. Cultured stem cells, especially embryonic stem (ES) cells, exhibit a high rate of proliferation and a short cell cycle time (10–12 hours). Studies by S. Dalton (Athens, GA, USA) showed that ES cells have a unique cell cycle that lacks complete G1 and G2 gap phases (Stead et al, 2002). The activity of cyclin-dependent kinases (Cdks) is constitutively high relative to their activity in somatic cells such as mouse embryo fibroblasts. Genes that are the target of the E2F transcription factor are also constitutively active, consistent with the negligible activity of the retinoblastoma (Rb) protein pathway in these cells. Analysis of Cdk activity in mouse ES cells reveals a novel, constitutively active Cdk6–cyclin D3 complex that is rapidly downregulated following ES cell differentiation. Importantly, this Cdk–cyclin complex is insensitive to the defined Cdk inhibitors such as p16. Taken together, these results suggest that rapid cell division in ES cells is driven by the high activity of novel Cdk–cyclin complexes. Primordial germ cells (PGCs) are multipotent precursors of the gametes of the adult animal. They are also the cell of origin for testicular teratomas. Culturing PGCs with a cocktail of growth factors including fibroblast growth factor 2 (FGF2), leukaemia inhibitory factor (LIF) and the c-Kit ligand gives rise to pluripotent embryonic germ (EG) cells. P. Donovan (Philadelphia, PA, USA) used retroviral-mediated gene transfer to demonstrate an important role for the Akt kinase in PGC proliferation and survival (De Miguel et al, 2002). Using a genetic approach with mice bearing a PGC-specific deletion of the phosphatase and tensin homologue (PTEN) gene, T. Nakano (Osaka, Japan) showed that PTEN-null PGCs exhibited an increased ability to proliferate and enhanced formation of EG cell colonies. Thus, components of the phosphatidylinositol-3-OH-kinase (PI(3)K) pathway, including a key effector pathway (Akt) and a negative regulator of the pathway (PTEN), are important for controlling the survival and proliferation of EG cells in a similar way to their role in non-stem-cell systems. The in vivo microenvironment that controls the self-renewal and maintenance of stem cells is termed the 'stem-cell niche'. It has been difficult to identify stem-cell niches for tissue stem cells in mammalian systems, but they have been elegantly modelled in the Drosophila ovary and testis by H. Lin (Durham, NC, USA; Lin, 2002). Within the Drosophila ovary, specific support cells, such as CAP cells and inner sheath (IS) cells, provide key instructional cues for the maintenance of germline stem cells and somatic stem cells (Fig 1). Studies so far have highlighted the requirement for E-cadherin and β-catenin-containing adherens junctions between the support cells and the stem cells for the maintenance of the latter. Lin presented research on the Piwi family of proteins, which have an evolutionarily conserved role in stem-cell maintenance from plants to humans. The piwi gene encodes a highly basic protein the precise biochemical function of which remains ill-defined. The mouse homologue, miwi, is a protein that is associated with the endoplasmic reticulum and has RNA-binding activity. It may be involved in RNA silencing and translational regulation of specific mRNAs.
Figure 1.
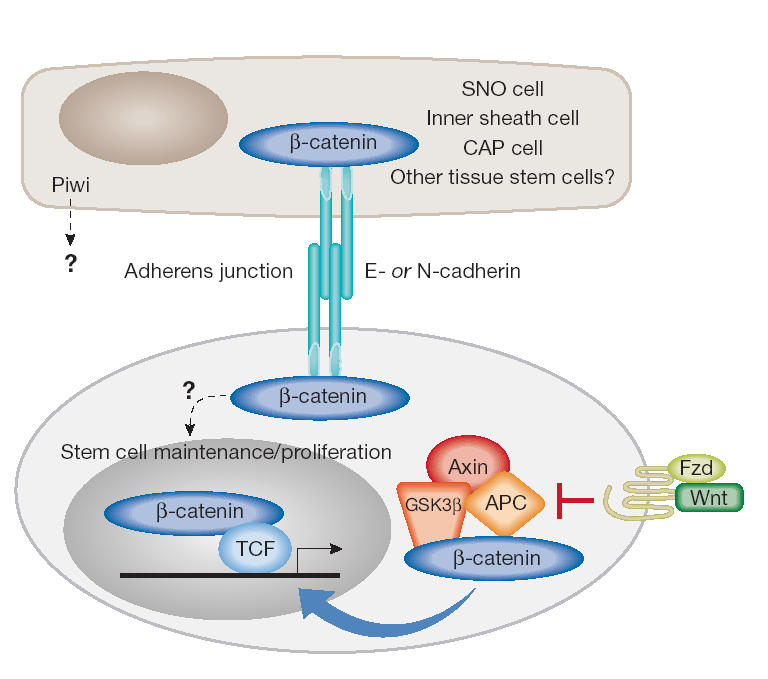
Putative role of β-catenin in stem-cell renewal and maintenance through cadherin-containing adherens junctions and Wnt signalling. Support cells such as CAP cells, inner sheath cells or spindle-shaped N-cadherin+CD45− osteoblastic (SNO) cells form adherens junctions with germline stem cells, somatic stem cells or haematopoietic stem cells (HSCs), respectively, through E-cadherin or N-cadherin and β-catenin (Lin, 2002). In addition, β-catenin can be stabilized as a result of Wnt signalling through the frizzled (Fzd) receptors leading to the inhibition of the APC–axin–GSK3-β complex, allowing transcriptional activation of target genes by T-cell factor (TCF)/β-catenin complexes.
