Abstract
The biochemistry of most metabolic pathways is conserved from bacteria to humans, although the control mechanisms are adapted to the needs of each cell type. Oxygen depletion commonly controls the switch from respiration to fermentation. However, Saccharomyces cerevisiae also controls that switch in response to the external glucose level. We have generated an S. cerevisiae strain in which glucose uptake is dependent on a chimeric hexose transporter mediating reduced sugar uptake. This strain shows a fully respiratory metabolism also at high glucose levels as seen for aerobic organisms, and switches to fermentation only when oxygen is lacking. These observations illustrate that manipulating a single step can alter the mode of metabolism. The novel yeast strain is an excellent tool to study the mechanisms underlying glucose-induced signal transduction.
Keywords: metabolism, respiration, hexose transport, glycolysis, signalling
Introduction
The glycolytic pathway and its individual enzymes are conserved during evolution, although mechanisms controlling carbon and energy metabolism have adapted to the needs of each species or cell type. Aerobic organisms respire pyruvate completely to CO2 with oxygen (O2) as the terminal electron acceptor, thereby making maximal use of energy transformations for ATP production. However, facultative aerobic organisms may add fermentation for fast energy production. For instance, glucose is fermented to lactate by human muscle cells. Similarly, the yeast Saccharomyces cerevisiae switches to a mixed respiro-fermentative metabolism, resulting in ethanol production, as soon as the external glucose concentration exceeds 0.8 mM (Verduyn et al, 1984). Hence, S. cerevisiae controls fermentation versus respiration primarily in response to the sugar level.
Aerobic ethanol production by S. cerevisiae is thought to depend on the relative capacities of the fermentative and respiratory pathways: high glucose levels result in a glycolytic rate exceeding that of the pyruvate dehydrogenase (Pdh) reaction, thereby generating an overflow towards pyruvate decarboxylase (Pdc) and hence ethanol production. At low external glucose levels and in the presence of oxygen, S. cerevisiae does not produce ethanol (Kappeli, 1986). Recent studies on human Pdh suggest that lactate accumulation in muscle cells is also due to an overflow of pyruvate towards lactate formation and is not a result of oxygen limitation in the mitochondria (Heigenhauser & Parolin, 1999; Parolin et al, 2000a, 2000b). Such overflow occurs during exercise, when glycogen is broken down to ensure rapid ATP production (Hargreaves, 2000).
The uptake of glucose into S. cerevisiae is controlled by multiple hexose transporters (Hxts) (Ozcan & Johnston, 1999), which have different substrate specificity and affinity, and are expressed under different, overlapping conditions (Reifenberger et al, 1997). The availability of a yeast strain that lacks all those Hxts (Wieczorke et al, 1999) and that does not take up glucose opens the possibility to study the role of uptake in glycolytic metabolism and glucose-induced signalling. We reasoned that chimeric sugar transporters composed of parts of the low-affinity Hxt1 and the high-affinity Hxt7 could result in transporters with novel properties. One of the chimaeras, when expressed as the only glucose transporter, conferred a respiratory metabolism even at high external glucose concentrations. At the same time, the strain maintained its ability to ferment under anaerobic conditions. The data presented here show that manipulating one individual step, glucose uptake, can alter the mode of metabolic control. The characterization of this yeast strain provides a novel insight into the control of metabolism and signalling.
Results and Discussion
An S. cerevisiae strain with a fully respiratory growth
During aerobic growth on fermentable carbon sources, S. cerevisiae controls fermentation versus respiration in response to the carbon source level or quality. Maximal ATP yield does not seem to be an issue for S. cerevisiae in its natural environment, and it has been interpreted that ethanol production instead provides a competitive advantage.
