Abstract
Dystroglycan is an important cell adhesion receptor linking the actin cytoskeleton, via utrophin and dystrophin, to laminin in the extracellular matrix. To identify adhesion-related signalling molecules associated with dystroglycan, we conducted a yeast two-hybrid screen and identified mitogen-activated protein (MAP) kinase kinase 2 (MEK2) as a β-dystroglycan interactor. Pull-down experiments and localization studies substantiated a physiological link between β-dystroglycan and MEK and localized MEK with dystroglycan in membrane ruffles. Moreover, we also identified active extracellular signal-regulated kinase (ERK), the downstream kinase from MEK, as another interacting partner for β-dystroglycan and localized both active ERK and dystroglycan to focal adhesions in fibroblast cells. These studies suggest a role for dystroglycan as a multifunctional adaptor or scaffold capable of interacting with components of the ERK–MAP kinase cascade including MEK and ERK. These findings have important implications for our understanding of the role of dystroglycan in normal cellular processes and in disease states such as muscular dystrophy.
Keywords: dystroglycan, ERK, MAP kinase, MEK, muscular dystrophy, utrophin
Introduction
Dystroglycan is an important cell adhesion receptor linking the actin cytoskeleton, via utrophin and dystrophin, to laminin in the extracellular matrix (Winder, 2001). Dystroglycan has a vital role in maintaining muscle integrity, its loss leading to muscular dystrophy (Cote et al, 1999; Parsons et al, 2002), and has recently been implicated in the maintenance of cell polarity (Muschler et al, 2002; Deng et al, 2003). Previous studies have also shown that cell adhesion leads to tyrosine phosphorylation of the β-dystroglycan cytoplasmic domain, mediated by Src family kinases (James et al, 2000; Sotgia et al, 2001), preventing its association with utrophin (James et al, 2000). In addition, tyrosine phosphorylation of dystroglycan in muscle cells leads to a potential differential regulation of interactions between dystrophin and caveolin-3 (Sotgia et al, 2000; Ilsley et al, 2001, 2002). In non-muscle cells, tyrosine phosphorylation of dystroglycan leads to the recruitment of SH2 domain-containing adaptor proteins such as Nck and Shc (Sotgia et al, 2001), and the SH2/SH3 domain-containing adaptor protein Grb2 also associates with β-dystroglycan (Yang et al, 1995; Cavaldesi et al, 1999; Russo et al, 2000), but in an SH3-dependent manner (Yang et al, 1995) that is also competitive with dystrophin binding (Russo et al, 2000). Despite the association of dystroglycan with a number of adaptor proteins involved in a variety of signalling cascades, and even reported interactions with tyrosine kinases including Src, Fyn and FAK (Cavaldesi et al, 1999; Sotgia et al, 2001), no definitive role for dystroglycan in signalling has so far been elucidated. More recently, it has been demonstrated that engagement of dystroglycan by laminin has an inhibitory effect on the activation of the ERK–MAP (ERK, extracellular signal-regulated kinase; MAP, mitogen-activated protein) kinase cascade in response to the binding of α6β1 integrin to laminin (Ferletta et al, 2003), although the precise function or mechanism whereby dystroglycan exerts this effect is not understood. We report here an association between dystroglycan and components of the ERK–MAP kinase cascade, and the differential targeting by dystroglycan of MAP kinase kinase (MEK) and active ERK to membrane ruffles and focal adhesions, respectively. The ability of dystroglycan to interact with several components of the ERK–MAP kinase cascade may be part of the mechanism involved in dystroglycan modulating ERK activity in response to integrin engagement on laminin.
