Summary
The membrane bending and GTPase-binding functions of proteins from the BAR-domain family
Keywords: BAR domain, dimerization, membrane curvature sensing and induction, small GTPases, intracellular transport, endocytosis
Abstract
BAR-domains recently took centre stage in science through a report on the crystal structure of this domain in Drosophila Amphiphysin. Though only weakly conserved at the sequence level, the structure of the BAR domain shows striking similarity to the GTPase-binding domain of Arfaptin 2, an effector of Rho- and Arf- GTPases. On the basis of this sequence and structural similarity, these two proteins have been classified as belonging to the same family, the BAR-domain family, and they probably also have similar functional characteristics. Presented here are the results of a database search for the sequence of the BAR domain of Amphiphysin and Arfaptin 2. This search identified a variety of related proteins, most of which are involved in intracellular transport and especially in endocytosis. For example, the BAR-domain family includes Endophilins, GTPase-activating proteins of the Centaurinβ family and Oligophrenins, the adaptor proteins APPL1 and APPL2 that were recently shown to interact with the small GTPase Rab5, as well as members of the Sorting nexin family. On the basis of the structures of Amphiphysin and Arfaptin 2 and the cellular role of Amphiphysins in the early steps of endocytosis, the functions of the BAR domain have been defined as a dimerization motif and as sensing and inducing membrane curvature. However, data on Arfaptin 2 and now also on the Adaptor proteins APPL1 and 2 suggest that another function of the BAR domain is to bind to small GTPases.
Introduction
Endocytosis and intracellular transport involve several mechanistic steps: (1) for the internalization of cargo molecules, the membrane needs to bend to form a vesicular structure, which requires membrane curvature and a rearrangement of the cytoskeleton; (2) following its formation, the vesicle has to be pinched off the membrane; (3) the cargo has to be subsequently transported through the cell and the vesicle must fuse with the correct cellular compartment.
Members of the Amphiphysin protein family are key regulators in the early steps of endocytosis. They are involved in the formation of clathrin-coated vesicles by promoting the assembly of a protein complex at the plasma membrane and directly assist in the induction of the high curvature of the membrane at the neck of the vesicle (Lee et al, 2002; Takei et al, 1999; reviewed in Wigge & McMahon, 1998). Amphiphysins contain a characteristic domain, known as the BAR (Bin–Amphiphysin–Rvs)-domain, which is required for their in vivo function and their ability to tubulate membranes (Takei et al, 1999). Other regulators of endocytosis include small GTP-binding proteins of the Ras superfamily (small GTPases) and their various effectors, which regulate cargo uptake, intracellular transport (the Rab and Arf families) and also the rearrangement of the cytoskeleton (the Rho, Rac and Cdc42 family; Chavrier & Goud, 1999; Takai et al, 2001). Recently, the crystal structure of Amphiphysin was solved. This structure revealed a striking similarity to the GTPase-binding domain of Arfaptin 2/POR1, which is an effector of small GTPases of the Rho and Arf families and is involved in Rac- and Arf-mediated membrane ruffling (Peter et al, 2003; D'Souzaschorey et al, 1997; Tarricone et al, 2001). Even though only distantly related at the sequence level, the BAR domain of Amphiphysin and the GTPase-binding domain of Arfaptin 2 belong to the same protein family, and therefore probably have similar functional features.
Clearly, there are two functions associated with the BAR domain:(1) the sensing and/or induction of membrane curvature and (2) the binding to a small GTPase. The main questions that arise from the current data are whether and how these two functions can be combined in a single domain and whether they are connected to each other.
