Abstract
Normal embryo development is required for correct seedling formation. The Arabidopsis gurke and pasticcino3 mutants were isolated from different developmental screens and the corresponding embryos exhibit severe defects in their apical region, affecting bilateral symmetry. We have recently identified lethal acc1 mutants affected in acetyl-CoA carboxylase 1 (ACCase 1) that display a similar embryo phenotype. A series of crosses showed that gk and pas3 are allelic to acc1 mutants, and direct sequencing of the ACC1 gene revealed point mutations in these new alleles. The isolation of leaky acc1 alleles demonstrated that ACCase 1 is essential for correct plant development and that mutations in ACCase affect cellular division in plants, as is the case in yeast. Interestingly, significant metabolic complementation of the mutant phenotype was obtained by exogenous supply of malonate, suggesting that the lack of cytosolic malonyl-CoA is likely to be the initial factor leading to abnormal development in the acc1 mutants.
Keywords: very-long-chain fatty acids, embryo development, plant development, cell division
Introduction
Normal embryo development governs correct seedling formation. In Arabidopsis, the origin of seedling structures can be traced back to cell groups in the young embryo, due to the invariant pattern of cell division during early embryogenesis (Jürgens, 2001). First, the embryo develops through an apical–basal axis, which is partitioned into three major regions: apical, central and basal. Superimposed on this axis appears a radial pattern of concentric tissue layers established successively from the periphery to the centre (Jürgens, 1995). At the late-globular stage, the radial symmetry of the embryo is transformed into a bilateral symmetry by the enhanced dividing activity of cells at opposite sites of the apical region, which will determine the future cotyledons. How the apical region is partitioned between the presumptive shoot meristem and the two flanking cotyledon primordia is unknown. However, a number of genes (WUSCHEL, SHOOTMERISTEMLESS, CLAVATA1, CUPsHAPED COTYLEDON 1) were shown to affect the establishment and the development of this region (for reviews, see Souter & Lindsey, 2000; Jürgens, 2001). gurke mutants were isolated on the basis of apical defects with the cotyledons missing or reduced to small knob-like structures (Torres-Ruiz et al, 1996). Mutations in the GURKE gene were mapped to chromosome 1. Likewise, the PASTICCINO genes, which are involved in the control of cell division and differentiation, are required for normal organization of the apical region in the embryo (Faure et al, 1998). pasticcino mutants, which belong to three complementation groups (pas1, pas2, pas3), produce abnormal embryos with altered cotyledon primordia leading to a flat apex. The PAS1 gene was found to encode an immunophilin-like protein of the FK506 binding protein (FKBP) family (Vittorioso et al, 1998), and the PAS2/PEPINO gene was shown to encode a tyrosine phosphatase-like protein (Bellec et al, 2002; Haberer et al, 2002), but their functions remain unknown. pas3, which is epistatic to pas1 and pas2, has not yet been identified. However, genetic analyses demonstrated that it is located near the centromere of chromosome 1 (Faure et al, 1998).
We recently characterized acetyl-CoA carboxylase 1 (acc1) mutants impaired in embryo morphogenesis (Baud et al, 2003). Acetyl-CoA carboxylase (ACCase; EC 6.4.1.2) catalyses the ATP-dependent formation of malonyl-CoA from acetyl-CoA and bicarbonate. In dicots, ACCases are present in two structurally distinct forms, a multifunctional homodimeric form in the cytosol and a dissociable, multisubunit heteromeric form in the plastids (Alban et al, 2000). Activity of these ACCases is then able to generate two independent malonyl-CoA pools. The plastidic pool contributes to the de novo fatty acid synthesis, and the cytosolic pool is required for a wide range of reactions and pathways including very-long-chain fatty acid (VLCFA ⩾C20) elongation. These VLCFAs are incorporated into triacylglycerides, cuticle, waxes or sphingolipids. Two genes, ACC1 and ACC2, encode such cytosolic ACCases in Arabidopsis, although ACC2 transcripts are barely detectable (Baud et al, 2003). acc1 mutant embryos exhibited cucumber-like structures lacking cotyledons. acc1-1 and acc1-2 alleles are devoid of cytosolic ACCase protein and are considered as null alleles. In the search for new alleles exhibiting leaky phenotypes, we identified the Arabidopsis gurke and pas3 mutants as new acc1 alleles. Like acc1, the pas3 and gk alleles also showed a reduced VLCFA accumulation in seeds. Moreover, a significant metabolic complementation of the acc1 phenotype could be obtained with exogenous malonate, suggesting that the lack of malonyl-CoA is responsible for the developmental defect observed in acc1/gk/pas3 mutants.
