Abstract
The anthrax lethal factor (LF) has a major role in the development of anthrax. LF is delivered by the protective antigen (PA) inside the cell, where it exerts its metalloprotease activity on the N-terminus of MAPK-kinases. PA+LF are cytotoxic to macrophages in culture and kill the Fischer 344 rat when injected intravenously. We describe here the properties of some polyphenols contained in green tea as powerful inhibitors of LF metalloproteolytic activity, and how the main catechin of green tea, (−)epigallocatechin-3-gallate, prevents the LF-induced death of macrophages and Fischer 344 rats.
Keywords: anthrax, lethal toxin, metalloprotease, green tea extract, inhibitor
Introduction
The spores of toxigenic strains of Bacillus anthracis, once inhaled, are phagocytosed by alveolar macrophages and transported to the regional lymph nodes where the bacteria multiply; their subsequent diffusion causes massive bacteraemia and toxaemia, which develop into a rapid and often fatal disease of several vertebrate species, including humans (Dixon et al, 1999; Mock & Mignot, 2003). Penetration of spores via skin lesions or the gastrointestinal tract is less dangerous and results mainly in a localized disease.
B. anthracis secretes the anthrax lethal factor (87 kDa, LF) together with the protective antigen (83 kDa, PA) and the oedema factor (89 kDa, EF) (Lacy & Collier, 2002; Mock & Mignot, 2003). PA binds to rather ubiquitous cellsurface proteins (Lacy & Collier, 2002; Bradley & Young, 2003), and it is rapidly nicked by surface-bound proteases (Klimpel et al, 1992) to a 63 kDa form, which oligomerizes and becomes competent for binding LF or EF (Lacy & Collier, 2002; Mock & Mignot, 2003). Such binding is followed by the recruitment of PA+LF (lethal toxin, LeTx) into rafts (Abrami et al, 2003) and endocytosis within acidic compartments. LeTx then undergoes a low-pH-driven conformational change, which mediates the transfer of LF from the lumen of a late endocytic compartment to the cytoplasm (Menard et al, 1996). Here, LF displays its metalloproteolytic activity directed towards the N-terminus of the mitogen-activated protein kinase kinase (MAPKK) family of proteins (Duesbery et al, 1998; Vitale et al, 1998, 2000).
LeTx is cytotoxic to macrophages (Friedlander, 1986), whereas in lipopolysaccharide (LPS)-timulated macrophages it also induces apoptosis (Park et al, 2002). Recent evidence indicates that LeTx depresses the immune reactions within the lymph nodes by acting on macrophages and dendritic cells (Pellizzari et al, 1999; Agrawal et al, 2003). Such activity is thought to be essential for the development of systemic anthrax, as it permits an undisturbed proliferation and dissemination of the bacilli from the lymph nodes to the blood.
LF consists of four domains, one of which (the C-terminal) is a zinc-dependent endopeptidase (Pannifer et al, 2001). Using coloured peptide substrates suitable for large LF activity screenings, we tested compounds known to be inhibitors of various metalloprotease families (Tonello et al, 2002). We have now assayed some phytofactors found abundantly in green tea and known to inhibit the activity of some matrix metalloproteases: MMP-2, MMP-9, MMP-12 and MT1-MMP (Garbisa et al, 1999, 2001; Maeda-Yamamoto et al, 1999; Demeule et al, 2000; Dell'Aica et al, 2002). Two compounds that are very strong inhibitors of the biochemical reactions catalysed by LF have been identified. One of these, identified as (−)epigallocatechin-3-gallate (EGCG), has also been found to inhibit the intracellular metalloproteolytic activity of LF and its macrophage cytotoxicity. EGCG also prevents or delays the death induced by LeTx in the Fischer 344 rat.