In this study, we are presenting an S. cerevisiae strain that switches to fermentation only when oxygen is removed. The strain we generated relies for sugar uptake on a chimeric sugar transporter composed of the low-affinity (100 mM) and the high-affinity (1–2 mM) transporters Hxt1 and Hxt7. They are 72% identical and are predicted to consist of 12 membrane-spanning domains (TMDs) (Ozcan & Johnston, 1999). The construct was integrated into the genome of the hxt null strain KOY.VW100 behind the truncated HXT7 promoter (Hauf et al, 2000). A fusion in TMD6 (TM6) generated a nonfunctional glucose transporter. A serendipitous mutation introduced by PCR (TM6*, S279Y) generated a transporter that mediated growth of strain KOY.TM6*P (from here onwards called the TM6* strain) on glucose. Hence, single point mutations can render a nonfunctional chimaera functional, and this approach could be instrumental in future structure–function analysis.
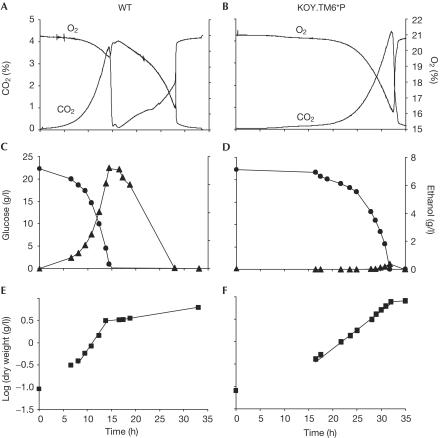
The wild type showed a carbon dioxide production and oxygen consumption profile (Fig 1A) typical for aerobic diauxic growth by S. cerevisiae (Fiechter et al, 1981; Diderich et al, 2001). Glucose was first catabolized fermentatively to carbon dioxide and ethanol plus minor amounts of other by-products (e.g. glycerol and acetate). A fraction of the sugar was catabolized respiratorily or used for biomass production. In the following phase, ethanol was respired to carbon dioxide and water, and partly incorporated into biomass (Fig 1C,E). The wild type had a respiratory quotient (RQ) of 3.4 (Table 1). In contrast, the TM6* strain exhibited a single growth phase (Fig 1B). The RQ of this strain was 1.0 (Table 1), consistent with an exclusively respiratory metabolism (Fig 1D). The strain relying on Tm6* for glucose transport produced only negligible amounts of ethanol (8 mM) and other by-products during the entire growth phase. Consequently, the TM6* strain attained a higher overall biomass yield (Fig 1E,F). Similar results were obtained at even higher glucose levels (5%, data not shown). Although the glucose consumption rate, that is, glycolytic rate, of TM6* was only about 20% of that of the wild type (Table 1), the specific growth rate was as high as 70% (Table 2). The wild type exhibited low and the TM6* strain high glucose affinity of the sugar uptake (Table 2). Vmax was only about 10% of that of the wild type (Table 2), consistent with glucose consumption data (Table 1).
Figure 1.
Glucose, ethanol, dry weight and gas measurements during aerobic batch cultures of the wild type (WT) and TM6* in 2% glucose. (A,B) Oxygen consumption and carbon dioxide evolution. (C,D) Glucose (filled circle) and ethanol (filled triangles). (E,F) Dry weight (filled squares).
Table 1.
Metabolic characteristics of the wild type and the TM6* strain in aerobic batch with 2% glucose and after shift to anaerobicity
| Strain |
Aerobically |
Anaerobically |
||||
|---|---|---|---|---|---|---|
| Glucose consumption rate (mmol/g h) | Ethanol production rate (mmol/g h) | RQ | Glucose consumption rate (mmol/g h) | Ethanol production rate (mmol/g h) | RQ | |
| Wild type |
16±2 |
20±3 |
3.4 |
20±1 |
39±7 |
∞ |
| TM6* | 3.5±0.5 | 0.0 | 1.0 | 2.8–1.0 | 5.0–1.0 | ∞ |
Values are mean±s.d. g, dry biomass; RQ, respiratory quotient.
Table 2.