Results And Discussion
Dystroglycan interacts with MEK
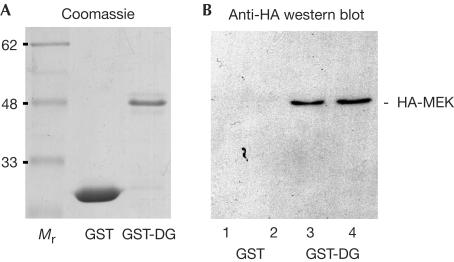
In a yeast two-hybrid screen against a HeLa cell library, using the cytoplasmic tail of β-dystroglycan as bait, we identified two independent full-length clones corresponding to the MAP kinase kinase 2 (MEK2) out of a total of 29 verified positive hits from 106 clones screened. To substantiate the yeast two-hybrid interaction by other means, we overexpressed haemagglutinin (HA)-tagged MEK1 and MEK2 in Cos-7 cells and subjected lysates from these cells to pull-down experiments with either glutathione-S-transferase (GST) or GST-dystroglycan cytoplasmic domain (GST-DG) (Fig 1). As can be seen, GST-DG was able to pull down specifically HA-MEK1 and HA-MEK2 from these extracts, suggesting a bona fide interaction between these two proteins. It has recently been suggested that in certain cells dystroglycan can act antagonistically to other adhesion molecules, such as integrins, and suppresses the activation of downstream kinase cascades (Ferletta et al, 2003). Dystroglycan was able to suppress the activation of the ERK–MAP kinase cascade induced by the interaction of integrin α6β1 with laminin (Ferletta et al, 2003), suggesting the possibility of an interaction between β-dystroglycan and components of the ERK–MAP kinase cascade. The direct interaction of dystroglycan with MEK might be sufficient to sequester and inactivate or prevent the activation of MEK and therefore reduce the activity of its downstream kinase ERK. Furthermore, it has previously been reported that the SH2/SH3 adaptor protein Grb2 also associates with β-dystroglycan (Yang et al, 1995; Russo et al, 2000). Grb2 is capable of linking SOS (Son of Sevenless), a guanine nucleotide exchange factor for Ras and the upstream activator of the Raf–MEK–ERK–MAP kinase cascade, to membrane receptors (Lowenstein et al, 1992; Buday & Downward, 1993; Egan et al, 1993). We have been unable to demonstrate any association between β-dystroglycan and SOS by immunoprecipitation (S.J. Winder, unpublished observations), and it remains unclear as to what the potential binding partner for Grb2 might be. Nevertheless, previous published data and our finding that MEK associates with dystroglycan suggest a potential mechanism whereby dystroglycan may modulate adhesion-mediated signalling from integrins to the ERK–MAP kinase cascade.
Figure 1.
Interaction of MEK with β-dystroglycan. (A) Coomassiestained gel of purified GST and GST-DG; Mr represents molecular mass markers. (B) Extracts from Cos-7 cells expressing HA-tagged MEK1 or MEK2 were incubated with either GST alone or GST-DG. GST alone was unable to pull down either HA-tagged MEK1 or MEK2 (lanes 1 and 2), whereas GST-DG was able to pull down both HA-MEK1 and MEK2 (lanes 3 and 4).