Results And Discussion
gk and pas3 mutants are allelic to acc1 mutants
acc1-1 and acc1-2 embryos exhibit an early developmental arrest, leading to cucumber-like structures deprived of cotyledons (Baud et al, 2003). The pattern defect of these embryos resembles the patterns exhibited by the severe gurke and pas3 alleles (Torres-Ruiz et al, 1996; Faure et al, 1998). gk and pas3 mutations were both mapped to chromosome 1, near the centromere (Torres-Ruiz et al, 1996; Faure et al, 1998) and not far from ACC1. As the mutants display a similar phenotype and close genetic location, allelism tests were carried out (see Methods). No complementations were obtained, thus demonstrating that acc1, gk and pas3 mutants were allelic. The large number of alleles (six pas3 alleles, ten gk alleles and two acc1 alleles) isolated so far in various screens is consistent with the large size of the ACC1 gene (about 9 kb).
Molecular characterization of the gk and pas3 alleles
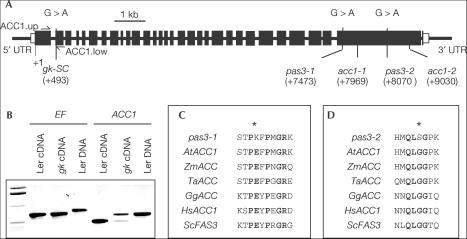
To gain further insights into the molecular defects of the gk and pas3 alleles, ACC1 was sequenced in pas3-1, pas3-2 and gk-SC (Stock Center N8148) alleles. The three mutants were obtained by ethyl methane sulphonate (EMS) mutagenesis of Col0 (pas3-1, pas3-2) and Ler (gk-SC). In all cases, single point mutations were identified in the ACC1 gene (Fig 1A). In gksC, a G-to-A transition at position 493 altered a splicing site at the 3′ end of the first intron (Fig 1A). Reverse transcription (RT)–PCR experiments using primers designed on both sides of this intron were performed on homozygous embryos, demonstrating a splicing defect in the mutant background (Fig 1B). Two distinct RT–PCR products were obtained and sequenced. The higher and stronger band corresponded to ACC1 cDNA in which intron 1 was not spliced out. Unspliced intron 1 led to a frameshift and a predicted protein 163 amino acids (aa) shorter than wild-type (WT) protein (2,212 aa). The lower and weaker band corresponded to ACC1 cDNA in which intron 1 was incorrectly spliced: exon 2 was initiated at position 522 instead of 494 in the WT. Incorrect splicing led to a frameshift and a predicted protein of 131 aa. In pas3-1, a G-to-A transition at position 7473 led to the substitution of an E residue to a K residue at amino-acid position 1581. Transition of G to A at position 8070 in pas3-2 caused a substitution of a G residue to a S residue at amino-acid position 1780. In both cases, a highly conserved amino-acid residue of the carboxyltransferase (CT) domain of ACC1 was thus mutated (Fig 1C,D).
Figure 1.