BAR-domain proteins and intracellular transport
Even though the BAR domain of the Amphiphysins shows rather distant homology to the Arfaptins at the sequence level, the crystal structures of these molecules display a striking similarity (Fig 1; Peter et al, 2003). Both proteins form a crescent-shaped dimer of a three-helix coiled coil. Rac binds to the centre of the V-shaped Arfaptin 2 dimer. Iterated sequence profile searches with either the BAR domain of Amphiphysin or the GTPase-interacting BAR domain of Arfaptin 2 reveal a large range of proteins, most of which are involved in intracellular transport and especially in endocytosis (Fig 2 and supplementary Fig S1 online; Peter et al, 2003). For example, apparent homologues that contain a BAR domain include the Endophilins, the islet cell autoantigen Ica69 family, the GTPase-activating proteins of the Oligophrenin and Centaurinβ family, the Rac–interacting protein Bap2α/IRSp53, as well as the adaptor proteins APPL1/Dip13α and APPL2/Dip13β, and the guanine exchange factor Tuba. In addition, members of the Sorting nexin family (Snx1, Snx2, Snx4-8, Snx9 and Snx18) were identified, all of which belong to the subclass that contains a characteristic carboxy (C)-terminal coiled-coil region (Worby & Dixon, 2002). On the basis of the sequence alignment of the BAR domain, the domain is about 200 amino acids in length, which is in accordance with the GTPase-binding interface of Arfaptin 2 and the structure of the Amphiphysin BAR domain. The domain displays a coiled-coil-like nature with a characteristic set of conserved hydrophobic, aromatic and hydrophilic amino acids (supplementary Fig S1 online). A wide variety of accessory domains can be identified within BAR-domain family members, which reflects their diverse nature and cellular functions (supplementary Fig S2 online; Peter et al, 2003).
Figure 1.
Crystal structures of Arfaptin 2 complexed with Rac and Amphiphysin. Both proteins form a crescent-shaped dimer. Rac (light grey) binds to the centre of the Arfaptin 2 dimer. One monomer of each homodimer is highlighted in red. Protein Data Bank accession numbers: Arfaptin 2:Rac1, ; Amphiphysin, .
Figure 2.
Phylogenetic tree of proteins belonging to the BAR-domain family. Six families can be distinguished: the Arfaptin family, which contains the Arfaptins, Protein kinase C-binding protein 1 (PICK1) and the islet cell antigen Ica69; the Amphiphysin family, which contains Amphiphysin I and II, as well as their paralogue BRAP1 and the RhoGEF Tuba; the third family is composed of the GTPase-activating proteins Centaurinβ 1, β 2 and β 5, Oligophrenins and the adaptor proteins APPL1 and APPL2; the Rac-binding proteins Bap2α and Bap2-like seem to form a separate family; the Sorting nexins (1, 2, 4, 5, 6, 7, 8, 9 and 18), which are the most divergent members of the BAR-domain family; and finally the Endophilin and Nadrin family, which includes Endophilin I, II and III, Endophilin B, as well as Nadrin and SH3-BP1. Those depicted in red indicate experimentally determined GTPase-binding, double circles indicate experimentally proven dimerization. Helices indicate helical fold as determined by the program 3D-PSSM (Kelley et al, 2000). When the structure of Arfaptin 2 (1I4T) was identified as the top hit, a red helix is shown. When related structures (Phospholipase Cβ (1JAD), Alpha-Spectrin (1CUN), Syntaxin 1A (1DN1) and Interferon-induced guanylate-binding protein 1 (1DG3)) were identified, a grey helix is shown. For details of database searching and accession numbers, see supplementary information online.
Dimer formation by BAR-domain-containing proteins
On the basis of structural studies of Arfaptin 2 and the Amphiphysin BAR domain, dimerization is a minimal function of BAR-domain-containing proteins. Arfaptin 2 itself forms a homodimer, which is a prerequisite for its binding to small GTPases (Tarricone et al, 2001). Amphiphysins also dimerize and it was suggested that the V-shaped dimer is able to sense and/or induce membrane bending (Peter et al, 2003). Among the proteins that contain a BAR domain, there is evidence that they can and sometimes must form homo- or heterodimers. This is the case for the Endophilin and Sorting nexin families (Ringstad et al, 2001; Worby & Dixon, 2002), as well as for the adaptors APPL1 and APPL2 (E. Yus, M. Miaczynska and M. Zerial, personal communication). Dimer formation by these protein families depends on their predicted BAR domain. The Snxs, for instance, dimerize via their characteristic C-terminal coiled-coil region, which represents their BAR domain. Heterodimers are mostly formed between closely related family members, such as between Amphiphysin I and II, APPL1 and 2, or between different members of the Snx family (such as Snx1 and 2, or Snx5 and 6). Heterodimerization, however, has also been observed for distant members of the BAR-domain family, for example Amphiphysin II was shown to heterodimerize with Snx4, an interaction that may link early steps in endocytosis to intracellular transport (Leprince et al, 2003). Therefore, heterodimer formation between distantly related members of the BAR-domain family may offer some interesting new insights into the crosstalk between different functional pathways.