Association of dystroglycan with active ERK
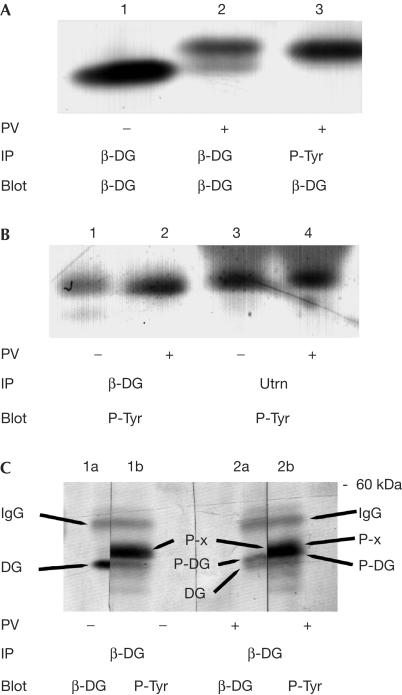
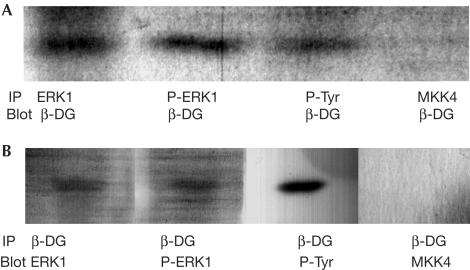
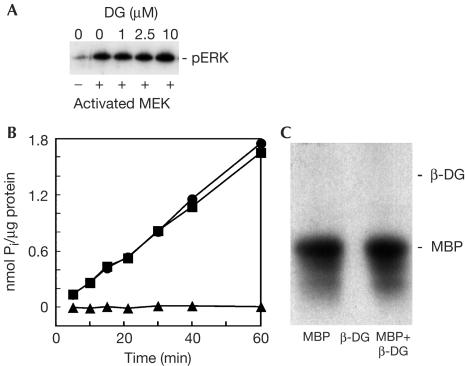
We have previously used cell adhesion and/or peroxyvanadate treatment to increase the phosphotyrosine content of β-dystroglycan (James et al, 2000; Ilsley et al, 2001), an approach used here to enrich possible interactions between potential SH2 adaptor proteins involved in anchoring MAP kinase components to β-dystroglycan. As we have observed previously, peroxyvanadate treatment of HeLa cells leads to an electrophoretic mobility shift in β-dystroglycan, consistent with its being phosphorylated on tyrosine residues, as determined by immunoprecipitation with antiphosphotyrosine antisera and western blotting for β-dystroglycan (James et al, 2000; Fig 2A). However, when the immunoprecipitation was first performed with β-dystroglycan or utrophin antisera and then blotted with antiphosphotyrosine antisera, a phosphotyrosine-containing band of ∼45 kDa was apparent in both peroxyvanadate-treated and untreated samples (Fig 2B). To further confirm that the ∼45 kDa phosphotyrosine-containing band was not in fact β-dystroglycan phosphorylated on tyrosine, or multiply phosphorylated on tyrosine and serine/threonine residues, we carried out immunoprecipitation of β-dystroglycan from untreated and peroxyvanadate-treated cells as before. In extracts from untreated cells, β-dystroglycan antisera immunoprecipitated a band of 43 kDa corresponding to β-dystroglycan itself (Fig 2C, lane 1a) and a higher band of ∼45 kDa, which was recognized with antiphosphotyrosine antisera (Fig 2C, lane 1b). In extracts from peroxyvanadate-treated cells, two weaker bands of β-dystroglycan immunoreactivity were evident (Fig 2C, lane 2a) corresponding to unphosphorylated (lower band) and tyrosine-phosphorylated β-dystroglycan (upper band). In the part developed with antiphosphotyrosine antiserum (lane 2b), there is a broad band, only part of which coincides with the higher migrating phosphorylated β-dystroglycan band. Given the previously identified association between dystroglycan and Grb2 (Yang et al, 1995), and the interaction between dystroglycan and MEK described above, we investigated the possibility that the phosphotyrosine-containing band associated with β-dystroglycan was another component of the Ras–Raf–MEK–ERK cascade. The most obvious candidate for the 45 kDa phosphoprotein was the downstream kinase of MEK, ERK, which is phosphorylated by the dualspecificity kinase MEK on both threonine and tyrosine residues, whereas MEK itself is phosphorylated only on serine residues (for review, see Pearson et al, 2001). Using antisera specific for inactive and activated forms of ERK MAP kinase, we identified activated p44 MAPK or ERK1 as the phosphotyrosine-containing β-dystroglycan-associated band (Fig 3A,B). Antisera against ERK, phospho-ERK or phosphotyrosine were all able to immunoprecipitate the endogenous β-dystroglycan, whereas antisera against MKK4 did not (Fig 3A). Conversely, β-dystroglycan antisera were able to immunoprecipitate an endogenous ∼45 kDa band, which was recognized by antisera against ERK, phospho-ERK or phosphotyrosine, but not MKK4 (Fig 3B), confirming that the band was the active phosphorylated