Results and Discussion
Catechins block in vitro metalloprotease activity of LF
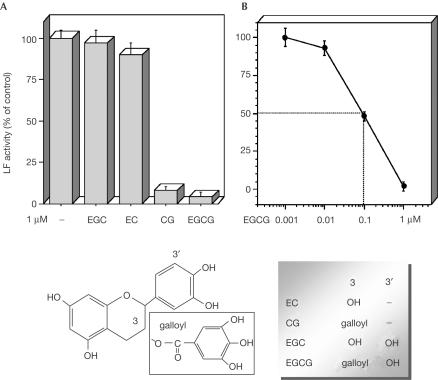
Some polyphenols present in green tea extracts (GTEs) are effective inhibitors of metalloproteases (Garbisa et al, 1999, 2001; Maeda-Yamamoto et al, 1999; Demeule et al, 2000; Dell'Aica et al, 2002), and were thus tested here on the metalloproteolytic activity of LF. Figure 1 gives the chemical formulae of the compounds used; two of them, catechin-gallate (CG) and EGCG, exert powerful inhibition of the LF proteolytic activity assayed under conditions of linearity. The curve used to determine the IC50 value of EGCG, the more powerful inhibitor, is shown in Fig 1B, from which a value of 97±17 nM was obtained. By contrast, CG and EGCG were found not to be effective inhibitors of tetanus and botulinum type A neurotoxins, which are also bacterial toxins with intracellular metalloproteolytic activity (not shown).
Figure 1.
Inhibition of LF proteolytic activity by catechins. LF (1 nM) was preincubated for 5 min at 37°C with (A) 1 μM of each catechin (EGC, epigallocatechin; EC, epicatechin; CG, catechin-gallate; EGCG, (−)epigallocatechin-3-gallate), or (B) increasing concentrations of EGCG; the mixture was then added to 5 μM p-nitroaniline-conjugated substrate. The reaction was monitored for 5 min at 405 nm. The bars are the average of the absorbance values at 5 min (triplicate experiments±s.d.). The lower part of the figure shows the chemical structure of the compounds used here.
We performed a kinetics analysis of its mechanism of inhibition and found that it does not act as a competitive inhibitor. However, we were not able to identify its precise mode of action, and such work is currently in progress. Recently, other polyphenols have been found to alter profoundly the activity of sirtuins (Howitz et al, 2003) and the tetanus neurotoxin metalloprotease (Satoh et al, 2002), and these actions were attributed to undefined binding site(s) different from the active site.
EGCG inhibits the LF-induced cleavage of MAPKK
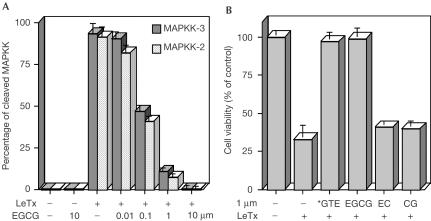
The only known natural substrate of LF is the MAPKK proteins, which are present in several different isoforms within the cell (Duesbery et al, 1998; Vitale et al, 1998, 2000). MAPKK cleavage within LFsensitive macrophage cells in culture is associated with their death (Friedlander, 1986). Inhibitors of LF proteolytic activity have to be not only strong but also permeable to the cell plasma membrane to be effective in preventing MAPKK cleavage and macrophage death. Fig 2A shows that EGCG, when preincubated with LF, is a powerful inhibitor of the intracellular cleavage of MAPKK-2 and MAPKK-3, taken here as indicative and representative of the intracellular action of LF on the MAPKK family of proteins (Pellizzari et al, 1999; Vitale et al, 2000; Chopra et al, 2003).
Figure 2.
Inhibition of cell death and cleavage of MAPKK by LF in the RAW264.7 macrophage cell line. (A) The cells were treated for 4 h with LF preincubated with EGCG, dissolved with Laemmli sample buffer, and the content of each well was loaded in SDS–PAGE gel. After electrophoresis, the proteins were blotted on nitrocellulose paper and stained with anti-MAPKK-2 and -3 antibody. The percentage of cleaved MAPKK was obtained by analysing the ECL film as described in Methods; the bars are the average of triplicate experiments±s.d. (B) The cells were plated onto 96-well plates at 2 × 104/well in DMEM with FCS and treated for 4 h with LF (400 ng/ml), which was preincubated for 15 min at 37°C with GTE, purified EGCG, EC or CG, and then mixed with PA (800 ng/ml). Cell viability was determined after 4 h. In all the experiments with cells, controls with GTE or purified catechins alone always maintained 100% viability. The bars are the average of triplicate experiments; errors shown are ±s.d. *For GTE, the value 1 μM refers to its EGCG concentration.
Under the same conditions, EGCG and decaffeinated GTE proved to be very effective in preventing the LF-induced death of RAW264.7 macrophage cells, measured after 4 h of incubation (Fig 2B), with a potency similar to that found for their inhibition of LF metalloprotease activity. By contrast, CG, a powerful inhibitor of LF enzymatic activity in vitro (Fig 1A), appears to confer less protection on the cells (Fig 2B), perhaps because of a lower membrane permeability or a higher rate of cell-induced modification. When the cells were maintained for a longer time period (24 h) in the presence of PA+LF preincubated with 1 μM EGCG, a very high protection (94% viability) was also registered (not shown).