Kinetic data (Vmax and Km) of glucose transport, specific growth rates (μ) and oxygen consumption rates of the wild type and the TM6* strain
| Strain | Vmax (nmol/(mg protein)min) | Vmax (%) | Km (mM) | μ (h−1) | μ (%) | Oxygen consumption rate (mmol/(g biomass)h) |
|---|---|---|---|---|---|---|
| Wild type |
663±31 |
100 |
76±9.2 |
0.35 |
100 |
1.4±0.3 |
| TM6* | 61±2.1 | 9 | 3.5±0.5 | 0.23 | 66 | 6.3±0.4 |
Values are mean±s.d.
Following the concept that alcoholic fermentation occurs once the glycolytic rate exceeds the capacity of the Pdh reaction, it appears that the low sugar uptake in TM6* keeps the pyruvate formation rate below the critical value (Kappeli, 1986). In addition, Pdc expression is stimulated by glucose to a lower degree in the TM6* strain (Table 3 and Fig 3). The respiratory rate is 4.5 times higher in the TM6* strain than in the wild type (Table 2).
Table 3.
Specific activity (mU/mg protein) of invertase and Pdc for the wild type and the TM6* strain
| Strain | Carbon source | Invertase | Pdc |
|---|---|---|---|
| Wild type |
Ethanol |
680±100 |
100±10 |
| |
Glucose |
330±10 |
1,470±190 |
|
TM6* |
Ethanol |
620±100 |
120±1 |
| Glucose | 4,240±700 | 430±12 |
Values are mean±s.d. Pdc, pyruvate decarboxylase.
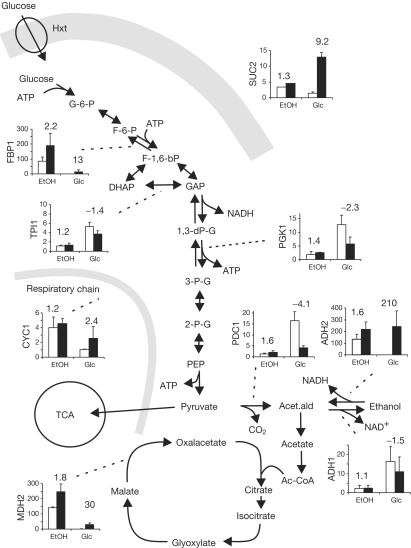
Figure 3.
Relative expression levels in the wild type (open bars) and TM6* (closed bars) under derepressed (ethanol growth) and repressed (glucose growth) conditions. Numbers: fold differences in expression in TM6* compared with the wild type either at ethanol (EtOH) or glucose growth. Bars indicate mean±s.d. SUC2, invertase; FBP1, fructose 1,6 bisphosphatase; TPI1, triose-phosphate isomerase; PGK1, phosphoglycerate kinase; PDC1, pyruvate decarboxylase1; ADH1/2, alcohol dehydrogenase 1/2; CYC1, iso-1-cytochrome C; MDH2, malate dehydrogenase 2; TCA, tricarboxylic acid cycle.
Fermentative growth under anaerobic conditions
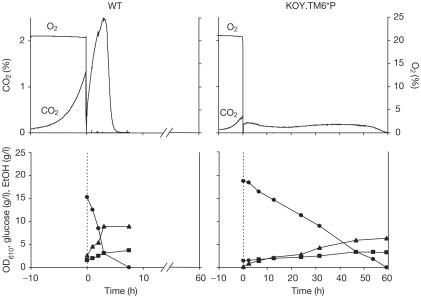
When shifted to anaerobic conditions, both the wild type and the TM6* strain show a transient drop in gas production (Fig 2). The TM6* strain continues to consume glucose and switches to fermentative metabolism, albeit at a much slower rate than the wild type (Fig 2 and Table 1). The increase in biomass (120%) observed for the TM6* strain was accompanied by only a 30% increase in cell number and a 75% decrease in budding index. Hence cells had ceased dividing and uncoupled glycolytic rate and growth.
Figure 2.