Haematopoietic stem cells (HSCs) are multipotential, self-renewing stem cells that can form all blood cell types (Kondo et al, 2003). The precise in vivo regulatory environment within the bone marrow and the cues that control HSC proliferation and renewal have remained poorly defined. Using mice with a conditional disruption of the bone morphogenetic protein (BMP) receptor type IA, L. Li (Kansas City, MO, USA) showed that these animals had increased numbers of HSCs. This observation was not due to increased HSC self-renewal or an inhibition of differentiation, but rather to change in the microenvironment or niche size (Zhang et al, 2003). Further experiments revealed that the HSCs are found attached to spindle-shaped N-cadherin+CD45− osteoblastic (SNO) cells. Interestingly, the junction between the HSC and the SNO cells contained N-cadherin and β-catenin (Fig 1). Thus, SNO cells lining the bone surface function as a key compartment of the microenvironment that supports HSCs. In another presentation about HSC self-renewal, I. Weissman (Stanford, CA, USA) showed that the Wnt pathway has an important role in HSC renewal and proliferation. Overexpression of activated β-catenin, a key mediator of Wnt signalling, expands HSCs in long-term in vitro culture (Reya et al, 2003). In addition, the lymphoid enhancer factor 1 and T-cell factor (LEF1/TCF) mediate Wnt-inducible transcription and a transfected LEF1/TCF-dependent reporter is activated in HSCs that are present in their normal niche. Finally, transfection of molecular inhibitors of the Wnt pathway reduced HSC growth in vitro. Taken together, these two talks suggest that β-catenin function is regulated through an N-cadherin-dependent, SNO cell-specific interaction as well as through secreted Wnt proteins that regulate HSC renewal within the niche. The requirement for adherens junctions comprised of β-catenin and cadherin proteins in the fly germline stem cell and HSC niches is intriguing. The signalling pathways that are engaged through adherens junctions have been actively investigated (Juliano, 2002), but not yet applied to these stem-cell systems. The increased molecular understanding of the niches for the fly germline stem cells and mammalian HSCs may provide a template for exploring the nature of the niches for neural, hepatic and epithelial tissue stem-cell systems.