The BAR domain as a sensor of membrane curvature
BAR-domain-containing proteins have been shown to bind to lipids and to bend membranes. Amphiphysins and Endophilins, the first such proteins to be discovered (Farsad et al, 2001; Takei et al, 1999), respectively form clathrin-coated vesicles and induce membrane curvature. Amphiphysins mediate the formation of clathrin-coated vesicles by assembling at the plasma membrane a protein complex that is required for disconnecting vesicles from the plasma membrane (reviewed in Wigge & McMahon, 1998; Zhang & Zelhof, 2002). Furthermore, on the basis of their ability to tubulate membranes in vitro and in vivo, Amphiphysins are thought to take part in the formation of a narrow tubular neck at the point of contact of the vesicle and the membrane (Lee et al, 2002; Takei et al, 1999). In the recent study of Peter et al (2003), in vitro membrane tubulation activity was also shown for Arfaptin 2, Centaurinβ 2, Oligophrenin and Nadrin. Initial work on Amphiphysins and Endophilins suggested that a short sequence stretch, adjacent to the amino (N)-terminus of the BAR domain is essential for lipid-binding and tubule formation by the Amphiphysins and Endophilins (Farsad et al, 2001). This sequence stretch at the N-terminal end was shown to form an amphipathic helix, thereby extending the helical backbone of the dimer at the tips. Together with the BAR domain, this sequence motif is termed N-BAR and can be found in a subgroup of the BAR-domain family, including the Amphiphysins, Endophilins and Nadrin (Peter et al, 2003). N-BAR is also detectable in APPL1 and 2, as well as in some members of the Snx family (Snx1, 2, 4, 5 and 6). Recent data now suggest that the N-BAR is only required for binding to lipids and not for the tubule forming activity of the BAR domain, even though tubule formation is more effective in its presence. Next to the N-BAR, in vitro mutagenesis studies indicate that two patches of positively charged amino acids in the second helix and the adjacent loop are essential for in vitro tubule formation (Fig 3; Peter et al, 2003). The proposed model of the BAR domain as a sensor of membrane curvature implies that the V-shaped structure of the dimer preferentially binds to curved rather than flat membranes, thereby either sensing (as a means for localization) or even bending membranes (as a means for active membrane deformation). Interestingly, the adaptor proteins APPL1 and 2 not only require the Pleckstrin Homology (PH) domain but also the adjacent BAR domain and presumably the N-BAR for proper localization to membranes (Miaczynska et al, 2004). Pursuing this train of thought, it could also be possible that the extent of membrane curvature negatively influences the association of a BAR domain with the membrane, as the curvature could increase to such a point that association of the dimer with the membrane is sterically no longer feasible.
Figure 3.
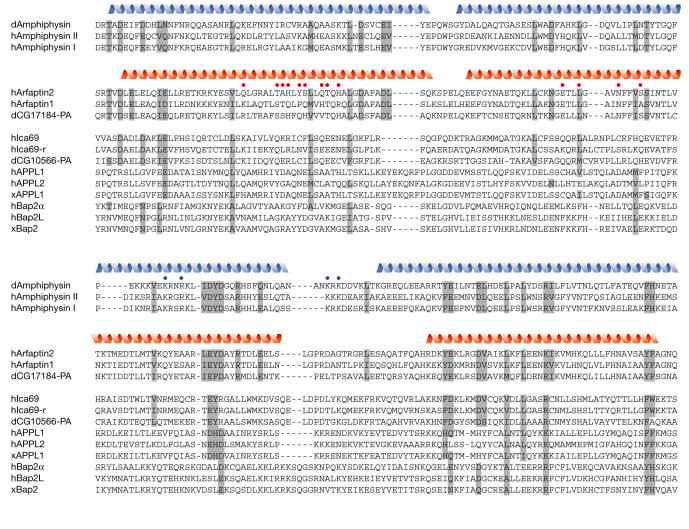
Multiple sequence alignment of the BAR domains from Amphiphysins and from the subset of BAR domain family members that were shown to bind to a small GTPase. Residues with related sequences in the Amphiphysins and the Arfaptins are highlighted in grey; secondary structure elements, as determined from the crystal structures, are indicated at the top of the Amphiphysin and Arfaptin families, respectively as blue and red helices. Red dots indicate GTPase-binding sites of Arfaptin 2, blue dots indicate residues in Amphiphysin involved in the formation of tubules in the membrane. The alignment was generated on the basis of a structural alignment between Amphiphysin and Arfaptin 2, using the COMPARER server (Sali & Blundell, 1990). The remaining sequences were aligned manually. d, Drosophila melanogaster; h, human; x, Xenopus laevis. An alignment of human orthologues of all BAR domain-containing proteins is available in the supplementary information online.