The wild type (WT) and TM6* were grown aerobically to an OD610 of 1–1.5 before shift to anaerobicity at time zero. Glucose (filled circles) and ethanol (EtOH; filled triangles) concentrations and optical density (filled squares) were followed. Oxygen consumption and carbon dioxide evolution were followed.
The basis for aerobic fermentation by yeast has been the subject of many studies. Our data show that altering one single metabolic step, glucose uptake, is enough to switch the mode of metabolism from fermentation to respiration. The behaviour of the TM6* strain furthermore suggests that during evolution yeast has precipitously increased the capacity of glucose uptake and the glycolytic pathway, whereas that for the tricarboxylic acid cycle has not followed. The driving force for that evolution may have been a competitive advantage in the yeast's natural environment. It should be noted that the observed amplification of hexose transporter genes does not seem to contribute much to the increased uptake capacity, as single Hxts can sustain almost wild-type uptake capacity (Reifenberger et al, 1997). Rather, the differently expressed transporters allow the cell to adjust sugar affinity to substrate availability.
Glucose-induced gene expression
The presence of glucose, the preferred carbon and energy source, mediates a large number of changes in yeast and all other organisms investigated so far. A major issue in this regard is whether sensing occurs at the cell surface by membrane-localized receptors or internally; that is, at the level of metabolism. Both modes of sensing seem to exist in yeast (Rolland et al, 2002). To exemplify the usefulness of the TM6* strain to study glucose-induced regulatory effects, we have looked at two different phenomena, glucose repression and glucose induction of gene expression during derepressed (ethanol growth) and repressed (glucose growth) conditions. Different regulatory pathways mediate glucose repression itself. Repression of the SUC2 gene at high glucose levels is controlled by the Snf1–Mig1/Mig2 system (Gancedo, 1998), whereas low glucose levels stimulate SUC2 expression (Ozcan et al, 1997). In the TM6* strain, expression of SUC2 is induced about tenfold by glucose levels that mediate repression in the wild type (Fig 3). This observation suggests that the TM6* strain perceives 2% glucose as less than about 0.2% glucose. Consistent with previous evidence (Gancedo, 1998), this is only possible if sensing occurs after the glucose uptake step at the metabolic level by the Snf1–Mig1 system.
Expression of the gluconeogenetic genes FBP1 and MDH2 is controlled by the Snf1–Mig1 system and an induction mechanism involving the Cat8 and Sip4 regulators (Schuller, 2003). Significant expression of both genes was detectable in the TM6* strain on glucose (Fig 3), consistent with sensing inside the cell and with the notion that this strain perceives only a very low glucose level. Expression of ADH2, which is dependent on Snf1 for derepression and activated by Cat8 and Adr1 (Schuller, 2003), is completely derepressed in TM6* during glucose growth. Surprisingly, on ethanol, absolute expression levels of FBP1, MDH2 and ADH2 are higher in the TM6* strain than in the wild type (Fig 3). Perhaps the Tm6*p hexose transporter causes a different efflux of glucose produced in gluconeogenesis and/or trehalose turnover, resulting in various metabolic pool sizes.
Derepression of CYC1, which encodes iso-1-cytochrome C in respiration, is Snf1 dependent (Wright & Poyton, 1990) and expression is induced by the activator Hap1 (Pfeifer et al, 1987). Glucose repression of CYC1 expression is largely abolished in the TM6* strain (Fig 3), suggesting that also the Hap1 system senses carbon source availability internally rather than via membrane receptors.
The expression of genes encoding glycolytic and fermentative enzymes such as PGK1, TPI1, PDC1 and ADH1 is induced to a different degree by glucose (Fig 3). Their expression requires the regulator complex Rap1–Gcr1–Gcr2 (Chambers et al, 1995). The extent of induction depends on the glucose level and probably on glucose being taken up and metabolized (Muller et al, 1995). Our observations in the TM6* strain confirm this notion, as expression of these genes on glucose medium is diminished to different degrees.
Taking these results together, the TM6* and other similar strains (data not shown) are most useful tools to study glucose-induced regulatory effects and they will prove highly instrumental in further analyses.