form of ERK. ERK antisera raised against the inactive enzyme cannot distinguish between inactive and active ERK. Thus as ERK sera immunoprecipitated only the higher form of ERK corresponding to the band detected by the P-ERK antiserum, only active ERK appears to associate with β-dystroglycan. Despite the presence of both ERK1 and ERK2 in these cells, we were only able to detect an association between β-dystroglycan and ERK1, implying a specificity and distinct role for ERK1 binding to dystroglycan. Dystroglycan is therefore able to interact with both MEK and active ERK, as well as the adaptor Grb2. A conserved docking motif for MAP kinases comprising three or four basic residues has previously been described (Tanoue et al, 2000). These basic stretches serve as docking sites not only between the kinases themselves but also their substrates and phosphatases that inactivate them (Tanoue et al, 2000). The cytoplasmic tail of β-dystroglycan contains such a motif that may be involved in the docking of ERK. Furthermore, the primary sequence of the β-dystroglycan cytoplasmic tail is very proline-rich and contains several consensus sites for phosphorylation by ERK. We therefore investigated whether β-dystroglycan, as well as being associated with active ERK1, might also be a substrate for ERK1. However, the cytoplasmic tail of β-dystroglycan was neither itself phosphorylated, nor had it any effect on the ability of activated ERK to phosphorylate myelin basic protein, nor did it have any effect on the activity of MEK as determined by the ability of MEK to phosphorylate ERK (Fig 4). Thus, the association of β-dystroglycan with active ERK or MEK would simply appear to be as a membrane anchor rather than as a potential substrate, and under in vitro conditions, ERK was unable to phosphorylate β-dystroglycan.
Figure 2.
Immunoprecipitation of a tyrosine phosphoprotein with β-dystroglycan and utrophin. Cell extracts from adherent HeLa cells, untreated (−) or treated (+) with peroxyvanadate (PV), were immunoprecipitated (IP) with antisera against either β-dystroglycan (β-DG), phosphotyrosine (P-Tyr) or utrophin (Utrn) followed by western blotting (Blot) with sera against either β-DG or P-Tyr. (A) Peroxyvanadate caused a reduction in electrophoretic mobility of immunoprecipitated β-DG (compare lanes 1 and 2); a band corresponding to the higher migrating species could also be immunoprecipitated with P-Tyr antiserum (lane 3). (B) β-DG and utrophin are both able to immunoprecipitate a band of ∼45 kDa from HeLa cell extracts, which is recognized by antiphosphotyrosine sera, whether the cells were first treated with PV or not. (C) Untreated HeLa cell extracts (−) or PV-treated extracts (+) were immunoprecipitated with β-DG, separated by SDS–PAGE, transferred to PVDF and each single gel lane was cut in half. Each half lane was probed with either β-DG or P-Tyr antisera and developed as above. Cut halves were re-aligned to reveal four identifiable bands: namely IgG, upper band across all lanes; and unphosphorylated β-DG (lane 1a, lower band in lane 2a); tyrosine-phosphorylated β-DG (upper band in lane 2a, lower part of main band in lane 2b); and the ∼45 kDa phosphotyrosine band (lane 1b, upper part of lane 2b), arrowed as DG, P-DG and P-x, respectively.
Figure 3.