For many BAR-domain family members, lipid binding has already been demonstrated or is in accordance with their proposed cellular functions. Even though some members contain additional lipid-binding domains, for example the APPL proteins, these might not be sufficient for proper localization of the molecule in vivo. Furthermore, the proposed model of curvature sensing by the BAR domain introduces another level of selectivity of recruitment, because membrane association of a BAR domain would not only be influenced by the lipid composition of the membrane, but also by the membrane's curvature.
The BAR domain as a binding platform for small GTPases
Several proteins that have been predicted to have a BAR domain have been shown to interact with small GTPases. The BAR domain of the Arfaptins was shown to bind to the small GTPases Rac, Arf1, Arf3 and Arf6, as well as to Arl1 (Lu et al, 2001; Tarricone et al, 2001; Van Aelst et al, 1996; Williger et al, 1999). So far, at least in vivo, all known functions of the Arfaptins depend on the interaction with a small GTPase. The involvement of Arfaptin 2 in membrane ruffling, for instance, seems to be mediated by a direct interaction with small GTPases and is thought to work on a regulatory level through the remodelling of the actin cytoskeleton (D'Souzaschorey et al, 1997; Tarricone et al, 2001). The residues involved in its interaction with Rac1 are located along the first and second helix of Arfaptin 2, in a region that shows surprisingly low sequence conservation (Fig 3). Nevertheless, several other members of the BAR-domain family have also been reported to bind to small GTPases. Bap2α/IRSp53 has been shown to be the intermediate between Rac and WAVE in regulating membrane ruffling through its direct interaction with both proteins, and binding to Rac is mediated by its N-terminal BAR domain (Miki et al, 2000). Ica69 has been characterized as an Arfaptin-related molecule that interacts directly with a small GTPase of as yet unknown nature (Spitzenberger et al, 2003). Furthermore, a recent paper reports the interaction of APPL1 and APPL2 with the small GTPase Rab5 via their N-terminal BAR domain (Miaczynska et al, 2004). Even though the adjacent PH domain seems to be required for in vitro binding of the proteins to Rab5, deletion of only the BAR domain in APPL1 abolishes binding to the small GTPase in vivo, suggesting that the PH domain has a stabilizing function on the dimer. The APPL proteins associate with Rab5:GTP on distinct endosomal structures. On stimulation with epidermal growth factor (EGF), the EGF receptor is partly internalized through APPL-positive endosomes and APPL1 and 2 translocate to the nucleus. There they interact with the nucleosome remodelling and histone deacetylase complex NuRD/MeCP1 and thus regulate cell proliferation. The release from endosomes and the subsequent translocation of the APPL proteins to the nucleus takes place in a Rab5-dependent fashion, and GTP hydrolysis by Rab5 is required for releasing APPL. APPL1 and 2 therefore represent classical effectors of the small GTPase Rab5.
Even though only four members of the BAR-domain family have so far been shown to bind to small GTPases, the idea of a general binding platform for small GTPases is intriguing and, owing to the homology of the BAR domain to the Arfaptins, is clearly worth pursuing. On the basis of our current knowledge it may be that many of the BAR-domain family members bind to and are regulated by small GTPases. What makes this domain interesting is the rather broad specificity of Arfaptin 2 to the different subclasses of small GTPases of the Arf and Rho families. It has been suggested that Arfaptin 2 is a mediator between the Arf and Rac pathways, so it may be that the BAR domain takes part in crosstalk between signalling pathways that are regulated by different classes of small GTPases.
Bending and/or binding?