Identification of gksC, pas3-1 and pas3-2 mutations in ACC1 gene. (A) Structure of ACC1 gene and position of the point mutations identified in gk-SC, pas3-1 and pas3-2 mutants; acc1-1 and acc1-2 mutations. Closed boxes represent exons, whereas open boxes stand for untranslated regions (UTRs). Nucleotide positions are relative to the translational start site. (B) Characterization of abnormal ACC1 transcripts in gk-SC embryos. Primer sets were used to amplify EF (EF1.up and EF1.low; control) and ACC1 (ACC1.up and ACC1.low) on cDNA from excised Ler and gk-SC embryos aged 15 DAA and on Ler DNA. ACC1.up was designed in the first exon of ACC1 gene, and ACC1.low in the second exon. (C) Amino-acid sequence alignments of a conserved motif in the CT domain of ACCases. Bold residues indicate positions where amino acids are highly conserved. The asterisk indicates the conserved amino-acid residue mutated in pas3-1. (D) Amino-acid sequence alignments of a conserved motif in the CT domain of ACCases. Bold residues indicate positions where amino acids are highly conserved. The asterisk indicates the conserved amino-acid residue mutated in pas3-2. AtACC1, A. thaliana ACCase 1 (At1g36160); ZmACC, Zea mays ACCase (U19183); TaACC, Triticum aestivum ACCase (U10187); GgACC, Gallus gallus ACCase (J03541); HsACC, Homo sapiens ACCase 1 (U19822); ScFAS3, S. cerevisiae FAS3 (M92156).
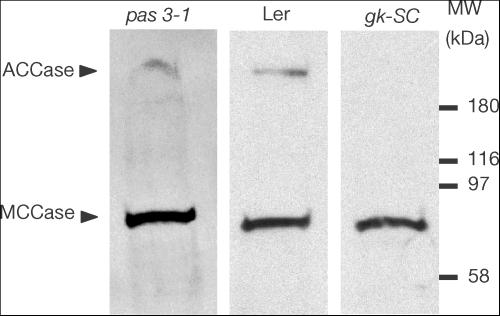
A western blot strategy using streptavidin was then applied to detect the presence of a biotinylated multifunctional (cytosolic) ACCase in excised embryos (see Baud et al, 2003). In WT embryos, a biotinylated polypeptide with a molecular mass of 220 kDa was detected, which corresponds to the multifunctional ACCase. The gksC mutation resulted in a lack of biotinylated multifunctional ACCase in isolated embryos (Fig 2). The truncated peptides corresponding to the unspliced mRNA or to the incorrectly spliced mRNA are indeed deprived of the central biotin-carboxyl carrier (BCC) domain that normally binds biotin. By contrast, the protein was detected in both pas3-1 and pas3-2 (Fig 2 and data not shown). This is consistent with point mutations identified in pas3 alleles that are likely to alter the protein activity because they affect highly conserved motifs in the CT domain of the enzyme (Fig 1).
Figure 2.
Multifunctional ACCase detection in excised pas3 and gksC embryos. Fresh seeds were removed from heterozygous siliques and mutant embryos excised from severely wrinkled seeds were sampled. Total proteins of excised embryos aged 15 DAA were submitted to SDS–polyacrylamide gel electrophoresis on a 4–15% polyacrylamide gel. A nitrocellulose blot was probed with peroxidase-conjugated streptavidin, which binds biotin cofactor. Biotinylated polypeptides such as methylcrotonoyl-CoA carboxylase (MCCase) or ACCase were thus revealed.
Modification of fatty acid composition and consequences
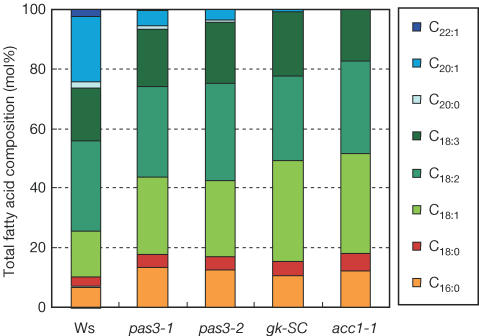
To measure the impact of the mutations on plant metabolism, the fatty acid composition of mature mutant seeds was analysed. acc1-1 and acc1-2 mutations resulted in a lack of VLCFAs, the elongation of which requires cytosolic malonyl-CoA (Bao et al, 1998). The synthesis of shorter acyl chains, requiring solely plastid malonyl-CoA, was not prevented in the mutant. The level of VLCFAs was significantly lower in pas3, whereas only traces of VLCFAs were found in gksC (Fig 3). Compared with WT, C22:1 was missing in pas3 and gksC as in acc1; C20:1 was almost absent in gk-SC and strongly reduced in pas3. Conversely, C16:0 and C18:1 were increased compared with WT in pas3 and gk-SC mutant as in acc1. In all cases, the biochemical analyses performed confirmed that cytosolic ACCase activity was impaired in the pas3 and gk mutants. Consistently, the decrease in VLCFA level correlated with the strength of each allele. acc1-1 and gk-SC alleles, which are embryo lethal, had the lowest VLCFA level, whereas pas3 embryos, which are viable and able to germinate, exhibited an intermediate VLCFA level (Fig 4B).