Catechins are known anti-oxidants (Lambert & Yang, 2003), and oxygen radical intermediates have been implicated in LF-induced macrophage cell death (Hanna et al, 1994). However, the inhibitory effect of EGCG on macrophage cytotoxicity of LF is hardly attributable to its anti-oxidant activity because, were this the case, the other catechins should also have been found to be inhibitory.
EGCG protects macrophages from LF-induced death
To assay the potential of EGCG not only in the prevention but also in the therapy of anthrax, we have considered different protocols of addition of the catechins to LF and to cells. In fact, the concept is now emerging that LF has a major role in anthrax pathogenesis at the very first stages of the infection, that is, when spores and bacteria are still in the mediastinal lymph nodes and no symptoms are apparent (Pellizzari et al, 1999; Agrawal et al, 2003).
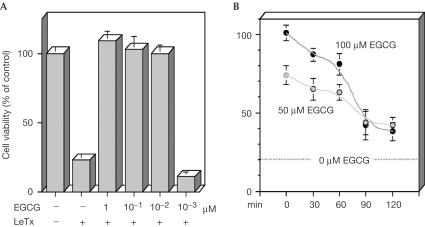
We therefore considered protocols where the cells were treated with EGCG before, at the same time as, or after addition of the toxin. Fig 3A shows the effect of decreasing concentrations of EGCG, added to the macrophages twice a day for 5 days at the given concentrations, without changing the medium; continuous addition of catechin—within the physiologically reachable serum concentration, 0.1–1 μM (Lambert & Yang, 2003)—somehow mimics the repeated daily ingestion of EGCG by green-tea drinkers. Moreover, our previous experience has shown that the effectiveness of the catechins is lowered by fetal calf serum (FCS), and progressive addition of EGCG to the culture partly overcomes this drawback.
Figure 3.
Inhibition of cytotoxicity of LF on the RAW264.7 macrophage cell line by EGCG added before or after the toxin. (A) The cells were seeded at 5 × 103 per well in DMEM with FCS in 96-well plates and treated with EGCG for 5 days (two additions/day) before the addition of LeTx (400 ng/ml LF, 800 ng/ml PA), and cell viability was determined after 4 h. The given EGCG concentrations refer to each single addition, performed without changing the medium, and therefore the final concentration is tenfold (not taking into consideration its decrease due to metabolism and oxidation). (B) The cells were treated with LeTx (as above), and EGCG was added with the delay indicated on the abscissa; the cell viability was determined 4 h after LeTx addition. Experiments were performed in triplicate and bars represent ±s.d.
Under this regimen, the cells were completely preserved from LeTx-induced lysis even with ten single very small additions of EGCG, which gave a concentration in the culture medium of 10 nM each time. The most effective inhibition was obtained by adding EGCG before LeTx (Fig 3A). When the inhibitor was added after the toxin, it was less powerful but nonetheless very effective. Fig 3B shows that the longer the delay between the addition of LeTx and that of the inhibitor, the lower the extent of inhibition of LeTx cytotoxicity achieved. This result is expected, but the kinetics of the phenomenon indicate that the cell penetration of EGCG is very rapid; the loss of effectiveness after 90 min is probably due to the time period being sufficient for LF to cleave a large part of cell MAPKKs, a figure in agreement with previously found kinetics of cleavage (Pellizzari et al, 1999; Agrawal et al, 2003). In addition, EGCG is shown to act by inhibiting LF metalloproteolytic activity and not the process of entry of LF into macrophages.
These findings qualify EGCG as the most powerful inhibitor of LF known so far, and make it a novel and powerful tool for studying the biochemical and cellular mechanisms of LF action both in vitro and in vivo.
EGCG protects Fischer 344 rats from LF-induced death
The best animal model to test the activity of LeTx is the Fisher 344 rat, which dies 40–60 min after intravenous injection of the toxin (Ezzell et al, 1984). The lethal activity of LeTx depends on PA and on the metalloproteolytic activity of LF, as inactive mutants of LF are nontoxic (Brossier et al, 1998; Tonello et al, 2004).