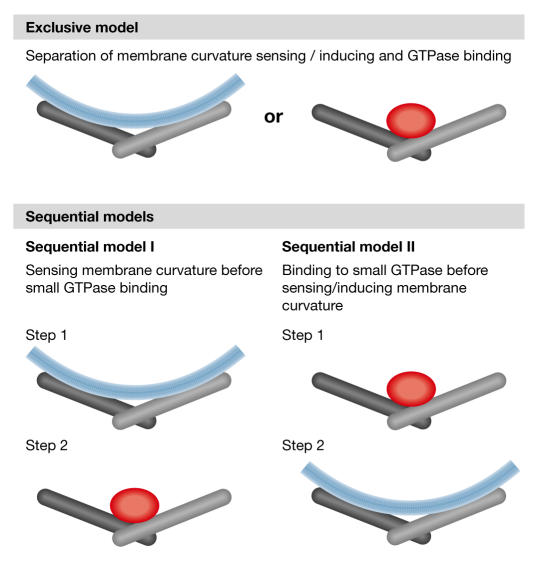
How can the two proposed functions of the BAR domain now be combined? Membrane curvature sensing and induction by the direct interaction of the V-shaped dimer with a curved membrane is not feasible when a small GTPase is bound to the dimer. In other words, the two proposed functions are mutually exclusive, at least at the same time. Several possibilities exist for a separation or an interplay between the two functions (Fig 4). In the simplest case (exclusive model), the two functions are separate: GTPase binding is a newly acquired function that only a subgroup of the family has at the expense of membrane interaction along the dimer. In this scenario, the BAR domain of a protein would either be able to sense and induce membrane curvature or else bind to small GTPases, such that if the ability to sense membrane curvature has been lost, membrane association could be mediated through binding to a small GTPase. In a second scenario (sequential model I), membrane curvature sensing and binding to a small GTPase could be associated functions that occur successively, initially through the localization of the protein to the correct compartment and then through its subsequent interaction with a small GTPase. In this respect and in the case of the APPL proteins, sensing membrane curvature by the BAR domain could be a means for proper localization prior to binding to Rab5. Even though APPL seems to require active Rab5 for proper localization to endosomal structures (Miaczynska et al, 2004) and Arfaptin 1 localization to Golgi membranes is also dependent on active Arf3 (Kanoh et al, 1997), one should keep in mind that the lipid composition and therefore curvature of the membrane is influenced by the small GTPases (Miaczynska & Zerial, 2002; Powner & Wakelam, 2002). The localization of BAR-domain proteins to a specific membrane could therefore be independent of a primary direct interaction with a small GTPase. Even though purely speculative at the moment, a BAR domain could similarly unite active membrane bending and GTPase binding as subsequent events, whereby one of the two functions could regulate the other (sequential model II). A candidate for a BAR-domain-containing protein that would unite both is obviously Arfaptin 2, because it has been shown to interact with small GTPases and is, at least in vitro, capable of tubulating membranes. However, it remains to be shown whether Arfaptin 2 has an in vivo function that is independent of a direct interaction with a small GTPase. Finally, it should be considered that the BAR domain might also influence the curvature of a membrane by directly interacting with the GTPase rather than the membrane. It could be required to stabilize the association of the active or inactive GTPase to a membranous compartment and in this way influence the lipid composition—and curvature—of the vesicle.
Figure 4.
Models for the separation or the interplay between membrane curvature sensing and bending and small GTPase-binding of BAR-domain proteins. In the exclusive model, the BAR domain is either a sensor of membrane curvature or a binding platform for a small GTPase. In the sequential model I, sensing membrane curvature takes place before interaction with a small GTPase, possibly as a means for proper localization. In the sequential model II, GTPase binding would take place before inducing membrane curvature.
Conclusion
The BAR-domain family contains a variety of members, most of which have a role in transport and endocytosis, with the BAR domain as their common factor. Whereas some of them have been shown to participate directly in vesicle formation and membrane bending, others act as effectors of small GTPases and both functions are dependent on the BAR domain. In the light of current data, the function of the BAR domain may therefore not be limited to deforming membranes or sensing membrane curvature. From experimental data and also from the sequence homology between BAR-domain members it is not clear whether GTPase binding and membrane curvature sensing and induction form a common feature of all BAR-domain family members. A lot of work still needs to be done to elucidate the functions of BAR-domain family members in sensing and inducing lipid curvature and in binding to small GTPases. Furthermore, such studies should discriminate between different models of the interplay between the two functions, particularly when they are combined in a single protein.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Material

Acknowledgments
The author thanks M. Miaczynska, P. De Camilli and M. Zerial for helpful discussions, and M. Miaczynska and P. De Camilli for critical reading of the manuscript. The author also thanks A. Henschel for assistance in database searching.