Immunoprecipitation of active ERK and β-DG. HeLa cell extracts were immunoprecipitated with antisera against β-DG, phosphotyrosine (P-Tyr), ERK, active ERK or MKK4 followed by western detection with the complementary sera. (A) Immunoprecipitation (IP) with either ERK, P-ERK or P-Tyr antisera reveals a 43 kDa band recognized by β-DG antisera corresponding to β-dystroglycan, but not with MKK4. (B) β-DG immunoprecipitation followed by western blotting with ERK, P-ERK and P-Tyr antisera all revealed a band of ∼45 kDa corresponding to phosphorylated ERK1 but not with MKK4.
Figure 4.
Effect of β-DG on activity of MEK and ERK. (A) GST-MEK was activated using activated Raf and then used to further activate GST-ERK (Yeung et al, 1999), either alone or in the presence of increasing concentrations of β-dystroglycan cytoplasmic domain (DG). ERK activation was detected by antisera for p-ERK (Sigma). Dystroglycan cytoplasmic domain had no effect on ERK activation. (B) Activated ERK was used to phosphorylate directly either myelin basic protein (MBP; squares), β-dystroglycan cytoplasmic domain (β-DG; triangles) or a mixture of the two proteins (circles). Only MBP and not β-DG was phosphorylated by ERK, and the presence of β-DG did not affect ERK phosphorylation of MBP. (C) This was confirmed by autoradiography of the final reaction mixtures showing that 32P label was only incorporated into MBP.
Differential localization of dystroglycan with ERK and MEK
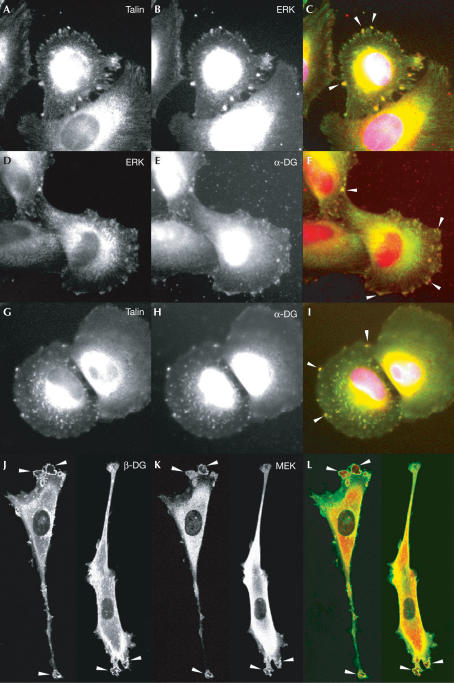
We next studied the localization of dystroglycan and both MEK and active ERK in cells to determine whether they were all localized to the same cellular structures, which might suggest a scaffold role for β-dystroglycan such as that seen with the adaptor protein MP1 (Schaeffer et al, 1998), or whether MEK and ERK localized with dystroglycan to different structures. Consistent with our inability to immunoprecipitate a ternary complex of DG–MEK–ERK, and the likely dissociation of ERK from MEK upon ERK activation (Wilsbacher et al, 1999), active ERK and MEK localized with dystroglycan to different cellular compartments. Active ERK localized with dystroglycan or talin at sites of cell–substrate adhesion, known as focal adhesions (Fig 5A–I), and MEK localized with dystroglycan in prominent ruffling membrane structures (Fig 5J–K). Dystroglycan therefore appears to associate with both MEK and active ERK in the cell, but in distinct compartments where it may perform different functions in either targeting or anchoring components of the MAP kinase cascade to specific cellular locations, or for specific functions. The ability of β-dystroglycan to target MEK to membrane ruffles and not to focal adhesions is consistent with the ability of dystroglycan to suppress the activation of MEK and ERK in response to integrin engagement (Ferletta et al, 2003). Dystroglycan may simply sequester MEK away from the rest of the Ras–RAF MAP kinase cascade machinery, preventing the efficient phosphorylation of ERK by MEK. But once ERK is activated, it is also able to interact with dystroglycan where it is efficiently targeted to focal adhesion structures induced by laminin (Fig 4). We have previously shown that activated ERK is targeted to focal adhesions by integrin engagement on laminin (Fincham et al, 2000), although how active ERK remained localized to adhesions was not clear. The ability of active ERK to associate with dystroglycan (Figs 2, 3 and 5), and its presence in adhesions (Belkin & Burridge, 1995; Khurana et al, 1995), provides a mechanism for the localization of active ERK in adhesions. The association of dystroglycan with components of the Ras-RAF MAP kinase cascade, including Grb2, MEK and ERK, and the tyrosine phosphorylation of