Metabolic engineering
Over the last two decades, numerous attempts have been reported to redirect the flux of glucose towards respiration and to avoid ethanol production at high extracellular glucose concentrations. Eliminating the pathway from pyruvate to ethanol has failed, not least because Pdc is necessary for the production of cytosolic acetyl-CoA (Flikweert et al, 1996). A mutant devoid of the four most important alcohol dehydrogenases still produced ethanol (Drewke et al, 1990). It has also been attempted to increase respiratory activity by alleviating glucose repression. This resulted in a shift towards fermentation initiated at higher glucose levels and a relief from glucose repression but only a partial redirection of metabolism to respiration (Diderich et al, 2001). A similar result was obtained by different expression levels of the high-affinity transporter Hxt7 (Ye et al, 1999). The availability of the TM6* strain hence constitutes a breakthrough because it demonstrates that the mode of metabolism can be completely altered by engineering a single metabolic step—uptake. It provides research with a novel unique tool to study metabolic control in the yeast model system. This strain offers the possibility to study glucose signalling also at high extracellular glucose concentrations and maintaining respiratory growth. It also provides industry with a fully respiratory strain, which gives a higher biomass yield and potentially increased heterologous protein production in simple batch cultures.
Methods
Yeast strains
All strains were derived from CEN.PK2-1C (MATa, leu2-3, 112 ura3-52 trp1-289 his3-Δ MAL2-8cSUC2 hxt17Δ) (van Dijken et al, 2000) in which the auxotrophic markers HIS3, TRP1, LEU2 and URA3 had been reintroduced. In EBY.VW4002, all relevant hexose transporters have been deleted (Wieczorke et al, 1999) and the markers HIS3, TRP1 and LEU2 have been re-introduced resulting in the hxt null strain KOY.VW100. In EBY.VW4002, the HXT3-HXT6-HXT7 locus has been replaced by a cassette comprising the truncated HXT7 promoter (Hauf et al, 2000) followed by the KlURA3 gene and the HXT7 terminator.
Construction of TM6* strain
TM6 was generated using overlap extension PCR (Ho et al, 1989). The HXT1 (bp 1–741) portion was amplified from the plasmid pHXT1-2 (Reifenberger et al, 1997) and the HXT7 (bp 742–1713) portion from p21 (Reifenberger et al, 1995). The construct was integrated by recombination into KOY.VW100, replacing the KlURA3 marker. Transformants were selected first on 2% YPD plates and then on 1% maltose, 5-fluoroorotic acid YNB plates. Sequencing of TM6 revealed a point mutation (S279Y, TM6*). The strain was finally made prototrophic by integration of the URA3 marker. The TM6* construct was amplified from the TM6* strain and re-transformed into a new KOY.VW100. The same phenotype was obtained.
Medium and cultivation in fermentors
Bioreactor fermentations were performed in 1.5 l of 2 × concentrated complete minimal medium (Verduyn et al, 1992), 2% glucose and polypropylene glycol P2000 as anti-foam at 30°C, 1,500 r.p.m., pH 5.0, and an air flow of 0.75 l/min. Carbon dioxide production and oxygen consumption were measured on-line (type 1308, Bruel and Kjaer). The RQ was calculated as the ratio of the carbon dioxide production rate to the oxygen consumption rate.
Oxygen consumption rate
The oxygen consumption rate was measured in a Cyclobios oxygraph (A. Paar, Austria) during logarithmic growth on glucose by transferring 2.2 ml of cells directly into the oxygraph.
Anaerobic shift
Cultures were grown as described above until an OD610 of 1–1.5. The culture was shifted to anaerobicity (N2 gas) and Ergosterol/Tween 80 (10 μg/l/0.42 μg/l) was added.
Biochemical determinations
For dry weight, 5 ml of the culture was centrifuged, washed twice in MilliQ water and dried for 24 h at 110°C. The glucose, ethanol, glycerol and acetate concentrations in the medium supernatant were determined using enzymatic combination kits.