LeTx is usually injected into a caudal vein, and Table 1 shows that a 5-min preincubation of LeTx with EGCG or GTE abolishes its toxicity, as judged from successful survival for the 1-week observation period. Nevertheless, with this procedure, repeated injections are difficult to perform, and on several occasions this prevented us from testing the effect of EGCG injected separately and after the toxin.
Table 1.
Protective effect of EGCG or GTE on Fischer 344 rats treated with LeTx (12 μg LF+30 μg PA)
| Symptoms | ||
|---|---|---|
| (a) Caudal vein | ||
| [LeTX] preincub. 5 min |
Yes |
|
| [LeTx+50 μM EGCG] preincub. 5 min |
No |
|
| [LeTx+100 μM EGCG] preincub. 5 min |
No |
|
| [LeTx+100 μM GTEa] preincub. 5 min |
No |
|
| (b) Femoral vein | ||
|
Right leg (first) |
Left leg (after 5 min) |
Symptoms |
| LeTx |
— |
Yes |
| 50 μM EGCG |
LeTx |
Delayed |
| 100 μM EGCG |
LeTx |
Delayed |
| 100 μM GTEa | LeTx | Delayed |
(a) The i.v. injection of LeTx or LeTx preincubated with green tea compounds was performed into the caudal vein. Control animals developed symptoms within 1 h of injection; all the others were protected over the 1-week observation period (three animals per group). (b) The first injection was performed in the right, and the second in the left, femoral vein of six animals per group. Control animals developed symptoms within 1 h of injection; in the others, the symptoms were delayed as specified in the Results and discussion section.
aFor GTE, the value 100 μM refers to the estimated circulating EGCG concentration.
We then chose to inject a second group of animals via the femoral veins uncovered under anaesthesia. A total of 12 Fischer 344 rats were injected with LeTx in one leg and with EGCG in the other, to give estimated circulating concentrations of 50 and 100 μM. Of the six rats of the 50 μM group, four developed symptoms 1–2 h after the controls; in two animals of the 100 μM group, the delay exceeded 5 h, and two rats recovered completely. Similar effects were registered following injection of GTE. This partial protective effect can be explained in several different ways, including reduced availability of EGCG following metabolism, absorption by proteins and tissues, etc. Moreover, EGCG is labile and very sensitive to oxidation. It is noteworthy that EGCG has a very low toxicity in animals and humans (Yamada, 1998), and indeed here, over a 1-week period, rats and mice injected with only EGCG or GTE showed no adverse reactions.
The present data show the potential value of these natural compounds for the prevention and therapy of anthrax. However, testing the potential of these molecules on human anthrax is a difficult task because animal models of human anthrax are not yet well defined. Therefore, experimental tests will have to be performed on a range of animal species, including large animals such as sheep, which are very susceptible to B. anthracis. This requires the preparation of large amounts of inhibitors, which is currently under way. The in vivo effectiveness of EGCG and related molecules might be improved by association with compounds that are able to increase their lifetime and by designing chemical variants endowed with better pharmacokinetic properties.
Methods
Reagents. EGCG was from Calbiochem, code 324880. Decaffeinated GTE was supplied lyophilized by SOFAR (Trezzano Rosa, Milan, Italy), and contained 50% EGCG, 86% total catechins (including EGCG) and 0.5% caffein (HPLC titration by SOFAR). Anti-MAPKK-2 and MAPKK-3 rabbit polyclonal antibodies (Abs) were from Santa Cruz, and peroxidase-conjugated goat anti-rabbit IgG was from Sigma.
Assay of the enzymatic activity of LF. The samples were solubilized in 25 mM Na2HPO4 and 15 mM NaCl (pH 7.4), and the enzymatic reactions were performed at 25°C with 1 nM LF and 5 μM AcGYβARRRRRRRRVLRpNA substrate (Tonello et al, 2002). The release of p-nitroaniline by LF was monitored in a cuvette at 405 nm with a Perkin-Elmer lambda 5 spectrophotometer (ɛ405=9,920 M−1 cm−1) and the absorbance values after 5 min of reaction were taken; within this time period, the reaction was linear. Control buffer was the same mixture without LF; there was no appreciable hydrolysis of the substrate within 5 min at pH 7.4. Triplicate experiments were run, and the results were expressed as means±s.d. taking the value without inhibitors as 100%. Enzymatic reactions at progressive dilutions of EGCG were performed to determine its IC50 value. The LF-induced hydrolysis and its inhibition were also measured in 96-well plates with a Packard Spectracount plate reader; very similar results were obtained.