Figure 3.
Fatty acid composition of mature mutant seeds. Total lipid extraction and fatty acid transmethylation were carried out on batches of 20 seeds. Total fatty acid composition (in mol%) of seeds was determined by GC analyses. Results are the mean of three independent measurements; s.d. was always less than 1% of the mean. Fatty acid composition of WT Ws (acc1-1 background), Ler (gk background) and Col0 (pas3 background) seeds was analysed; the results presented for the Ws seeds are representative of the three ecotypes.
Figure 4.
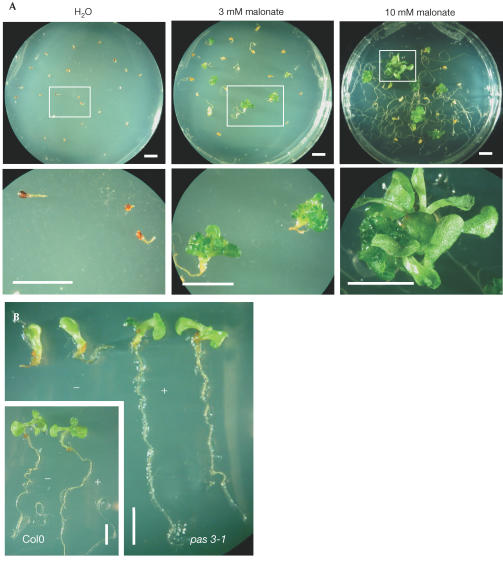
Complementation of acc1 mutations by exogenous supply of malonate. (A) Response of immature acc1-1 mutant embryos. Arrested embryos were removed from immature siliques of heterozygous plants at 15 DAA, plated on basal medium, treated every 2 days with malonate and observed after several weeks of culture. Pictures on the bottom row are close-ups of embryos or plantlets presented in pictures on the top row. The development of WT Ws embryos was unaffected by malonate supply (not shown). (B) Response of pas3-1 embryos during germination. Col0 and pas3-1 seeds were germinated on basal and enriched (1 mM malonate) medium and observed after several weeks of culture. Scale bars, 5 mm.
Although VLCFAs (mainly C20:1) are mainly incorporated into triacylglycerol molecules stored during seed maturation, some VLCFAs (from C26 to C32) are also required for cuticle and wax synthesis (Kolattukudy, 1980). Mutation in ALE1, a subtilisin-like serine protease required for epidermal surface formation in Arabidopsis embryos, demonstrated that a normal cuticle is required for separation of the endosperm from the embryo (Tanaka et al, 2001). Abnormal adherence observed between endosperm and embryo in acc1 alleles (data not shown) seems to indicate that cuticle formation was altered in these mutant embryos. Moreover, organ fusion was reported in leaky gk and pas3 alleles (Torres-Ruiz et al, 1996; Faure et al, 1998), and this phenotype could be mimicked (data not shown) in WT plants sprayed with quizalofop, a specific inhibitor of multifunctional ACCase (Dehaye et al, 1994). Interestingly, mutations in ALE1 or FIDDLEHEAD, a gene encoding a fatty acid elongase involved in cuticle biosynthesis, resulted in a similar phenotype (Yephremov et al, 1999). Taken together, these results suggested that cuticle formation is severely altered in acc1, gk and pas3 alleles. However, the phenotype of mutants with altered cuticle formation, which have been previously described, suggests that this specific defect is not responsible for the developmental phenotype observed in acc1 embryos.