Sugar uptake analysis
Sugar uptake of 14C-glucose was assayed on logarithmic cells at a cell density of 7.5% w/v (Walsh et al, 1994). Protein was determined (Lowry et al, 1951) with bovine serum albumin (BSA) as a standard. Data from at least two independent experiments (performed in duplicate) were analysed using computer-assisted nonlinear regression. The calculations indicated one-component Michaelis–Menten uptake kinetics.
Activity measurements
Pdc activity (Schmitt et al, 1983) and invertase activity (Goldstein & Lampen, 1975) were measured according to established protocols. Protein was measured using the Bio-Rad DC protein assay with BSA as a standard.
mRNA and quantitative real-time PCR
Cells were cultivated in fermentors with 1% ethanol and 5 × concentrated minimal medium (Verduyn et al, 1992) and pulsed with 5% glucose at an OD610 of 1–1.5. Samples were taken during ethanol growth and at glucose concentrations between 15 and 25 g/l, mRNA was extracted, DNase treated and controlled on agarose gel. Reverse transcription (Superscript II, Invitrogen) using pd(T)12–18 as primers and quantitative real-time PCR assays were performed in an iCycler (Bio-Rad). PCR products were checked by agarose gel and melting curve analysis. Expression data were normalized against IPP1 and ACT1. Experiments were repeated at least twice and verified by northern blot analysis.
Acknowledgments
This work was supported by European Commission contract BIO4-CT98-0562, Swedish National Energy Administration (P1009-5), Swedish Council for Forestry and Agricultural Research (52.0609/97) and Swedish Research Council (621-2001-1988 to L.G.; research position to S.H.). Dr A. Kruckeberg is acknowledged for discussions.
References
- Chambers A, Packham EA, Graham IR (1995) Control of glycolytic gene expression in the budding yeast (Saccharomyces cerevisiae). Curr Genet 29: 1–9 [DOI] [PubMed] [Google Scholar]
- Diderich JA, Raamsdonk LM, Kruckeberg AL, Berden JA, Van Dam K (2001) Physiological properties of Saccharomyces cerevisiae from which hexokinase II has been deleted. Appl Environ Microbiol 67: 1587–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewke C, Thielen J, Ciriacy M (1990) Ethanol formation in adh0 mutants reveals the existence of a novel acetaldehyde-reducing activity in Saccharomyces cerevisiae. J Bacteriol 172: 3909–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiechter A, Fuhrmann GF, Kappeli O (1981) Regulation of glucose metabolism in growing yeast cells. Adv Microb Physiol 22: 123–183 [DOI] [PubMed] [Google Scholar]
- Flikweert MT, Van Der Zanden L, Janssen WM, Steensma HY, Van Dijken JP, Pronk JT (1996) Pyruvate decarboxylase: an indispensable enzyme for growth of Saccharomyces cerevisiae on glucose. Yeast 12: 247–257 [DOI] [PubMed] [Google Scholar]
- Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Biol Rev 62: 334–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Lampen JO (1975) β-D-Fructofuranoside fructohydrolase from yeast. Methods Enzymol 42: 504–511 [DOI] [PubMed] [Google Scholar]
- Hargreaves M (2000) Skeletal muscle metabolism during exercise in humans. Clin Exp Pharmacol Physiol 27: 225–228 [DOI] [PubMed] [Google Scholar]
- Hauf J, Zimmermann FK, Muller S (2000) Simultaneous genomic overexpression of seven glycolytic enzymes in the yeast Saccharomyces cerevisiae. Enzyme Microb Technol 26: 688–698 [DOI] [PubMed] [Google Scholar]
- Heigenhauser GJ, Parolin ML (1999) Role of pyruvate dehydrogenase in lactate production in exercising human skeletal muscle. Adv Exp Med Biol 474: 205–218 [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59 [DOI] [PubMed] [Google Scholar]