Cell culture and LeTx cytotoxicity. The RAW264.7 mouse macrophage cell line is commonly used to test LF cytotoxicity. Cells were grown in DMEM supplemented with 10% FCS and antibiotics, and incubated in 5% CO2 in air at 37°C. Three different experiments were run in triplicate as follows, and the results were expressed as means±s.d.
Preincubation of LF with EGCG: The cells were plated onto 96-well plates at 2 × 104 per well in DMEM with FCS, and used after 24 h. LF (400 ng/ml) was preincubated for 15 min at 37°C with GTE or purified EGCG, epicatechin (EC) or CG, and then mixed with PA (800 ng/ml); this mixture was added to the cells, and after 4 h the cell viability was determined by CellTiter 96® assay (Promega).
Pretreatment of cells with EGCG: The cells were plated onto 96-well plates at 5 × 103 per well in DMEM with FCS, and pretreated with EGCG for 5 days (two additions per day, without changing the medium); LeTx was then added (400 ng/ml LF, 800 ng/ml PA) and cell viability was determined after 4 h.
Delayed addition of EGCG: The cells were plated onto 96-well plates at 2 × 104 per well in DMEM with FCS, and used after 24 h. LeTx (as above) was added to the culture, and then EGCG was added with a progressive delay (0, 30, 60, 90 and 120 min). The cell viability was determined (as above) after 4 h from LeTx addition.
Western blotting. The cells treated with LF preincubated with increasing concentrations of EGCG (as described in (i)) were lysed in Laemmli sample buffer, and the content of each well was loaded in SDS–PAGE gel. After electrophoresis, the proteins were blotted on nitrocellulose paper and stained overnight at 4°C with a rabbit anti-MAPKK-2 or -3 antibody (Santa Cruz Biotechnology) at a dilution of 1:2,000, followed by washings and a 1 h incubation at 22°C with the secondary anti-rabbit immunoglobulin peroxidase-conjugated antibody (Calbiochem) at a 1:2,000 dilution and developed with the enhanced chemiluminescence (ECL) assay (Amersham). The ECL film obtained was analysed with the program ImageJ 1.30v (National Institutes of Health, USA, http://rsb.info.nih.gov/ij/) to calculate the percentage of cleaved MAPKK.
Animal treatment with LeTx. In a first set of experiments, LeTx (12 μg LF+30 μg PA) or LeTx preincubated for 5 min at 37°C with green tea compounds (50 and 100 μM) were injected into one of the caudal veins of Fisher 344 rats (weighing around 250 g), and the animals (three per group) were monitored for symptoms for up to 1 week.
In a second set of experiments, femoral intravenous injections were used. The rats were anaesthetized with isoflurane, the femoral veins were uncovered, and 200 μl of EGCG or GTE was injected into the vein of the right leg; after 5 min, 200 μl of LeTx (as above) was injected into the vein of the left leg. The concentration of EGCG was estimated assuming a total circulating fluid volume of 25 ml.
Acknowledgments
This work was supported by funds from the University of Padova, the Associazione Italiana per la Ricerca sul Cancro, and by the Armenise-Harvard Medical School Foundation.