dystroglycan in response to cell adhesion with recruitment of SH2 domain-containing adaptor proteins such as Shc and Nck and kinases of the Src family including Src, Fyn and FAK point to dystroglycan's having a key function in the transduction and modulation of various signalling cascades. A recent report also suggested Grb2- and SOS-dependent signalling to the Jun kinase cascade via the dystrophin–dystroglycan complex in myotubes (Oak et al, 2003), and JNK activity is elevated in dystrophic muscle (Kolodziejczyk et al, 2001), although dystroglycan was not implicated directly in either of these studies. Perturbation of dystroglycan function, and loss of its role in modulating signalling cascades, may also be an important factor in the aetiology of muscular dystrophies or in muscle cell survival, and these possibilities warrant further investigation.
Figure 5.
Colocalization of dystroglycan with active ERK and MEK in cells. REF52 rat embryo fibroblast cells were grown on laminin and fixed, permeabilized and stained for talin (A,G) active ERK (B,D) and dystroglycan (E,H). Talin and ERK, dystroglycan and ERK, and talin and dystroglycan are all found localized in discrete patches throughout the cells, with colocalization to prominent adhesions at the cell periphery seen as yellow (arrows in C,F,I). Co-transfected GFP-tagged dystroglycan and HA-MEK were visualized by the GFP signal for dystroglycan (J) and counterstained with anti-HA antisera for MEK (K). Dystroglycan-GFP staining was evident throughout the cell, with some perinuclear staining, but also prominent staining in membrane ruffles and other surface protrusions (J). A similar staining pattern was also seen with HA-MEK (K), which coincided with dystroglycan-GFP in the prominent membrane ruffles (L). Images (A,D,G,J) are the green channel and images (B,E,H,K) are the red channel in the respective merged images (C,F,I,L).
Methods
Expression constructs, yeast two-hybrid screen and protein expression. The cytoplasmic domain of mouse β-dystroglycan (residues 775–899) was generated by PCR with 5′ NdeI and BamHI restriction sites and 3′ stop codon and SalI site, and cloned into the BamHI and SalI sites of pGEX-2T and subcloned into pSJW1 (Winder & Kendrick-Jones, 1995) using NdeI and SalI. For yeast two-hybrid analyses, dystroglycan (residues 775–889) was cloned into pAS2 and screened against a HeLa cell cDNA library using the Matchmaker system (Clontech). For cellular studies, a full-length mouse dystroglycan cDNA was cloned into a pEGFP vector (Clontech). HA-tagged MEK1 and MEK2 were a gift from W. Kolch (Yeung et al, 1999). GST or the cytoplasmic domain of β-dystroglycan fused to GST (GST-DG) was expressed in Escherichia coli and purified on glutathione–Sepharose according to the manufacturer's instructions (Amersham). β-Dystroglycan cytoplasmic domain nonfusion protein expressed from pSJW1 was purified by cation exchange, HA and gel filtration chromatography.
Tissue culture, microscopy and pull-down assays. Culture of REF52, HeLa and Cos-7 cells, growth of cells on laminin, peroxyvanadate treatment and immunoprecipitation assays were carried out as described previously (James et al, 2000). Transfection of Cos-7 cells with HA-MEK or dystroglycan-GFP (GFP, green fluorescent protein) plasmids were performed using Lipofectamine, processed for microscopy, and stained for talin and p-ERK as described previously (Fincham et al, 2000). Cells were counterstained for α-dystroglycan with a sheep polyclonal serum (1:100) raised against residues 62–163 of mouse α-dystroglycan. To colocalize dystroglycan-GFP and HA-MEK, the GFP signal was visualized directly for dystroglycan and counterstained with anti-HA antisera (Roche, 1:100) and rhodamine secondary antibody.