- Kappeli O (1986) Regulation of carbon metabolism in Saccharomyces cerevisiae and related yeasts. Adv Microb Physiol 28: 181–209 [DOI] [PubMed] [Google Scholar]
- Lowry O, Rosebrough N, Farr A, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275 [PubMed] [Google Scholar]
- Muller S, Boles E, May M, Zimmermann FK (1995) Different internal metabolites trigger the induction of glycolytic gene expression in Saccharomyces cerevisiae. J Bacteriol 177: 4517–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan S, Johnston M (1999) Function and regulation of yeast hexose transporters. Microbiol Mol Biol Rev 63: 554–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan S, Vallier LG, Flick JS, Carlson M, Johnston M (1997) Expression of the SUC2 gene of Saccharomyces cerevisiae is induced by low levels of glucose. Yeast 13: 127–137 [DOI] [PubMed] [Google Scholar]
- Parolin ML, Spriet LL, Hultman E, Hollidge-Horvat MG, Jones NL, Heigenhauser GJ (2000a) Regulation of glycogen phosphorylase and PDH during exercise in human skeletal muscle during hypoxia. Am J Physiol Endocrinol Metab 278: E522–E534 [DOI] [PubMed] [Google Scholar]
- Parolin ML, Spriet LL, Hultman E, Matsos MP, Hollidge-Horvat MG, Jones NL, Heigenhauser GJ (2000b) Effects of PDH activation by dichloroacetate in human skeletal muscle during exercise in hypoxia. Am J Physiol Endocrinol Metab 279: E752–E761 [DOI] [PubMed] [Google Scholar]
- Pfeifer K, Prezant T, Guarente L (1987) Yeast HAP1 activator binds to two upstream activation sites of different sequence. Cell 49: 19–27 [DOI] [PubMed] [Google Scholar]
- Reifenberger E, Freidel K, Ciriacy M (1995) Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol Microbiol 16: 157–167 [DOI] [PubMed] [Google Scholar]
- Reifenberger E, Boles E, Ciriacy M (1997) Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem 245: 324–333 [DOI] [PubMed] [Google Scholar]
- Rolland F, Winderickx J, Thevelein JM (2002) Glucosesensing and -signalling mechanisms in yeast. FEM Yeast Res 2: 183–201 [DOI] [PubMed] [Google Scholar]
- Schmitt HD, Ciriacy M, Zimmermann FK (1983) The synthesis of yeast pyruvate decarboxylase is regulated by large variations in the messenger RNA level. Mol Gen Genet 192: 247–252 [DOI] [PubMed] [Google Scholar]
- Schuller HJ (2003) Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae. Curr Genet 43: 139–160 [DOI] [PubMed] [Google Scholar]
- Walsh MC, Smits HP, Scholte M, van Dam K (1994) Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J Bacteriol 176: 953–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijken JP et al. (2000) An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb Technol 26: 706–714 [DOI] [PubMed] [Google Scholar]
- Verduyn C, Zomerdijk TPL, Van Dijken JP, Scheffers WA (1984) Continuous measurement of ethanol production by aerobic yeast suspension with an enzyme electrode. Appl Microbiol Biotechnol 19: 181–185 [Google Scholar]
- Verduyn C, Postma E, Scheffers WA, Van Dijken JP (1992) Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8: 501–517 [DOI] [PubMed] [Google Scholar]
- Wieczorke R, Krampe S, Weierstall T, Freidel K, Hollenberg CP, Boles E (1999) Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett 464: 123–128 [DOI] [PubMed] [Google Scholar]
- Wright RM, Poyton RO (1990) Release of two Saccharomyces cerevisiae cytochrome genes, COX6 and CYC1, from glucose repression requires the SNF1 and SSN6 gene products. Mol Cell Biol 10: 1297–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Kruckeberg AL, Berden JA, van Dam K (1999) Growth and glucose repression are controlled by glucose transport in Saccharomyces cerevisiae cells containing only one glucose transporter. J Bacteriol 181: 4673–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]