Partial complementation of the phenotype by malonate
As cytosolic ACCase is involved in malonyl-CoA synthesis, we investigated whether the developmental defect observed in acc1 mutants could be compensated for by exogenous malonyl-CoA. Owing to membrane impermeability to CoA-bound compounds, malonate was supplied to immature embryos (Fig 4A). Malonyl-CoA synthetase (MCS) was shown to catalyse the formation of malonyl-CoA directly from malonate and CoA in microorganisms such as Bradyrhizobium japonicum (Koo & Kim, 2000). We expected that an A. thaliana MCS homologue (encoded by At3g16170) could use malonate to generate the malonyl-CoA lacking in the mutant embryos. When WT embryos excised at 15 days after anthesis (DAA) were cultured in vitro on a basal medium, they quickly generated green plantlets with small leaves and a branched root system. However, acc1-1 embryos exhibited a slight elongation of the radicle and then died. When treated every 2 days with 3 mM malonate, mutant embryos exhibited a significant increase in root elongation, whereas callus-like structures appeared in the apical region of some of these embryos (Table 1). When treated with 10 mM malonate, branched root systems were formed, and mutant embryos occasionally developed into leafy plantlets (Fig 4A). As pas3-1 mutation was not embryo lethal, germination experiments were conducted to test the effect of malonate. Seedlings were germinated on 1 mM malonate and, after 10 days of culture, the root growth was completely restored, leaf fusion was prevented and the apical part of the seedling was partly restored (Fig 4B).
Table 1.
Response of acc1-1 embryos cultured in vitro to exogenously supplied malonate
| Concentration of malonate (mM) | Embryos cultured |
Embryo response after 2 weeks in culture (%) |
|||
|---|---|---|---|---|---|
| Dead embryos | Yellow calli | Green calli | Leafy plantlets | ||
| 0 |
65 |
100 |
0 |
0 |
0 |
| 3 |
62 |
10 |
66 |
24 |
0 |
| 10 | 110 | 6 | 41 | 45 | 8 |
Mutant embryos excised at 15 DAA were cultured on basal medium and sprayed every 2 days with malonate.
In both experiments, exogenously supplied malonate compensates for at least part of the mutant developmental defect. These data suggest that the lack of cytosolic malonyl-CoA is likely to be the main factor leading to this strong phenotype. acc1/pas3/gk mutations are the first mutations affecting ACCase described in a multicellular eukaryotic organism. If acc1-1 and acc1-2 lethal mutations allowed the key role of ACC1 during embryo morphogenesis to be demonstrated, identification of leaky alleles now emphasizes the role of ACC1 in the development of the plant.
Speculation
Further biochemical and transcriptional analyses are needed to elucidate which specific biosynthetic pathway(s) requiring cytosolic malonyl-CoA is (are) involved in this developmental process (Baud et al, 2003). Recently, a Saccharomyces cerevisiae mutant strain defective in ACCase was shown to be blocked at the G2/M phase of the cell cycle (Al-Feel et al, 2003). The authors pointed to the role in the cell cycle of ceramides, a basic component of all sphingolipids the biosynthesis of which requires VLCFA. Interestingly, in developing gk seeds exhibiting strong phenotypes, the number of cell divisions in rudimentary cotyledon primordia was severely reduced (Torres-Ruiz et al, 1996), whereas pasticcino mutants were shown to display uncoordinated cell divisions enhanced by cytokinin (Faure et al, 1998). Studying sphingolipid content in mutant backgrounds and considering their putative role in the control of cell division could thus shed new light on the acc1 phenotype. Sphingolipids have been reported to interact with cholesterol to form clusters of raft-like domains in mammalian membranes (Carl & Hetherington, 2001). FACKEL, a gene involved in sterol biosynthesis in Arabidopsis, was shown to be required for proper cell division and expansion in the embryo (Schrick et al, 2000). Some membrane sphingolipids and phytosterols may be important for normal organization of the embryo through the regulation and coordination of cell division during its morphogenesis, thus pointing out a novel role for lipids in the embryogenesis of plants.