References
- Chavrier P, Goud B ( 1999) The role of ARF and Rab GTPases in membrane transport. Curr Opin Cell Biol 11: 466–475 [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Boshans RL, McDonough M, Stahl PD, Van Aelst L ( 1997) A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J 16: 5445–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P ( 2001) Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol 155: 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh H, Williger BT, Exton JH ( 1997) Arfaptin 1, a putative cytosolic target protein of ADP-ribosylation factor, is recruited to Golgi membranes. J Biol Chem 272: 5421–5429 [DOI] [PubMed] [Google Scholar]
- Kelley LA, MacCallum RM, Sternberg MJ ( 2000) Enhanced genome annotation using structural profiles in the program 3D-PSSM. J Mol Biol 299: 499–520 [DOI] [PubMed] [Google Scholar]
- Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P ( 2002) Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 297: 1193–1196 [DOI] [PubMed] [Google Scholar]
- Leprince C, Le Scolan E, Meunier B, Fraisier V, Brandon N, De Gunzburg J, Camonis J ( 2003) Sorting nexin 4 and amphiphysin 2, a new partnership between endocytosis and intracellular trafficking. J Cell Sci 116: 1937–1948 [DOI] [PubMed] [Google Scholar]
- Lu L, Horstmann H, Ng C, Hong W ( 2001) Regulation of Golgi structure and function by ARF-like protein 1 (Arl1). J Cell Sci 114: 4543–4555 [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Zerial M ( 2002) Mosaic organization of the endocytic pathway. Exp Cell Res 272: 8–14 [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M ( 2004) APPL proteins link Rab5 to signal transduction via an endosomal compartment. Cell 116: in press [DOI] [PubMed] [Google Scholar]
- Miki H, Yamaguchi H, Suetsugu S, Takenawa T ( 2000) IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature 408: 732–735 [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT ( 2003) BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303: 495–499 [DOI] [PubMed] [Google Scholar]
- Powner DJ, Wakelam MJ ( 2002) The regulation of phospholipase D by inositol phospholipids and small GTPases. FEBS Lett 531: 62–64 [DOI] [PubMed] [Google Scholar]
- Ringstad N, Nemoto Y, De Camilli P ( 2001) Differential expression of endophilin 1 and 2 dimers at central nervous system synapses. J Biol Chem 276: 40424–40430 [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL ( 1990) Definition of general topological equivalence in protein structures. A procedure involving comparison of properties and relationships through simulated annealing and dynamic programming. J Mol Biol 212: 403–428 [DOI] [PubMed] [Google Scholar]
- Spitzenberger F, Pietropaolo S, Verkade P, Habermann B, Lacas-Gervais S, Mziaut H, Pietropaolo M, Solimena M ( 2003) Islet cell autoantigen of 69 kDa is an Arfaptin-related protein associated with the Golgi complex of insulinoma INS-1 cells. J Biol Chem 278: 26166–26173 [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T ( 2001) Small GTP-binding proteins. Physiol Rev 81: 153–208 [DOI] [PubMed] [Google Scholar]
- Takei K, Slepnev VI, Haucke V, De Camilli P ( 1999) Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol 1: 33–39 [DOI] [PubMed] [Google Scholar]
- Tarricone C, Xiao B, Justin N, Walker PA, Rittinger K, Gamblin SJ, Smerdon SJ ( 2001) The structural basis of Arfaptin-mediated cross-talk between Rac and Arf signalling pathways. Nature 411: 215–219 [DOI] [PubMed] [Google Scholar]
- Van Aelst L, Joneson T, Bar-Sagi D ( 1996) Identification of a novel Rac1-interacting protein involved in membrane ruffling. EMBO J 15: 3778–3786 [PMC free article] [PubMed] [Google Scholar]
- Wigge P, McMahon HT ( 1998) The amphiphysin family of proteins and their role in endocytosis at the synapse. Trends Neurosci 21: 339–344 [DOI] [PubMed] [Google Scholar]
- Williger BT, Provost JJ, Ho WT, Milstine J, Exton JH ( 1999) Arfaptin 1 forms a complex with ADP-ribosylation factor and inhibits phospholipase D. FEBS Lett 454: 85–89 [DOI] [PubMed] [Google Scholar]
- Worby CA, Dixon JE ( 2002) Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol 3: 919–931 [DOI] [PubMed] [Google Scholar]
- Zhang B, Zelhof AC ( 2002) Amphiphysins: raising the BAR for synaptic vesicle recycling and membrane dynamics. Bin–Amphiphysin–Rvsp. Traffic 3: 452–460 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material