Regulation of cellular plasticity and pluripotency
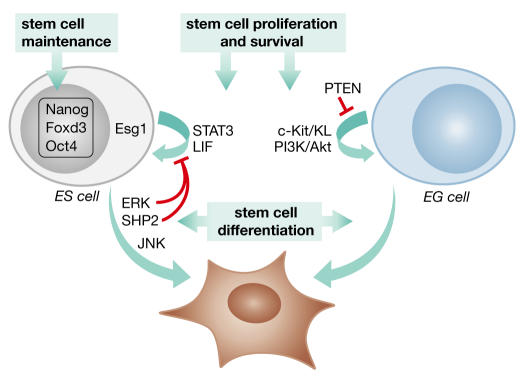
The pluripotency of stem cells, both embryonic and somatic, renders them highly suited for producing diverse differentiated cell types. As a remarkable example of this property in cultured ES cells, H. Schöler (Philadelphia, PA, USA) presented findings that mouse ES cells can develop into oocytes that enter meiosis, recruit adjacent cells to form follicle-like structures, and subsequently develop into blastocysts (Hubner et al, 2003). Developmental potential is ultimately dictated by gene expression patterns that are regulated by specific transcription factors, but also by epigenetic processes including DNA methylation and chromatin acetylation. A. Müller (Würzburg, Germany) showed that treating neural stem cells with inhibitors of DNA methylation or histone deacetylases increases their haematopoietic potential, supporting the role of epigenetic processes in the regulation of cell plasticity. As discussed by I. Wilmut (Edinburgh, UK), cloning by nuclear transfer from adult somatic cells is a striking demonstration of developmental plasticity, which is the ability of a cell to switch from one committed lineage to another. His presentation highlighted the achievements and present limitations of cloning by nuclear transfer. Significant advances have occurred but there has also been a high failure rate associated with frequent and severe defects in cloned animals. Wilmut suggested that the failings probably reflect epigenetic phenomena (Wilmut & Paterson, 2003). Identifying methods to enhance the ability of the oocyte cytoplasm to appropriately remodel the chromatin of the transferred nucleus is a high priority.The identification of the factors and signals that maintain pluripotency, a property of most stem cells, is a focus of many laboratories. LIF signalling through the signal transducer and activator of transcription 3 (Stat3) and the appropriate expression of the transcription factor Oct4 is well established for the maintenance of the pluripotent, undifferentiated state of mouse ES cells (Smith, 2001). The meeting highlighted the role of two recently defined transcription factors, Nanog, a homeobox transcription factor, and Foxd3, a winged-helix transcription factor of the forkhead family of transcription factors, in ES cell function (Fig 2). P. Labosky's (Philadelphia, PA, USA) presentation revealed the importance of Foxd3 for the maintenance of embryonic cells of the early mouse embryo (Hanna et al, 2002). Foxd3-null embryos die soon after implantation with a marked loss of epiblast cells, which are the cells within the inner cell mass (ICM) of the blastocyst that will form the three somatic germ layers. Significantly, Foxd3−/− ES cells could not be derived from blastocysts or by double-targeting approaches in ES cells, indicating the crucial requirement of Foxd3 for ES-cell maintenance. In a search for additional factors that promote pluripotency, S. Yamanaka (Nara, Japan) identified the homeoprotein, Nanog, which can maintain ES-cell self-renewal independently of LIF and Stat3 (Mitsui et al, 2003). Moreover, ICMs from Nanog-deficient blastocysts produced only parietal endoderm and Nanog−/− ES cells lost pluripotency and differentiated into extraembryonic endoderm. Thus, Nanog is another key transcription factor that is required for pluripotency. T. Tanaka (Baltimore, MD, USA) described another ES-cell-specific protein, Esg1, that is expressed in the ICM and the trophectoderm of the early embryo (Tanaka et al, 2002). The function of Esg1 is presently ill-defined, although it has a KH domain, a feature of many RNA-binding proteins. Reduction of Esg1 expression in blastocysts following siRNA injection into fertilized oocytes decreased the number of cells in the ICM of mouse blastocysts. Identifying the genes that are dependent on Oct4, Foxd3 and Nanog for their expression and dissecting Esg1 function at the molecular level will provide additional insight into pluripotency.
Figure 2.
Signalling pathways and transcription factors involved in the maintenance, proliferation, survival and differentiation of embryonic stem (ES) cells and embryonic germ (EG) cells. LIF,leukaemia inhibitory factor; PTEN, phosphatase and tensin homologue; PI(3)K, phosphatidylinositol-3-OH-kinase; Stat3, signal transducer and activator of transcription 3.
Signals regulating stem-cell differentiation
The identification of dominant signal pathways that regulate stem-cell differentiation will facilitate the generation of distinct cell types in vitro. Three examples in which loss of specific signalling enzymes in ES cells leads to alterations in the potential for differentiation were presented. The first concerned SHP2, an SH2-domain-containing protein tyrosine phosphatase that, paradoxically, functions to promote signalling from receptor tyrosine kinases to the Ras/Raf/MEK/ERK pathway. G.-S. Feng (La Jolla, CA, USA) presented findings that ES cells bearing homozygous SHP2 mutations exhibited decreased potential for haematopoiesis (Chan et al, 2003). This defect is associated with decreased differentiation to mesoderm lineages and to hemangioblasts. In another example, L. Heasley (Denver, CO, USA) presented findings with mouse ES cells in which specific cJun amino-terminal kinases (JNKs) were disrupted. Although the jnk−/− ES cells and wild-type ES cells exhibited equivalent abilities to form embryoid bodies in culture, microarray analysis revealed a decreased expression of a panel of marker genes for the extraembryonic endoderm lineages in jnk2−/− embryoid bodies. J. Chen (Urbana, IL, USA) discussed the role of the mammalian target of rapamycin (mTOR) pathway in skeletal muscle differentiation modelled in cultured C2C12 cells (Erbay & Chen, 2001). Interestingly, rapamycin-resistant mTOR rescues rapamycin-inhibited myogenesis in a novel kinase-independent manner. Target genes involved in mTOR-regulated C2C12 differentiation are likely to include insulin-like growth factors. It will be interesting to confirm these studies in muscle satellite cells, which are a self-renewing pool of stem cells that give rise to myogenic precursor cells.