Methods
Plant material and growth conditions. Arabidopsis thaliana of ecotypes Wassilewskija (Ws), Landsberg erecta (Ler) and Columbia (Col0) were obtained from the Station de Génétique et d'Amélioration des Plantes (INRA Versailles, France). acc1-1 and acc1-2 mutants (from the Ws ecotype) have been described previously (Baud et al, 2003). pas3-1 and pas3-2 were isolated from an EMS-mutagenized seed stock from the Col0 ecotype (Faure et al, 1998). The gksC allele, isolated from an EMS-mutagenized seed stock from the Ler ecotype, was obtained from the Nottingham Arabidopsis Stock Center (N8148). Seeds were surface sterilized and germinated on Murashige and Skoog (MS) medium (M02 555, pH 5.6; Duchefa, Haarlem, The Netherlands) solidified with 0.7% (w/v) agar. Culture conditions were the same as described previously (Baud et al, 2003). For allelism tests, reciprocal crosses between gksC and both acc1-1 and pas3-1 lines were performed. In reciprocal crosses between gk-SC and acc1-1, a segregation ratio of 22.9% was observed for wrinkled seeds, which was not different from the theoretical segregation ratio of 25% if no complementation occurs (χ2=0.507, two-sided P-value 0.476). In the reciprocal crosses between gk-SC and pas3-1, we observed a segregation ratio of 24.5% for wrinkled seeds, and again no complementation occurred (χ2=0.057, two-sided P-value 0.812). For complementation assays, the whole siliques were surface sterilized. Wrinkled mutant seeds were then removed from the silique under sterile conditions. Embryos were excised from the seeds and cultured as described above on MS medium complemented with Gln at 2.5 mM. Every 2 days, embryos were treated with water (control plants), or 3 mM or 10 mM malonate (pH 5.3).
DNA extraction and sequencing. Genomic DNA was extracted according to Oard & Dronavalli (1992). For molecular characterization of the gksC and pas3 mutation, DNA sequences were then carried out on PCR products purified with a Qiagen (Chatsworth, CA) Purification Kit, using the Applied Biosystems (Foster City, CA) DNA Sequencing Kit (Bigdye Terminator V.3.0) and ABI prism 310 genetic analyser.
RNA analyses. Frozen embryos harvested at 15 DAA were ground under liquid nitrogen and total RNA was extracted with the RNeasy Plant Mini Kit (Qiagen) according to the manufacturer's instructions. The extracts were treated with 30 U of RNase-free DNase I (Qiagen) and eluted with 40 μl of diethyl pyrocarbonate-treated water. For RT–PCR studies, DNA-free RNA was converted into first-strand cDNA using the SuperScript preamplification system for first-strand cDNA synthesis (Gibco BRL, Cergy Pontoise, France) with oligo(dT)22. Then, 35 cycles of RT–PCR were performed with different primer sets amplifying fragments in the EF1-ALPHA-A4 cDNA (EF1.Up, 5′-atgccccaggacatcgtgatttcat-3′; EF1.Low, 5′-ttggcggcacccttagctggatca-3′) or in ACC1 cDNA, on both sides of the first intron (ACC1.Up, 5′-atggctggctcggttaacgg-3′; ACC1.Low, 5′-accaatcttatctcccagtgc-3′).
Western blot. Approximately 20–50 isolated embryos were homogenized in 50 μl of sample buffer according to the Laemmli procedure (Laemmli, 1970). Extraction, electrophoresis, transfer and visualization were performed as described previously (Baud et al, 2003).
Lipid analyses. Pools of 20 mature seeds were ground in a glass reaction tube. Extraction and analyses of fatty acid methyl esters by gas chromatography were performed as described previously (Baud et al, 2002).
Acknowledgments
We are grateful to Basil Nikolau and Beth Fatland (Iowa State University) for their precious technical advice in malonate feeding experiments and to Dr Lynne F. Whitehead for kind improvement of the English language.