Extracts from Cos-7 cells expressing HA-tagged MEK1 or MEK2 (Yeung et al, 1999) were incubated with either GST alone or GST-DG and re-purified on glutathione–Sepharose before SDS-polyacrylamide gel electrophoresis (PAGE), transfer to polyvinylidene fluoride and western blotting with antisera against the HA epitope.
MEK and ERK assays. MEK activity assay was carried out as described previously (Yeung et al, 1999), but replacing RKIP with bacterially expressed β-dystroglycan cytoplasmic domain. Phosphorylation by activated ERK1 (Upstate) using [γ-32P]-ATP of bacterially expressed β-dystroglycan cytoplasmic domain (17 μM) and myelin basic protein (28 μM), either alone or together, was carried out according to the manufacturer's instructions (Upstate). Phosphorylation was quantified by scintillation counting and confirmed by SDS–PAGE and autoradiography of samples taken at the 60 min time point.
Acknowledgments
We are grateful to Louise Anderson and Glenn Morris for providing dystroglycan antisera, to Derek Blake for mouse dystroglycan cDNA and to Walter Kolch for the HA-MEK plasmid. This work was supported by Wellcome Trust and MRC grants to S.J.W.
References
- Belkin AM, Burridge K (1995) Localization of utrophin and aciculin at sites of cell–matrix and cell–cell adhesion in cultured cells. Exp Cell Res 221: 132–140 [DOI] [PubMed] [Google Scholar]
- Buday L, Downward J (1993) Epidermal growth factor regulates p21Ras through the formation of a complex of receptor, GRB2 adapter protein, and SOS nucleotide exchange factor. Cell 73: 611–620 [DOI] [PubMed] [Google Scholar]
- Cavaldesi M, Macchia G, Barca S, Defilippi P, Tarone G, Petrucci TC (1999) Association of the dystroglycan complex isolated from bovine brain synaptosomes with proteins involved in signal transduction. J Neurochem 72: 1648–1655 [DOI] [PubMed] [Google Scholar]
- Cote PD, Moukhles H, Lindenbaum M, Carbonetto S (1999) Chimeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nat Genet 23: 338–342 [DOI] [PubMed] [Google Scholar]
- Deng W-M, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, Ruohola-Baker H (2003) Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development 130: 173–184 [DOI] [PubMed] [Google Scholar]
- Egan SE, Geddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA (1993) Association of SOS Ras exchange protein with GRB2 is implicated in tyrosine kinase signal transduction and transformation. Nature 363: 45–51 [DOI] [PubMed] [Google Scholar]
- Ferletta M, Kikkawa Y, Yu H, Talts JF, Durbeej M, Sonnenberg A, Timpl R, Campbell KP, Ekblom P, Genersch E (2003) Opposing roles of integrin α6Aβ1 and dystroglycan in laminin-mediated extracellular signal-regulated kinase activation. Mol Biol Cell 14: 2088–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, James M, Frame MC, Winder SJ (2000) Active ERK/MAP kinase is targeted to newly forming cell–matrix adhesions by integrin engagement and vsrc. EMBO J 19: 2911–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilsley JL, Sudol M, Winder SJ (2001) The interaction of dystrophin with β-dystroglycan is regulated by tyrosine phosphorylation. Cell Signal 13: 625–632 [DOI] [PubMed] [Google Scholar]
- Ilsley JL, Sudol M, Winder SJ (2002) The WW domain: linking cell signalling to the membrane cytoskeleton. Cell Signal 14: 183–189 [DOI] [PubMed] [Google Scholar]