References
- Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG (2003) Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol 160: 321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lingappa J, Leppla SH, Agrawal S, Jabbar A, Quinn C, Pulendran B (2003) Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424: 329–334 [DOI] [PubMed] [Google Scholar]
- Bradley KA, Young JA (2003) Anthrax toxin receptor proteins. Biochem Pharmacol 65: 309–314 [DOI] [PubMed] [Google Scholar]
- Brossier F, Guidi-Rontani C, Mock M (1998) Anthrax toxins. C R Séanc Soc Biol Fil 192: 437–444 [PubMed] [Google Scholar]
- Chopra AP, Boone SA, Liang X, Duesbery NS (2003) Anthrax lethal factor proteolysis and inactivation of MAPK kinase. J Biol Chem 278: 9402–9406 [DOI] [PubMed] [Google Scholar]
- Dell'Aica I, Donà M, Sartor L, Pezzato E, Garbisa S (2002) Epigallocatechin-3-gallate directly inhibits MT1-MMP activity, leading to accumulation of nonactivated MMP-2 at the cell surface. Lab Invest 82: 1685–1693 [DOI] [PubMed] [Google Scholar]
- Demeule M, Brossard M, Page M, Gingras D, Beliveau R (2000) Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta 1478: 51–60 [DOI] [PubMed] [Google Scholar]
- Dixon TC, Meselson M, Guillemin J, Hanna PC (1999) Anthrax. N Engl J Med 341: 815–826 [DOI] [PubMed] [Google Scholar]
- Duesbery NS et al. (1998) Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280: 734–737 [DOI] [PubMed] [Google Scholar]
- Ezzell JW, Ivins BE, Leppla SH (1984) Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect Immun 45: 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander AM (1986) Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem 261: 7123–7126 [PubMed] [Google Scholar]
- Garbisa S, Biggin S, Cavallarin N, Sartor L, Benelli R, Albini A (1999) Tumor invasion: molecular shears blunted by green tea. Nat Med 5: 1216. [DOI] [PubMed] [Google Scholar]
- Garbisa S, Sartor L, Biggin S, Salvato B, Benelli R, Albini A (2001) Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer 91: 822–832 [DOI] [PubMed] [Google Scholar]
- Hanna PC, Kruskal BA, Ezekowitz RA, Bloom BR, Collier RJ (1994) Role of macrophage oxidative burst in the action of anthrax lethal toxin. Mol Med 1: 7–18 [PMC free article] [PubMed] [Google Scholar]
- Howitz KT et al. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196 [DOI] [PubMed] [Google Scholar]
- Klimpel KR, Molloy SS, Thomas G, Leppla SH (1992) Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci USA 89: 10277–10281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy DB, Collier RJ (2002) Structure and function of anthrax toxin. Curr Top Microbiol Immunol 271: 61–85 [DOI] [PubMed] [Google Scholar]
- Lambert JD, Yang CS (2003) Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res 523: 201–208 [DOI] [PubMed] [Google Scholar]
- Maeda-Yamamoto M, Kawahara H, Tahara N, Tsuji K, Hara Y, Isemura M (1999) Effects of tea polyphenols on the invasion and matrix metalloproteinases activities of human fibrosarcoma HT1080 cells. J Agric Food Chem 47: 2350–2354 [DOI] [PubMed] [Google Scholar]
- Menard A, Altendorf K, Breves D, Mock M, Montecucco C (1996) The vacuolar ATPase proton pump is required for the cytotoxicity of Bacillus anthracis lethal toxin. FEBS Lett 386: 161–164 [DOI] [PubMed] [Google Scholar]
- Mock M, Mignot T (2003) Anthrax toxins and the host: a story of intimacy. Cell Microbiol 5: 15–23 [DOI] [PubMed] [Google Scholar]
- Pannifer AD et al. (2001) Crystal structure of the anthrax lethal factor. Nature 414: 229–233 [DOI] [PubMed] [Google Scholar]
- Park JM, Greten FR, Li ZW, Karin M (2002) Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297: 2048–2051 [DOI] [PubMed] [Google Scholar]
- Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C (1999) Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNγ-induced release of NO and TNFα. FEBS Lett 462: 199–204 [DOI] [PubMed] [Google Scholar]
- Satoh E, Ishii T, Shimizu Y, Sawamura S, Nishimura M (2002) A mechanism of the thearubigin fraction of black tea (Camellia sinensis) extract protecting against the effect of tetanus toxin. J Toxicol Sci 27: 441–447 [DOI] [PubMed] [Google Scholar]
- Tonello F, Seveso M, Marin O, Mock M, Montecucco C (2002) Screening inhibitors of anthrax lethal factor. Nature 418: 386. [DOI] [PubMed] [Google Scholar]
- Tonello F, Naletto L, Romanello V, Dal Molin F, Montecucco C (2004) Tyrosine-728 and glutamic acid-735 are essential for the metalloproteolytic activity of the lethal factor of Bacillus anthracis. Biochem Biophys Res Commun 313: 369–375 [DOI] [PubMed] [Google Scholar]
- Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C (1998) Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun 248: 706–711 [DOI] [PubMed] [Google Scholar]
- Vitale G, Bernardi L, Napolitani G, Mock M, Montecucco C (2000) Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem J 352(Part 3): 739–745 [PMC free article] [PubMed] [Google Scholar]
- Yamada M (1998), In Functional Foods for Disease Prevention I: Fruits, Vegetables, and Teas (Acs Symposium Series no. 701), Schibamoto T, Terao J, Osawa T (eds) Vol 701, pp 217–224. Washington, DC: Oxford University Press [Google Scholar]