References
- Alban C, Job D, Douce R (2000) Biotin metabolism in plants. Annu Rev Plant Physiol Plant Mol Biol 51: 17–47 [DOI] [PubMed] [Google Scholar]
- Al-Feel W, DeMar JC, Wakil SJ (2003) A Saccharomyces cerevisiae mutant strain defective in acetyl-CoA carboxylase arrests at the G2/M phase of the cell cycle. Proc Natl Acad Sci USA 100: 3095–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Pollard M, Ohlrogge J (1998) The biosynthesis of erucic acid in developing embryos of Brassica rapa. Plant Physiol 118: 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Boutin J-P, Miquel M, Lepiniec L, Rochat C (2002) An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Phys Biochem 40: 151–160 [Google Scholar]
- Baud S, Guyon V, Kronenberger J, Wuillème S, Miquel M, Caboche M, Lepiniec L, Rochat C (2003) Multifunctional acetyl-CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J 33: 75–86 [DOI] [PubMed] [Google Scholar]
- Bellec Y, Harrar Y, Butaeye C, Darnet S, Bellini C, Faure J-D (2002) Pasticcino2 is a protein tyrosine phosphatase-like involved in cell proliferation and differentiation in Arabidopsis. Plant J 32: 713–722 [DOI] [PubMed] [Google Scholar]
- Carl K-YNG, Hetherington AM (2001) Sphingolipid-mediated signalling in plants. Ann Bot 88: 957–965 [Google Scholar]
- Dehaye L, Alban C, Job C, Douce R, Job D (1994) Kinetics of the two forms of acetyl-CoA carboxylase from Pisum sativum. Eur J Biochem 225: 1113–1123 [DOI] [PubMed] [Google Scholar]
- Faure J-D, Vittorioso P, Santoni V, Fraisier V, Prinsen E, Barlier I, Van Onckelen H, Caboche M, Bellini C (1998) The PASTICCINO genes of Arabidopsis thaliana are involved in the control of cell division and differentiation. Development 125: 909–918 [DOI] [PubMed] [Google Scholar]
- Haberer G, Erschadi S, Torres-Ruiz R (2002) The Arabidopsis gene PEPINO/PASTICCINO2 is required for proliferation control of meristematic and non-meristematic cells and encodes a putative anti-phosphatase. Dev Genes Evol 212: 542–550 [DOI] [PubMed] [Google Scholar]
- Jürgens G (1995) Axis formation in plant embryogenesis: cues and clues. Cell 81: 467–470 [DOI] [PubMed] [Google Scholar]
- Jürgens G (2001) Apical–basal pattern formation in Arabidopsis embryogenesis. EMBO J 20: 3609–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE (1980) Cutin, suberin and waxes. In The Biochemistry of Plants, Stumpf PK, Conn EE (eds) pp 571–645. New York, USA: Academic [Google Scholar]
- Koo HM, Kim YS (2000) Identification of activesite residues in Bradyrhizobium japonicum malonyl-Coenzyme A synthetase. Arch Biochem Biophys 378: 167–174 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Oard JH, Dronavalli S (1992) Rapid isolation of rice and maize DNA for analysis by random-primer PCR. Plant Mol Biol Rep 10: 236–241 [Google Scholar]
- Schrick K, Mayer U, Horrichs A, Kuhnt C, Bellini C, Dangl J, Schmidt J, Jürgens G (2000) FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes Dev 14: 1471–1484 [PMC free article] [PubMed] [Google Scholar]
- Souter M, Lindsey K (2000) Polarity and signalling in plant embryogenesis. J Exp Bot 51: 971–983 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Onouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Machida C, Machida Y (2001) A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development 128: 4681–4689 [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz RA, Lohner A, Jürgens G (1996) The GURKE gene is required for normal organization of the apical region in the Arabidopsis embryo. Plant J 10: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Vittorioso P, Cowling R, Faure J-D, Caboche M, Bellini C (1998) Mutation in the Arabidopsis pasticcino1 gene, which encodes a new FK506-binding protein, has a dramatic effect on plant development. Mol Cell Biol 18: 3034–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H (1999) Characterization of the FIDDLEHEAD gene of Arabidopsis revals a link between adhesion response and cell differentiation in the epidermis. Plant Cell 11: 2187–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]