A. Miyajima (Tokyo, Japan) presented studies in which fetal hepatocytes were differentiated with oncostatin M (OSM), a cytokine of the interleukin 6 family that stimulates expression of tyrosine aminotransferase and glucose 6-phosphatase and that represses D-type cyclins through a Stat3 signalling pathway (Matsui et al, 2002). By contrast, OSM induces, in a K-Ras-dependent manner, the formation of E-cadherin-based adherens junctions, which are essential structures for the organogenesis of epithelial tissues such as the liver. Apart from hepatic differentiation, the OSM signalling pathway is also likely to be a key contributor to liver regeneration.
A novel example of the determination of the fate of a cell through a specific transcription factor was presented by L. Sussel (Denver, CO, USA). The homeobox transcription factor Nkx2.2 is required to direct pancreatic progenitor cells to become insulin-producing beta cells as well as a subset of glucagon-producing alpha cells. As a result, the pancreatic islets of Nkx2.2-null mice contain a large number of cells that fail to synthesize any of the four known islet hormones. Gene profiling studies of mutant islets revealed marked induction of the ghrelin gene that encodes an appetite-stimulating gut peptide. A small number of ghrelin-positive cells are found in normal islets, but loss of Nkx2.2 results in the majority of the islets cells becoming ghrelin-positive. These results reveal that Nkx2.2 is required for specification of pancreatic progenitors to beta cells and suggests that a lack of Nkx2.2 allows differentiation to ghrelin cells.
Interface between stem-cell research and cancer research
The interface between stem-cell research and cancer research was much discussed at this meeting. If stem cells are defined as clonigenic cells that are capable of self-renewal as well as able to differentiate into mature cells appropriate for the specific tissue in which they reside, then cancer may represent the consequence of poorly regulated self-renewal of mutated stem cells with a limited potential for differentiation. In fact, cancer stem cells have long been suspected to be a source of leukaemias. More recently, it has been suggested that they may also be a source of cancer cells in solid tumours, including breast and brain cancers (Marx, 2003; Reya et al, 2001). I. Weissman (Stanford, CA, USA) presented studies revealing that leukaemias are generated by a limited number of leukaemia stem cells (LSCs) of heterogeneous origin (Passegue et al, 2003). LSCs can be either HSCs that have accumulated mutations to become leukemic or they may derive from more restricted progenitors that have reacquired self-renewal through mutation. C. Kim (Cambridge, MA, USA) presented findings that bronchiolar epithelial cells positive for markers of both bronchial Clara cells and alveolar type II cells might represent a putative lung stem cell that is the target for murine adenocarcinoma. Compared to normal murine lung, increased numbers of double-positive cells are observed in early adenomas and adenocarcinomas. The finding that double-positive cells can be sorted with Sca1, a stem-cell marker, will allow the mutation status of these cells to be defined relative to the cells that comprise the adenoma and adenocarcinoma. However, as a cautionary tale when considering cancer stem cells, M. Applebury (Boston, MA, USA) described the derivation of self-renewing human neural cell lines from retinoblastomas that exhibit some of the properties of multipotent retinal cell precursors. Although it is tempting to speculate that these cells represent retinoblastoma stem cells, the retinal cell precursors do not carry mutations in the Rb gene, indicating that the retinal precursor cells may simply persist in close association with the tumour cells that bear mutant Rb.
Many of the signalling pathways required for normal stem-cell function are subverted in cancer cells, thus stem-cell research and cancer research share common interests. For instance, constitutive Cdk/cyclinD activity leading to the inactivation of the Rb pathway, a common occurrence in various cancer cells, is observed in ES cells (S. Dalton). H. Lin noted that overexpression of the human homologues of Drosophila piwi genes, required for normal germline stem-cell function, is associated with human testicular tumours of germ cell origin (Qiao et al, 2002). Also, specific deletion in mice of the Bmp receptor type IA in hair follicles yielded an increased number of stem cells in this tissue and eventually led to tumours of the hair follicle (trichofolliculoma) (L. Li). Finally, mice in which PTEN was deleted specifically in primordial germ cells developed testicular teratomas (Kimura et al, 2003). This is consistent with the enhanced proliferation of PTEN-null primordial germ cells (T. Nakano).