- James M, Nuttall A, Ilsley JL, Ottersbach K, Tinsley JN, Sudol M, Winder SJ (2000) Adhesion-dependent tyrosine phosphorylation of β-dystroglycan regulates its interaction with utrophin. J Cell Sci 113: 1717–1726 [DOI] [PubMed] [Google Scholar]
- Khurana TS, Kunkel LM, Frederickson AD, Carbonetto S, Watkins SC (1995) Interaction of chromosome 6-encoded dystrophin related protein with the extracellular matrix. J Cell Sci 108: 173–185 [DOI] [PubMed] [Google Scholar]
- Kolodziejczyk SM, Walsh GS, Balazsi K, Seale SP, Sandoz J, Hierlihy AM, Rudnicki MA, Chamberlain JS, Miller FD, Megeney LA (2001) Activation of JNK1 contributes to dystrophic muscle pathogenesis. Curr Biol 11: 1278–1282 [DOI] [PubMed] [Google Scholar]
- Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Barsagi D, Schlessinger J (1992) The SH2 and SH3 domain containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell 70: 431–442 [DOI] [PubMed] [Google Scholar]
- Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ (2002) A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res 62: 7102–7109 [PubMed] [Google Scholar]
- Oak SA, Zhou YW, Jarrett HW (2003) Skeletal muscle signaling pathway through the dystrophin glycoprotein complex and Rac1. J Biol Chem 278: 39287–39295 [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Campos I, Hirst EMA, Stemple DL (2002) Removal of dystroglycan causes severe muscular dystrophy in zebrafish embryos. Development 129: 3505–3512 [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Gibson TB, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183 [DOI] [PubMed] [Google Scholar]
- Russo K, Stasio ED, Macchia G, Rosa G, Brancaccio A, Petrucci TC (2000) Characterisation of the β-dystroglycan–growth factor receptor 2 (Grb2) interaction. Biochem Biophys Res Commun 274: 93–98 [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Catling AD, Eblen ST, Collier LS, Krauss A, Weber ML (1998) MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281: 1668–1671 [DOI] [PubMed] [Google Scholar]
- Sotgia F et al. (2000) Caveolin-3 directly interacts with the c-terminal tail of β-dystroglycan: identification of a central WW-like domain within caveolin family members. J Biol Chem 275: 38048–38058 [DOI] [PubMed] [Google Scholar]
- Sotgia F, Lee H, Bedford M, Petrucci TC, Sudol M, Lisanti MP (2001) Tyrosine phosphorylation of β-dystroglycan at its WW domain binding motif, PPxY, recruits SH2 domain containing proteins. Biochemistry 40: 14585–14592 [DOI] [PubMed] [Google Scholar]
- Tanoue T, Adachi M, Moriguchi T, Nishida E (2000) A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol 2: 110–116 [DOI] [PubMed] [Google Scholar]
- Wilsbacher JL, Goldsmith EJ, Cobb MH (1999) Phosphorylation of MAP kinases by MAP/ERK involves multiple regions of MAP kinases. J Biol Chem 274: 16988–16994 [DOI] [PubMed] [Google Scholar]
- Winder SJ (2001) The complexities of dystroglycan. Trends Biochem Sci 26: 118–124 [DOI] [PubMed] [Google Scholar]
- Winder SJ, Kendrick-Jones J (1995) Protein production in 3 different expression vectors from a single PCR product. Anal Biochem 231, 271–273. Erratum published (1996) 236: 190 [DOI] [PubMed] [Google Scholar]
- Yang B, Jung D, Motto D, Meyer J, Koretzky G, Campbell KP (1995) SH3 domain-mediated interaction of dystroglycan and Grb2. J Biol Chem 270: 11711–11714 [DOI] [PubMed] [Google Scholar]
- Yeung K et al. (1999) Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401: 173–177 [DOI] [PubMed] [Google Scholar]