Perspectives
Researchers with a wide variety of interests in stem-cell biology, developmental biology and basic signal transduction gathered for this meeting. If stem cells are truly destined to become therapeutic agents for the treatment of disease, we will continue to require a wide range of different specialists to address the key questions that remain. For example, can the emerging molecular and cellular description of niches for germline stem cells and HSCs enhance the characterization of the specific niches for other tissue stem cells? If so, can tissue stem-cell niches eventually be manipulated in vivo to mobilize specific stem-cell populations? In many ways, the ES cell seems a more tractable system for the in vitro production of specific differentiated cell types. However, are the signalling pathways and cues that induce specific cell types in mouse ES cells similar in human ES cells? Finally, if various cancers do arise from rare cancer stem cells, can they be selectively targeted with therapeutics without destroying the normal tissue stem cells that are required for the maintenance of healthy tissues? Addressing these questions will certainly depend, to a large degree, on continued exploration of the basic properties of stem cells.


References
- Chan RJ, Johnson SA, Li Y, Yoder MC, Feng GS ( 2003) A definitive role of Shp-2 tyrosine phosphatase in mediating embryonic stem cell differentiation and hematopoiesis. Blood 102: 2074–2080 [DOI] [PubMed] [Google Scholar]
- De Miguel MP, Cheng L, Holland EC, Federspiel MJ, Donovan PJ ( 2002) Dissection of the c-Kit signalling pathway in mouse primordial germ cells by retroviral-mediated gene transfer. Proc Natl Acad Sci USA 99: 10458–10463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbay E, Chen J ( 2001) The mammalian target of rapamycin regulates C2C12 myogenesis via a kinase-independent mechanism. J Biol Chem 276: 36079–36082 [DOI] [PubMed] [Google Scholar]
- Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA ( 2002) Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev 16: 2650–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF 3rd, Boiani M, Scholer HR ( 2003) Derivation of oocytes from mouse embryonic stem cells. Science 300: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Juliano RL ( 2002) Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol 42: 283–323 [DOI] [PubMed] [Google Scholar]
- Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T ( 2003) Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development 130: 1691–1700 [DOI] [PubMed] [Google Scholar]
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL ( 2003) Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol 21: 759–806 [DOI] [PubMed] [Google Scholar]
- Lin H ( 2002) The stem-cell niche theory: lessons from flies. Nat Rev Genet 3: 931–940 [DOI] [PubMed] [Google Scholar]
- Marx J ( 2003) Cancer research. Mutant stem cells may seed cancer. Science 301: 1308–1310 [DOI] [PubMed] [Google Scholar]
- Matsui T, Kinoshita T, Hirano T, Yokota T, Miyajima A ( 2002) STAT3 down-regulates the expression of cyclin D during liver development. J Biol Chem 277: 36167–36173 [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S ( 2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113: 631–642 [DOI] [PubMed] [Google Scholar]
- Passegue E, Jamieson CH, Ailles LE, Weissman IL ( 2003) Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci USA 100 (Suppl 1): 11842–11849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Zeeman AM, Deng W, Looijenga LH, Lin H ( 2002) Molecular characterization of hiwi, a human member of the piwi gene family whose overexpression is correlated to seminomas. Oncogene 21: 3988–3999 [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL ( 2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105–111 [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL ( 2003) A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423: 409–414 [DOI] [PubMed] [Google Scholar]
- Smith AG ( 2001) Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol 17: 435–462 [DOI] [PubMed] [Google Scholar]
- Stead E, White J, Faast R, Conn S, Goldstone S, Rathjen J, Dhingra U, Rathjen P, Walker D, Dalton S ( 2002) Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene, 21: 8320–8333 [DOI] [PubMed] [Google Scholar]
- Tanaka TS, Kunath T, Kimber WL, Jaradat SA, Stagg CA, Usuda M, Yokota T, Niwa H, Rossant J, Ko MS ( 2002) Gene expression profiling of embryo-derived stem cells reveals candidate genes associated with pluripotency and lineage specificity. Genome Res 12: 1921–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmut I, Paterson L ( 2003) Somatic cell nuclear transfer. Oncol Res 13: 303–307 [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L ( 2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425: 836–841 [DOI] [PubMed] [Google Scholar]